Abstract
The application of Artificial intelligence (AI) and machine learning (ML) tools in total (TKA) and unicompartmental knee arthroplasty (UKA) emerges with the potential to improve patient-centered decision-making and outcome prediction in orthopedics, as ML algorithms can generate patient-specific risk models. This review aims to evaluate the potential of the application of AI/ML models in the prediction of TKA outcomes and the identification of populations at risk.
An extensive search in the following databases: MEDLINE, Scopus, Cinahl, Google Scholar, and EMBASE was conducted using the PIOS approach to formulate the research question. The PRISMA guideline was used for reporting the evidence of the data extracted. A modified eight-item MINORS checklist was employed for the quality assessment. The databases were screened from the inception to June 2022.
Forty-four out of the 542 initially selected articles were eligible for the data analysis; 5 further articles were identified and added to the review from the PUBMED database, for a total of 49 articles included. A total of 2,595,780 patients were identified, with an overall average age of the patients of 70.2 years ± 7.9 years old. The five most common AI/ML models identified in the selected articles were: RF, in 38.77% of studies; GBM, in 36.73% of studies; ANN in 34.7% of articles; LR, in 32.65%; SVM in 26.53% of articles.
This systematic review evaluated the possible uses of AI/ML models in TKA, highlighting their potential to lead to more accurate predictions, less time-consuming data processing, and improved decision-making, all while minimizing user input bias to provide risk-based patient-specific care.
Similar content being viewed by others
Introduction
Artificial intelligence (AI) and Machine learning (ML) tools in knee arthroplasty (KA) have the potential to improve patient-centered decision-making and outcome prediction in orthopedics. The application of ML in KA has been useful for predicting implant size, reconstructing data, and assisting with component positioning and alignment. ML implementation enhances surgical precision and can help predict parameters such as length of hospitalization, healthcare costs, and discharge disposition [1,2,3].
Additionally, ML algorithms have been proven, in more recent studies, to be useful when selecting the right drugs to treat prosthetic joint infection (PJI) to have a more patient specific approach to medicine; this was possible due to the development of a Random Forest (RF) model able to take notice of several risk variables, such as patients’ characteristics and comorbidities and using the, for the selection [4]. In data science theory, the quantity and quality of input parameters are crucial; therefore, the previously mentioned variables, if not selected by relevance to the topic of each study, although beneficial in theory, may hinder the full potential of ML algorithms for KA. This is because, analyzing all underlying relations between variables, with a large number of inputs the models may highlight irrelevant patterns, leading to a greater risk of overfitting: the algorithms perform significantly better with the training data in respect to the newly presented one [4, 5].
Moreover, patient satisfaction following primary KA is one of many outcome measures currently used to assess the efficacy of this procedure. Patients’ satisfaction is dependent on many factors such as age, gender, and the presence of comorbidities. Therefore, it is essential to understand the relationship between the variables underlying satisfaction to provide the best care and optimized postoperative care for KA patients. ML algorithms, capable of generating patient-specific risk models, appear to be very effective means to achieve this goal [6].
Overall, the application and use of ML and AI in orthopaedics are beneficial not only for the previously mentioned situations, but also for the identification of possible patients that are at high risk for severe walking limitations post-total knee arthroplasty [7], and the selection of high-risk patients who will require a blood transfusion after KA [8].
This review will focus on investigating which predictions are achievable by using AI and ML models in knee arthroplasty, identifying prerequisites for the effective use of this new approach. Moreover, the second aim is to highlight the latest findings of these technologies in predicting outcomes after KA.
Materials and methods
Study selection
The research question was defined by using a PIO approach: Population (P); Intervention (I); Comparison (C); Outcome (O). The objective of this systematic review was to investigate which outcomes can be assessed by using AI or ML models (I) in patients with knee osteoarthritis who underwent total (TKA) or unicompartmental (UKA) knee replacement (P). The following outcomes were considered: complications, costs, functional outcomes, revision rate, and postoperative satisfaction (O).
Inclusion criteria
Only articles that evaluated AI/ML-based applications in clinical decision-making in knee arthroplasty were considered. Only original clinical studies written in English, Spanish, or Italian were screened.
Exclusion criteria
Studies that did not evaluate AI/ML applications in KA. Studies with nonhuman subjects. Medical imaging analysis studies without explicit reference or application to KA. Inaccessible articles, conference abstracts, reviews, and editorials. No limits were placed on the level of evidence or publication date of the study.
