Abstract
Purpose
The risk factors for excessive blood loss and transfusion during total knee arthroplasty (TKA) remain unclear. The present study aimed to determine the risk factors for excessive blood loss and establish a predictive model for postoperative blood transfusion.
Methods
This retrospective study included 329 patients received TKA, who were randomly assigned to a training set (n = 229) or a test set (n = 100). Univariate and multivariate linear regression analyses were used to determine risk factors for excessive blood loss. Univariate and multivariate logistic regression analyses were used to determine risk factors for blood transfusion. R software was used to establish the prediction model. The accuracy and stability of the models were evaluated using calibration curves, consistency indices, and receiver operating characteristic (ROC) curve analysis.
Results
Risk factors for excessive blood loss included timing of using a tourniquet, the use of drainage, preoperative ESR, fibrinogen, HCT, ALB, and free fatty acid levels. Predictors in the nomogram included timing of using a tourniquet, the use of drainage, the use of TXA, preoperative ESR, HCT, and albumin levels. The area under the ROC curve was 0.855 (95% CI, 0.800 to 0.910) for the training set and 0.824 (95% CI, 0.740 to 0.909) for the test set. The consistency index values for the training and test sets were 0.855 and 0.824, respectively.
Conclusions
Risk factors for excessive blood loss during and after TKA were determined, and a satisfactory and reliable nomogram model was designed to predict the risk for postoperative blood transfusion.
Similar content being viewed by others
Introduction
Total knee arthroplasty (TKA) is a common and reliable procedure for the treatment of end-stage knee osteoarthritis (OA) [1]. Successful TKA not only requires effective relief of joint pain and accurate alignment of the lower extremities but also minimizes tissue damage and blood loss. In previous investigations, the rate of blood transfusion in TKA procedures was 18.2% in a study involving 139,804 patients2 and 9.27% in another involving 949 patients [2]. Therefore, blood management is crucial for individuals undergoing TKA, and preventive measures are needed for those at a high risk for excessive blood loss to avoid or, at least mitigate, the adverse effects of this surgical complication.
Blood transfusion(s) itself is associated with several potential complications, including increased length of hospital stay (LOS), surgical site infection, pathogen transmission, hemolytic transfusion reactions, transfusion-induced coagulopathy, immunological reactions, and acute kidney injury [3,4,5]. These risks and the associated transfusion costs highlight the importance of minimizing the use of blood products in TKA [3].
Previous studies have reported that female sex, older age, longer operative duration, and lower preoperative hemoglobin levels are risk factors for perioperative transfusion after total hip arthroplasty (THA) [2, 6, 7]; however, risk factors for blood loss after TKA are not well understood. As such, the aim of the present study was to determine such risk factors for TKA and to establish a prediction model for blood transfusion, which is crucial for early identification and intervention in patients at high risk for excessive blood loss and blood transfusion.
Materials and methods
Patients and data collection
Data from patients, who underwent TKA for OA between September 2022 and May 2023 at the authors’ hospital, were enrolled. Patients with a history of coagulation disorders and those who received allogeneic blood transfusions within 30 days before TKA were excluded from the study. A total of 348 patients were enrolled in the study. After excluding 19 patients with incomplete HCT data, 329 patients were included, and all patients were randomly assigned to either the training group (N = 249) or the test group (N = 100) for further analysis and establishment of a prediction model for transfusion.
Surgical technique
All the surgeries were performed by the same experienced surgeon, who have performed over 5000 TKAs. The surgical procedures were performed under general anesthesia. All the participants used posterior stabilized knee prosthesis (Zimmer). The timing of using a tourniquet included early inflation (inflation before skin incision and release after wound closure) and late inflation (inflation before the placement of prothesis and release after the closure of joint capsule). Medial parapatellar approach was used. After the femur and tibia osteotomy, soft tissue was released to achieve balance between internal and external gaps and balance between flexion and extension gaps. After placing the prothesis, the incision was closed layer by layer. 1.5 g (15 ml) TXA or 15 ml normal saline was injected into the joint cavity after suturing the joint capsule. For patients with drainage tubes, the tubes were clamped for 2 h before opening, and was removed 24 h postoperatively. No intraoperative complication was observed. All patients followed the same rehabilitation program and analgesia pattern after surgery.