Search
Following the Preferred Reporting Items for Systematic Reviews and Meta-analysis (PRISMA) guidelines, a thorough literature search was conducted using the following string: ((((total) OR (unicompartmental or unicondylar)) AND (knee replacement)) AND (((artificial intelligence) OR (machine learning)) OR (algorithm))) AND ((((((((complications) OR ((blood) AND ((transfusion) OR (loss)))) OR (functional outcomes)) OR (revision)) OR (satisfaction)) OR (surgical technique)) OR ((length of stay) OR (hospitalization))) OR ((costs) OR (economic analysis))). The use of keywords was both combined and isolated. The following databases were used: MEDLINE (Medical Literature Analysis and Retrieval System Online), Scopus, Cinahl, Google Scholar, PUBMED, and EMBASE (Excerpta Medica Database). The reference lists of selected systematic reviews [2, 5] were searched for the selection of further studies. The authors (F.V. and M.V.C.) searched from June of 2022 to January 2024. The databases were screened from the inception to January 2024.
Data collection process
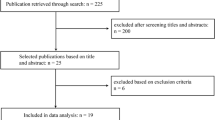
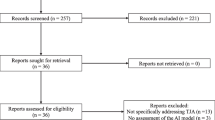
Two independent reviewers (F.V. and M.V.C.) collected the data, and mutual approval resolved differences. A third reviewer (S.D.S) was consulted in case of any disagreement. Title and abstract screening were the first steps, followed by the full-text evaluation of the selected articles. The inclusion and exclusion of the reviewed studies were displayed in the PRISMA flowchart, seen in Fig. 1.
Data items
A database was developed by collecting and categorizing the general study characteristics from the selected articles, which comprised: primary author, year of publication, study design, level of evidence, study duration, AI/ML methods, data source, input variables, output variables, sample size, average patient age, percentage of female patients, Area Under the Receiving Operating Characteristic Curve (AUC-ROC), accuracy, sensitivity, specificity.
Risk of bias assessment
For the quality assessment, a modified eight-item Methodological Index for Non-Randomized Studies (MINORS) checklist was employed to evaluate the selected articles. The eight-item checklist included: disclosure, study aim, input feature, output feature, validation method, dataset distribution, performance metric, and AI model. Each item was scored using the following binary scale: 0 (not reported or unclear) and 1 (reported and adequate). The following criteria were used as a guide when assessing the quality of each publication:
Disclosure: Scored 1 if clearly reported possible conflicts of interest, funding, or ethical considerations, scored 0 if not reported or unclear. Study aim: scored 1 if the research question and/or objective were clearly reported, scored 0 if unclear or not reported. Input feature: scored 1 if variables were clearly reported, scored 0 if unclear or not reported. Output feature: scored 1 if clearly reported, scored 0 if unclear or not reported. The validation method involves the evaluation of the AI/ML model’s performance by specific methods: scored 1 if the tools external validation, cross-validation, and/or bootstrapping were used and clearly reported, scored 0 if not reported nor used. Dataset distribution: scored 1 if the phases of training, testing, and validation for the AI/ML methods were clearly reported, scored 0 if unclear or not reported. Performance metric: scored 1 if the study clearly reported the metrics accuracy, sensitivity, specificity, and/or AUC-ROC for assessing the AI/ML model performance, scored 0 if unclear or not reported. AI model: scored 1 if clearly stated the specific AI/ML algorithm used by the study, scored 0 if not clearly stated.
Compared to the original MINORS checklist, this modified version, proposed by [9], provides a more accurate grading tool for studies focused on applying AI/ML methods in medical research and diagnostic studies within the medical field. Two independent reviewers (F.V. and M.V.C.) evaluated each publication individually.
Results
Study selection
The initial search identified 654 studies. After the duplicate removal, 479 studies were screened from which 402 articles were excluded after the title/abstract examination, resulting in 77 records for the full-text evaluation. After the full-text assessment, 49 studies were included in the data analysis (Fig. 1). Of these excluded articles, 9 studies did not evaluate AI/ML application in knee arthroplasty, 8 were medical imaging analysis studies without explicit reference or applications to knee arthroplasty, 2 used non-human objects, and 9 were inaccessible articles or systematic reviews.
Study characteristics
A total of 2,595,780 patients were identified from 48 of the 49 studies included, with one study [10] not providing the sample size. Thirty-seven of the 49 studies stated the percentage of female patients, adding up to 1,435,218 female patients, which account for 55.29% of the total patients. The overall average age of the patients was 70.2 years ± 7.9 years old, with 33 out of 49 articles providing an average age of the study population. The study which had the highest number of patients was Hyer et al., 2020 [11] with 1,049,160 patients (40.41% of all the patients included in the studies). All the study characteristics are reported in Table 1.