Potential predictive factors
Data were obtained from the hospital’s electronic information system. Baseline (age, gender, height, weight, body mass index (BMI), and blood pressure), intraoperative (operation time, timing of using a tourniquet, the use of MAKO robot, the use of drainage), and preoperative laboratory investigation results (hemoglobin (HGB) level, red blood cell count (RBC), hematocrit (HCT), platelet count (PLT), erythrocyte sedimentation rate (ESR), plasma albumin (ALB) and globulin, blood glucose, blood uric acid, blood calcium, blood potassium, blood lipids and hemagglutination index) were also obtained. Hematocrit (HCT) levels were also measured on the morning of postoperative day 1.
Outcome measures
Total blood loss (TBL) was calculated using the following methods. The estimated blood volume (EBV) was calculated according to Gross formula: EBV = K1 × height (m)^3 + K2 × weight (kg) + K3. When the patient is male, K1 = 0.3669, K2 = 0.03219, K3 = 0.6041, and when the patient is female, K1 = 0.3561, K2 = 0.03308, K3 = 0.1833 [8]. Then, TBL was also calculated according to Gross’s formula: TBL = EBV× (preoperative HCT- postoperative HCT)/average value of HCT [9] The TBL/estimated blood volume (EBV) ratio was calculated as an indicator of evaluate the effect of blood loss. Patients with a TBL/EBV ratio > 20% underwent postoperative blood transfusion(s).
Statistical analysis
Data were analyzed using SPSS (IBM Corporation, Armonk, NY, USA) (Version 25.0; IBM) and R version 4.1.2 (R Foundation for Statistical Computing, Vienna, Austria) (R Foundation for Statistical Computing). Categorical variables were analyzed using the χ [6] test, and continuous variables were analyzed with the independent samples t-test or rank sum test. Differences with P < 0.05 were considered to be statistically significant.
Identification of risk factors for intra-and postoperative excessive blood loss
Univariate and multiple linear regression analyses were performed to determine risk factors for excessive blood loss during and after TKA. The dependent variable was EBL/TBV in the univariate and multiple linear regression analyses. Variables were initially screened by univariate linear analysis; those with P < 0.1 were included in multivariate linear analysis. Variables with P < 0.05 in multivariate linear analysis were regarded as independent risk factors for excessive blood loss during and after TKA. Detailed steps for identifying the risk factors for excessive blood loss during and after TKA are presented in Fig. 1.
Establishment of the nomogram prediction model for postoperative blood transfusion
To identify risk factors for postoperative blood transfusion and establish a predictive model. Data from the 329 enrolled patients were randomly divided into training and test sets (N = 98). The dependent variable was whether the patients received postoperative blood transfusions in the univariate and multivariate logistic regression analyses. Binary univariate logistic regression analysis was first performed on the potential predictive factors, and those with P < 0.1 in the univariate analysis were included in the stepwise backward likelihood ratio (LR) multivariate analysis. Variables with P < 0.05 were regarded as independent risk factors for postoperative blood transfusion. Variance inflation factors (VIFs) and tolerances were calculated to assess the collinearity assumption, with a VIF of < 5 and tolerance > 0.1 considered to indicate no significant collinearity. These variables were used to establish a nomogram prediction model.
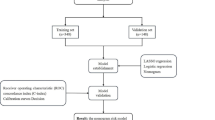
Validation of the nomogram prediction model
The reliability of the internal validation was assessed using the bootstrap method with 1000 replicates. The discrimination of the nomogram model was evaluated using the consistency index (C-index) and receiver operating characteristic (ROC) curve analysis. Calibration curves were drawn, and decision curve analysis (DCA) was performed. The construction and validation of the nomogram prediction model were completed through R language code packages including “rms”, “calibrate”, and “regplot”. The steps for establishing and validating the nomogram prediction model are detailed in Fig. 2.
Results
Patient characteristics
A total of 329 patients who fulfilled the inclusion criteria were enrolled in this study. The mean (± SD) age of the cohort was 68.00 ± 6.69 years, and the mean body mass index (BMI) was 27.62 ± 3 0.77 kg/m2. Among the 329 patients, 73 received transfusion (mean age, 68.27 ± 6.03 years; mean BMI, 27.27 ± 3.04 kg/m2), while 256 did not receive transfusion (mean age, 67.92 ± 6.88 years; mean BMI, 27.80 ± 3.56 kg/m2). Other descriptive data for all patients are summarized in Table 1.
Incidence and risk factors for excessive blood loss
Univariate linear regression analysis
According to univariate linear analysis, there was no significant correlation between excessive blood loss and age, sex, Kellgren-Lawrence (K-L) grading scale, blood pressure, or preoperative laboratory investigation results, including red blood cells and HGB, PLT, CRP, total cholesterol, lipoprotein A1, lipoprotein B, APOB/APOA, high-density lipoprotein, low-density lipoprotein, lipoprotein A, PT, TT, D-dimer levels. However, there were statistically significant correlation of excessive blood loss with BMI, timing of using a tourniquet, the use of drainage, operation time, and preoperative laboratory test results including HCT, blood calcium, ESR, INR, fibrinogen, blood glucose, activity of antithrombin III, ALB, globulin, triglyceride and free fatty acids. (P < 0.1). Analysis results of the measurements and count data are summarized in Table 2.
Multivariate linear regression analysis
Multivariate linear regression analysis including 15 statistically significant variables as independent variables, and TBL/EBV as the dependent variable revealed that TBL/EBV was associated with timing of using a tourniquet (P < 0.001), drainage (P = 0.048), preoperative erythrocyte sedimentation rate (ESR) (P < 0.001), fibrinogen (P = 0.029), HCT (P < 0.001), albumin (ALB) (P < 0.002), and free fatty acid levels (P = 0.007) (Table 2).
Incidence and risk factors for blood transfusion
Univariate logistic regression analysis
Factors with P < 0.1 in the univariate analysis were regarded to be significant. According to univariate logistic analysis, 13 variables were identified as significant factors for transfusion, including the use of drainage, timing of using a tourniquet, the use of TXA, and preoperative HCT, ESR, blood calcium, APTT ratio, fibrinogen, activity of antithrombin III, ALB, globulin, triglyceride and free fatty acid levels, whereas other variables did not exhibit significant differences between the transfusion and non-transfusion groups (Table 3).
Multivariate logistic regression analysis
Multivariate logistic regression analysis of 13 statistically significant variables revealed that transfusion was associated with timing of using a tourniquet (odds ratio [OR] = 0.142, 95% confidence interval [CI] = 0.059–0.338, P < 0.001), drainage, (OR 2.324, 95% CI 1.088–4.965, P = 0.029), the use of TXA (OR 0.175, 95% CI 0.084–0.362, P < 0.001), preoperative ESR (OR 1.122, 95% CI 1.070–1.177, P < 0.001), HCT (OR 1.193, 95% CI 1.066–1.335, P = 0.002), and ALB level (OR 1.260, 95% CI 1.091–1.454, P = 0.002) (Table 3).
Development and validation of nomogram for transfusion
Independent risk factors were included in the logistic regression prediction model and the nomogram was generated. The integral value of each included variable is reported in Fig. 3 and, by combining the integral values of all variables, the total score and corresponding probability can be calculated. The area under the ROC curve (AUC) (Fig. 4) was 0.855 (95% confidence interval [CI] 0.800–0.910) for the training set and 0.824 (95% CI 0.740–0.909) for the test set, indicating satisfactory discrimination performance of the prediction model. Furthermore, the C-index values from the calibration curves were 0.855 for the training set and 0.824 for the test set, and the 95% CI obtained using the bootstrap method with 1000 replicates was 0.800–0.910 for the training model and 0.740–0.909 for the test model. To identify the potential clinical benefits of the designed nomogram, DCA was performed using this dataset. DCA for this model, demonstrating its superiority over “treat all” or “treat none” strategies when threshold probabilities range from 0 to 1 is presented in Fig. 4. The calibration curve demonstrated good agreement with the observed probabilities of blood transfusion in this study (Supplementary file 1). These findings indicate that the prediction model was satisfactory.