The five most common AI/ML models used were: RF, used in 19 articles; Gradient Boosting Machine (GBM), used in 18 articles (including less generalized versions such as Extreme Gradient Boosting (XGBoost) and Stochastic Gradient Boosting (SGB)); Artificial Neural Network (ANN) used in 17 articles; Logistic regression (LR), used in 16 articles (together with less generalized versions such as Elastic-net penalized logistic regression (EPLR)); and Support Vector Machine (SVM) used in 13 articles.
Regarding the variables reported, the most common input variables were: Age [38, 41, 45, 47, 49, 50, 52] (44 articles), Sex (33 articles), Comorbidities (29 articles), BMI (27 articles), Race/ ethnicity (26 articles), ASA classification (10 articles). The most common output variables provided by the studies were: post-surgical complications (11 articles), Probability of TKA (7 articles), and length of stay (4 articles).
This review included studies with level of evidence II-IV. Level of evidence II studies consist of Randomized controlled trials (RCTs) and are considered one of the strongest study designs, second only to reviews and meta-analysis which are considered as level of evidence I; Level of evidence III studies are composed of non-randomized controlled trials; the last category of evidence included in the review is Level IV: Case–control studies assessing associations between exposure and outcome.
The following level of evidence was included in the selected articles: 37 level III retrospective cohort studies [6, 8, 10,11,12,13,14,15,16,17,18,19, 23,24,25,26,27,28,29,30,31,32,33,34,35,36,37, 40, 48, 51]; three level III diagnostic studies [20,21,22, 54]; three level II prospective cohort studies [4, 39, 53]; one level II comparative studies [46]; three level IV cohort pilot studies [42, 44, 55], one level III multi-center retrospective study [47]. One study [43] did not present the level of evidence. All the characteristics are reported in Tables 1, 2 and 3.
AI and ML methods
The following section reports the AI and ML methods identified in the reviewed articles. Each section includes the number of articles that used each AI or ML method, its corresponding AUC value, and the evaluated output variable. Table 4 classifies each article regarding the output variable studies and presents the highest AUC score for the respective article.
Random forest
RF is a decision trees-based algorithm introduced in the 2000s and capable of handling a variety of data types; its implementation in many medical fields is sustained by its high performance with large datasets and its ability to integrate both clinical and imaging data to achieve more accurate predictions compared to older models such as LR. This ML method operates by constructing and averaging a multitude of decision tress, a simpler ML method, with each of the tress randomly analyzing selected subset variations of the original data, the model is capable to analyze large and complex subset of data, resulting in a more resistant model to overfitting, while also adding diversity in the analysis. It was the most common AI method, applied in 38.77% of the reviewed articles. Mainly it was used to evaluate outcomes, one of them being a technical outcome: TKA component size prediction (femoral and tibial) [35]. Eight publications implemented RF for the evaluation of clinical outcomes, some of them being: achievement of Minimal Clinically Important Differences (MCIDs), prediction of Patient Reported Outcomes (PROs), prolonged postoperative opioid prescription, improvement of Knee injury and Osteoarthritis Outcome Score (KOOS) to one-year, dissatisfaction, assessment of sensitization in patients with chronic pain after TKA, etc. [4, 6, 20, 26, 32, 33, 45, 53]. Only one article evaluated the post-walking limitation with RF, under the functional outcome category [7, 56].
RF was also utilized to analyze the surgical technique by two articles [15, 49], which considered the following outputs respectively: characterization of anatomical tissues and surgical corrections, the latter presenting the highest AUC (0.89) for this ML method. Postoperative length of stay (LOS) was predicted using RF only by one article [57], which presented an AUC of 0.71.
Another application of RF was regarding possible complications such as major complications after primary TKA, blood transfusion, surgical site infection, and disposition of patients at discharge [15, 25, 38]. Lastly, two reviewed articles implemented RF for predicting TKA risk depending on knee OA, evaluating both risk and time [16, 27].
Gradient boosting machine
The ML model GBM gained popularity in the 2000s due to the model’s high predictive accuracy even in settings with mixed data types and missing values. GBM works by building decision tress sequentially, rather than in parallel like RF, with each of the tress correcting the predicting errors made by the previous ones. This results in the model being able to analyse complex relationships in data and producing an accurate prediction, even if lacking the randomized selection or diversity of the RF model. It can be used for both classification and regression due to its ability to produce new decision trees by correcting the errors of the previous predictions, gaining more accuracy than popularly used models such as SVM.
It was used by 18 studies, one employing it to predict TKA component size [35]. The highest AUC value was applied by an article that evaluated the development of acute kidney infection (AKI) after TKA, AUC: 0.89 [34]. Other studies that evaluated complications with GBM comprised the following outputs: major complications after primary TKA, blood transfusion after TKA, surgical site infection, and disposition of patients at discharge [8, 17, 38]. One study used GBM for the prediction of LOS after TKA [37], a different study employed this method to evaluate functional outcome: post-TKA walking limitations [7].
In addition, GB was used by 7 articles to evaluate clinical outcomes: prediction of patient satisfaction, achievement of MCIDs in KOOS 1 year after TKA, prediction of PROs, extended prescription of postoperative opioids, MCIDs attainment 2 years after TKA [6, 18, 19, 22, 26, 32, 33, 53]. Only one study evaluated the use of SGB to predict the risk of TKA in comparison to other ML models, resulting in the highest performance together with RF among the algorithms observed, with an AUC: 0.83 [16].
Artificial Neural Network (ANN) /Multilayer perceptron
Although it originated in the 1940s, the ANN model gained prominence in the 2010s due to the application of deep learning in modeling complex relationships, making it suitable for a wide range of applications. ANN is a computational algorithm consisting of interconnected nodes organized in sequential layers, each analyzing the data to pass the prediction to the following one, mimicking the functioning of human neural network. This model was applied by 17 studies, one of them being for the prediction of LOS, inpatient charges, and discharge disposition before primary TKA [43]. Five articles analyzed clinical outcomes, the one having the highest AUC for this method (0.86) was regarding the prediction of PROs [26]; other outputs under this category were: prolonged postoperative opioid prescription, dissatisfaction after TKA, prediction of same-day discharge in patients undergoing TKA [6, 32, 33, 50]. One article applied ANN for TKA component size prediction (femoral and tibial) [44], and another study applied it for procedural cost prediction for TKA [31, 58].
Regarding complications, ANN was applied to evaluate the disposition of patients at discharge, post-surgical complications such as surgical site infection, and blood transfusion [38]. Additionally, two articles used this ML method to characterize tissues and surgical corrections based on patient-specific intra-operative assessment [15, 49]. Another application of ANN, by four other articles, was related to future clinical intervention outputs: effect of opioid use in risk of knee revision and manipulation in the first year after primary TKA [59]; identification of influential factors before surgery, and prediction of the risk of TKA surgery [23, 60].
Logistic regression
LR is a simply interpretable model for binary classification developed in the early twentieth century; being one of the oldest predictive models, its role is well established in the medical setting to estimate the probability of occurrence of different events. Although, it is to be considered that the advent of newer algorithms able to form wider and more complex associations between inputs and outputs causes this model to be more frequently relegated to a comparator role. The algorithm was used by 16 out of 49 articles. Four articles evaluated complications, which comprised the following outputs: disposition of patient at discharge, predictors of Allogenic Blood Transfusion (ALBT), and post-surgical complications [17, 25, 38]. The future clinical intervention was studied by three articles, specifically regarding the risk and time for a TKA in a patient presenting knee OA [27]. One article used this machine learning method for TKA component size prediction [35], and a different publication used it to evaluate post-TKA walking limitations, a type of functional outcome [7].
Regarding clinical outcome, LR was applied by 7 articles to study: achievement of MCIDs in KOOS 1 year after TKA, extended opioid prescription post-surgery, dissatisfaction after TKA, assessment of sensitization in patients with chronic pain after TKA, prediction of same-day discharge in patients undergoing TKA, and prediction of PROs [6, 22, 26, 32, 33, 45, 50]. The article that presented the highest AUC (0.88) evaluated the probability of TKA within 5 years [47].
Support vector machine
SVM is an effective model which can be used for both classification and regression; developed in the 1960s it still is one of the most popular algorithms used to classify disease progression based on imaging data. However, due to its low accuracy in performances with noisy datasets, newly developed algorithms such as K-Nearest Neighbors (kNN) are gaining prominence in this role. SVM is particularly effective when the number of features exceeds the number of samples in the data, being able to handle both linear and non-linear relationships in data. It was used by 13 articles, one of them evaluating the prediction of LOS and complications after TKA [29, 51]. Mainly to assess clinical outcomes such as: prolonged postoperative opioid prescription [32]; improvement of KOOS one year after TKA [33]; dissatisfaction after TKA [6]; attainment of MCIDs 2 years after TKA [20, 53]. SVM was also employed to analyze subtask segmentation of the TUG test for perioperative TKA [24]; Risk and Time of TKA in patients with knee OA [16, 27]; surgical corrections based on patient-specific intra-operative evaluation [49]. Additionally, one article used the algorithm to evaluate the characterization of tissues [15, 60] while another applied SVM in component sizing for TKA [35].