A nomogram based on the 6 independent predictors of transfusion. For the TXA or the Drainage, “0” refers to not use and “1” refers to use; For the Tourniquet, “early” refers to inflation before skin incision and release after wound closure, and “late” refers to inflation before the placement of prothesis and release after the closure of joint capsule. *P < 0.05; **P < 0.01; ***P < 0.001
Discussion
Blood loss is inevitable in patients undergoing TKA, and sometimes patients require allogeneic blood transfusion to maintain normal hemoglobin levels. However, several studies have suggested that transfusion increases the risk for complications, mortality, costs, and length of hospital stay [4, 5, 10, 11]. Therefore, it is essential to identify high-risk patients and intervene early to reduce the risk for transfusion. Some studies have investigated single factors that affect blood loss and transfusion. However, few investigations have screened and identified the risk factors for excessive blood loss or established a prediction model to predict the risk for transfusion after TKA.
We propose, for the first time, that a high preoperative HCT value is an independent risk factor for postoperative blood transfusion in TKA. We found that the preoperative HCT in the transfusion group was higher than that in the non-transfusion group. Our study was supported by another study suggesting that a high preoperative HCT value was a risk factor for increased hidden blood loss (HBL) in patients undergoing laparoscopy and laparotomy for cervical cancer treatment [12].
The purpose of tourniquet use in TKA is to reduce or―at least mitigate―intraoperative blood loss, provide a better surgical field of vision, and improve the integration of bone cement into the bone. However, some evidence has challenged the necessity and safety of tourniquets because they may induce vascular injury, thromboembolism, reduce range of motion (ROM), and aggravate postoperative pain postoperatively [13,14,15]. Some researchers argued that no reduction in postoperative transfusion rates is observed when a tourniquet is used. In fact, while the use of tourniquets reduces intraoperative blood loss [16], it significantly increases total blood loss (i.e., blood loss during the operation and in the postoperative period) [17]. Activation of fibrinolysis after tourniquet deflation may explain this phenomenon [18]. Our results indicated that early inflation of tourniquet was an independent risk factor for increased blood loss and postoperative transfusion, which was consistent with the aforementioned findings.
The use of surgical drains after TKA has been standard practice for many years [19]. It is believed that the reduced formation of hematomas may reduce swelling, reduce the nidus for infection, and improve ROM [20]. However, the necessity for drainage in TKA has been a challenged [21]. Some clinicians believe that drainage increases blood loss because it destroys the tamponade effect at the surgical site. Drainage yielded no significant improvement in terms of ROM, reduction in swelling, and LOS [22] and did not increase the risk for complication(s) after TKA [23]. Closed-suction drainage is also a risk factor for infection after TKA [24]. Our results indicated that drainage was an independent risk factor for increased blood loss and postoperative transfusion.
We found that high ALB levels were risk factors for increased blood loss and transfusion. A low ALB level was an independent risk factor for increased LOS in patients undergoing TKA and/or THA. One study investigating TKA suggested that preoperative ALB < 30 g/L could increase LOS [25]. Similar results were found in THA, as the ALB level of patients with LOS < 48 h was significantly higher than that of patients with LOS > 48 h [26]. However, the effect of preoperative ALB levels on blood loss has rarely been reported. One study suggested that ALB was not associated with HBL after posterior lumbar fusion surgery; however, it did not report a correlation between ALB and TBL [27]. Higher preoperative plasma ALB level was an independent risk factor for greater intraoperative blood loss during intracranial meningioma [28].
We found that high ESR was an independent risk factor for excessive blood loss and blood transfusion. Our result was supported by another study that proposed that a high ESR was one of the risk factors for blood loss in patients with ankylosing spondylitis with hip involvement undergoing THA [29]. ESR is regarded as a biomarker of inflammatory diseases, and its level reflects disease activity. Although OA has not been traditionally treated as an inflammatory disease, accumulating evidence suggests that inflammation plays a role in its pathogenesis and progression [30,31,32]. For patients with high preoperative ESR, we believe that systematic history taking is essential to exclude active inflammation. Good management of the preoperative ESR is not only useful for reducing the possibility of infection but also for reducing blood loss during the perioperative period of TKA.
The use of tranexamic acid (TXA) in TKA has been confirmed to be effective in reducing blood loss and the risk for blood transfusion [33]. Our study further confirmed this finding because the transfusion rate in the TXA group was lower than that in the non-TXA group. We did not observe any complications with the use of TXA, and its safety was confirmed in another study, which suggested that intravenous application of TXA within 24 h after TKA led to reduced HBL without an obvious increase in the incidence of VTE or thrombosis [34].
Nomograms are widely used for clinical diagnoses and predictions. In the present study, we established a nomogram prediction model for transfusion(s) after TKA. Based on this nomogram, multiple risk factors can be quantified according to transfusion risk. The AUC for the nomogram was 0.855 (95% CI 0.800–0.910), which was higher than the threshold for good performance (AUC > 0.8). Moreover, the nomogram was validated in an independent cohort, and the validation results confirmed that it was effective in predicting the need for perioperative transfusion after TKA.
There are some researches on the risk factors for blood transfusion in TKA. One research reported that preoperative Hb levels and use of TXA was associated with the Hb loss, and preoperative Hb levels was associated with blood transfusion [35]. However, in our study, no significant difference of the preoperative Hb levels between the transfusion group and the non-transfusion group was observed, while the preoperative HCT was associated with transfusion. Another study proposed that posterior cruciate retaining prosthesis and topical use of TXA were preferred to reduce total blood loss [36]. All the participants used posterior stabilized knee prosthesis in our study so we did not evaluate the effect of difference kind of prosthesis on the blood loss and transfusion. What we share in common with these researches are that the use of tourniquet could not reduce blood loss, the use of drainage could increase blood loss and the use of TXA could reduce blood loss. Apart from these, we also determined that high ALB levels and high ESR were independent risk factors for excessive blood loss and blood transfusion in TKA, which has not been reported previously.
However, this study had some limitations, the first of which was that the patient population was derived from a single center and the sample size was relatively small. Although the model demonstrated satisfactory performance on the internal validation set, it lacked an independent external validation set, which could potentially affect its generalizability. Large-scale, multicenter studies should be performed to further enhance and externally validate the model.
Conclusion
The present study identified risk factors for excessive blood loss and established a predictive model for postoperative transfusion after TKA. Risk factors for excessive blood loss included the timing of tourniquet use, drainage, preoperative ESR, fibrinogen, HCT, ALB, and free fatty acid levels. Predictors in the nomogram included the timing of tourniquet use, drainage, TXA, preoperative ESR, HCT, and ALB level. We established a satisfactory and reliable nomogram prediction model to predict the risk for postoperative blood transfusion and to guide clinical decision-making regarding reducing blood loss. For those patients with more risk factors of blood transfusion and high score on the nomogram, blood preparation preoperatively is recommended.
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
Singh JA, Yu S, Chen L, Cleveland JD. Rates of total joint replacement in the United States: future projections to 2020–2040 using the National Inpatient Sample. J Rheumatol. 2019;46(9):1134–40.
Frisch NB, Wessell NM, Charters MA, et al. Predictors and complications of blood transfusion in total hip and knee arthroplasty. J Arthroplasty. 2014;29(9 Suppl):189–92.
White CC, th, Eichinger JK, Friedman RJ. Minimizing blood loss and transfusions in total knee arthroplasty. J Knee Surg. 2018;31(7):594–9.
Hart A, Khalil JA, Carli A, et al. Blood transfusion in primary total hip and knee arthroplasty. Incidence, risk factors, and thirty-day complication rates. J Bone Joint Surg Am. 2014;96(23):1945–51.
Kim JL, Park JH, Han SB, Cho IY, Jang KM. Allogeneic blood transfusion is a significant risk factor for Surgical-Site infection following total hip and knee arthroplasty: a Meta-analysis. J Arthroplasty. 2017;32(1):320–5.
Bedard NA, Pugely AJ, Lux NR, et al. Recent trends in blood utilization after primary hip and knee arthroplasty. J Arthroplasty. 2017;32(3):724–7.
Song K, Pan P, Yao Y, Jiang T, Jiang Q. The incidence and risk factors for allogenic blood transfusion in total knee and hip arthroplasty. J Orthop Surg Res. 2019;14(1):273.
Nadler SB, Hidalgo JH, Bloch T. Prediction of blood volume in normal human adults. Surgery. 1962;51(2):224–32.
Gross JB. Estimating allowable blood loss: corrected for dilution. Anesthesiology. 1983;58(3):277–80.
Jiang T, Song K, Yao Y, Pan P, Jiang Q. Perioperative allogenic blood transfusion increases the incidence of postoperative deep vein thrombosis in total knee and hip arthroplasty. J Orthop Surg Res. 2019;14(1):235.
Everhart JS, Sojka JH, Mayerson JL, Glassman AH, Scharschmidt TJ. Perioperative Allogeneic Red Blood-Cell Transfusion Associated with Surgical site infection after total hip and knee arthroplasty. J Bone Joint Surg Am. 2018;100(4):288–94.
Zhao Y, Hu J, Xiang J, et al. Hidden blood loss and its risk factors in patients undergoing laparoscopy and laparotomy for cervical cancer management. Arch Gynecol Obstet. 2019;300(1):183–9.
Patil S, Allan DB, Quin R. Effect of total knee arthroplasty on blood flow to the lower limb: a prospective clinical study and review of literature. J Arthroplasty. 2002;17(7):882–6.
Tai TW, Chang CW, Lai KA, Lin CJ, Yang CY. Effects of tourniquet use on blood loss and soft-tissue damage in total knee arthroplasty: a randomized controlled trial. J Bone Joint Surg Am. 2012;94(24):2209–15.
Ledin H, Aspenberg P, Good L. Tourniquet use in total knee replacement does not improve fixation, but appears to reduce final range of motion. Acta Orthop. 2012;83(5):499–503.
Yi S, Tan J, Chen C, Chen H, Huang W. The use of pneumatic tourniquet in total knee arthroplasty: a meta-analysis. Arch Orthop Trauma Surg. 2014;134(10):1469–76.
Smith TO, Hing CB. Is a tourniquet beneficial in total knee replacement surgery? A meta-analysis and systematic review. Knee. 2010;17(2):141–7.
Aglietti P, Baldini A, Vena LM et al. Effect of tourniquet use on activation of coagulation in total knee replacement. Clin Orthop Relat Res 2000(371):169–77.
Waugh TR, Stinchfield FE. Suction drainage of orthopaedic wounds. J Bone Joint Surg Am. 1961;43–A:939–46.
Parker MJ, Livingstone V, Clifton R, McKee A. Closed suction surgical wound drainage after orthopaedic surgery. Cochrane Database Syst Rev. 2007;2007(3):CD001825.
Adalberth G, Bystrom S, Kolstad K, Mallmin H, Milbrink J. Postoperative drainage of knee arthroplasty is not necessary: a randomized study of 90 patients. Acta Orthop Scand. 1998;69(5):475–8.
Quinn M, Bowe A, Galvin R, Dawson P, O’Byrne J. The use of postoperative suction drainage in total knee arthroplasty: a systematic review. Int Orthop. 2015;39(4):653–8.
Jenny JY, Boeri C, Lafare S. No drainage does not increase complication risk after total knee prosthesis implantation: a prospective, comparative, randomized study. Knee Surg Sports Traumatol Arthrosc. 2001;9(5):299–301.
Minnema B, Vearncombe M, Augustin A, Gollish J, Simor AE. Risk factors for surgical-site infection following primary total knee arthroplasty. Infect Control Hosp Epidemiol. 2004;25(6):477–80.
Li G, Weng J, Xu C, et al. Factors associated with the length of stay in total knee arthroplasty patients with the enhanced recovery after surgery model. J Orthop Surg Res. 2019;14(1):343.
Wang H, Fan T, Li W, et al. A nomogram to predict the risk of prolonged length of stay following primary total hip arthroplasty with an enhanced recovery after surgery program. J Orthop Surg Res. 2021;16(1):716.
Lei F, Li Z, He W, et al. Hidden blood loss and the risk factors after posterior lumbar fusion surgery: a retrospective study. Med (Baltim). 2020;99(19):e20103.
Wang C, Li P. Risk factors for intraoperative blood loss in resection of intracranial meningioma: analysis of 530 cases. PLoS ONE. 2023;18(9):e0291171.
Li L, Fu J, Xu C, et al. Factors associated with blood loss in ankylosing spondylitis patients with hip involvement undergoing primary total hip arthroplasty: a cross-sectional retrospective study of 243 patients. J Orthop Surg Res. 2020;15(1):541.
Sanchez-Lopez E, Coras R, Torres A, Lane NE, Guma M. Synovial inflammation in osteoarthritis progression. Nat Rev Rheumatol. 2022;18(5):258–75.
Molnar V, Matisic V, Kodvanj I et al. Cytokines and chemokines involved in Osteoarthritis Pathogenesis. Int J Mol Sci 2021;22(17).
Knights AJ, Redding SJ, Maerz T. Inflammation in osteoarthritis: the latest progress and ongoing challenges. Curr Opin Rheumatol. 2023;35(2):128–34.
Fillingham YA, Ramkumar DB, Jevsevar DS, et al. The efficacy of Tranexamic Acid in total knee arthroplasty: a Network Meta-Analysis. J Arthroplasty. 2018;33(10):3090–e30983091.
Xue CX, Yao YF, Lv H, Cheng L, Jing JH. Efficacy and safety of postoperative intravenous tranexamic acid in total knee arthroplasty: a prospective randomized controlled study. Orthop Surg. 2021;13(8):2227–35.
Boutsiadis A, Reynolds RJ, Saffarini M, Panisset JC. Factors that influence blood loss and need for transfusion following total knee arthroplasty. Ann Transl Med. 2017;5(21):418.
Hu Y, Li Q, Wei BG, et al. Blood loss of total knee arthroplasty in osteoarthritis: an analysis of influential factors. J Orthop Surg Res. 2018;13(1):325.
Funding
This work was supported by the National Natural Science Foundation of China (NO. 81672197). Grant numbers: ¥520,000. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Author information
Authors and Affiliations
Contributions
Yikai Liu: Data collection, Writing – original draft; Jiangshan Ai: Investigation, statistical analysis. Xue Teng: Data curation, writing - review & editing. Zhenchao Huang: Statistical analysis; Haoshen Wu: Validation. Zian Zhang: Validation; Wenzhe Wang: Formal analysis; Chang Liu: Formal analysis; Haining Zhang: Conceptualization, Supervision, Funding.
Corresponding author
Ethics declarations
Conflicts of interest
None.
Ethics approval and consent to participate
This study was approved by the medical ethics committee of our hospital (QYFY WZLL 28091), and all patients written informed consent.
Consent for publication
Not applicable.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Liu, Y., Ai, J., Teng, X. et al. Risk factor analysis and establishment of a nomogram model to predict blood loss during total knee arthroplasty. BMC Musculoskelet Disord 25, 459 (2024). https://doi.org/10.1186/s12891-024-07570-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12891-024-07570-3