Other AI models
Two AI models were employed to evaluate major complications after primary TKA [17]: AutoPrognosis (AP) and AdaBoost. The ML method Decision tree was utilized in two studies for the analysis of the following outputs: gait comparison between UKA and TKA patient [30], and subtask segmentation of TUG test for perioperative TKA, the latter also being assessed by the methods: AdaBoost, kNN, Naïve Bayes Classifier (NB) [24].
Regarding the analysis of post-TKA walking limitation, the model SuperLearner was used [7]. Both the Cox-PH model and DeepSurv model were used to predict the risk and time of TKA in patients with knee osteoarthritis [27]; an Ensemble Deep Learning (DL) model based on the use of MRI and radiograph was also compared with traditional ML algorithms to predict the risk of TKA, obtaining promising results [40]. The prediction of PROs was assessed by the models: NB, kNN, and Multi-Step Adaptive Elastic-Net (MSAENET) [26].
The models Quadratic Discriminant Analysis (QDA) and LASSO regression were employed to evaluate MCIDs attainment after TKA in different periods. One of the studies made the assessment 1 year after TKA [22], other two articles made the evaluation 2 years after TKA [20, 53]. LASSO regression was also used to analyze mortality and complication after TKA, such as respiratory, cardiovascular, and nervous system and renal complications [21]. Regarding the prediction of clinical outcomes, the new Skeletal Oncology Research Group Machine Learning Algorithm (SORG-MLA) was validated for the identification of patients at risk of prolonged postoperative opioid use after TKA, obtaining an AUC: 0.75 [48].
Moreover, the models Linear Discriminant Analysis (LDA), Recursive Partitioning (RP), and NB were employed for the assessment of sensitization in patients with chronic pain after TKA [1]. The prediction of procedural cost after TKA, the DenseNet was used, presenting an AUC score of 0.813 [31].
Natural Language Processing Method (NLPM) was utilized to assess surgical technique, using the following outputs: category of surgery, implant model, presence of patellar resurfacing, constraint type, and laterality of surgery [46]. NLPM was also used to estimate ITS data [4] and analyze the alteration that opioid use can have in risk of knee revision and manipulation in the first year after primary TKA [12].
Lastly, the Stochastic Hill Climbing Complexity score was for the prediction of surgical 90-day morbidity, mortality, and complications [11]. NB was employed to analyze inpatient cost and LOS after TKA [32, 45].
Quality assessment by modified MINORS
All 49 of the reviewed articles were evaluated following the modified MINORS checklist to assess quality and risk of bias. All 49 articles clearly reported the study aim, however, 11 studies failed to report the performance metric. Two publications did not report the output feature, while 46 of the studies clearly stated the input feature, and 45 of the articles indicated disclosure. Regarding the item AI model, 45 of the reviewed articles fulfilled this criterion. These findings showed a relatively high degree of quality and low likelihood of bias, only two of the reviewed articles received a score of 5/8, five articles with 6/8 as a score, and the majority, 42 out of 49 publications, scored 7/8 and higher (Table 5).
Discussion
This systematic review evaluated the possible uses of AI/ML models in TKA, highlighting their potential in improving decision-making, component sizing, inpatient costs, perioperative planning, and streamlining the surgical workflow. Implementing these prediction models in TKA can ultimately lead to more accurate predictions, less time-consuming data processing, and higher precision in identifying patterns, all while minimizing user input bias to provide risk-based patient-specific care.
A key finding was the benefits of RF in aiding surgical decision-making when applied in intraoperatively collected surface models and patient-specific intraoperative assessments. RF outperformed both ANN and SVM not only when categorizing various types of anatomical tissue [15], but also when identifying populations at risk for TKA [16], and assessing balance and alignment during TKA surgery, aiding the surgeon regarding the optimal choice for the suitable bone recut or soft tissue adjustment [49, 61]. This review highlights how the application of RF in all the steps leading to TKA, perioperative and postoperative care can lead to optimal clinical and surgical outcomes, while reducing complications thanks to patient-specific planning. Moreover, by streamlining the surgical workflow and helping to select surgical corrections, this AI model can overcome the risk of data overload and the challenge of data interpretation, while being fast, cost-efficient, and accurate.
The SGB model presented promising results in the Kunze et al. (2021) study, by outranking RF, SVM, and EPLR for the prediction of the component sizing of the implant used in TKA. This model demonstrated the best overall performance regarding minimizing prediction error and maximizing accuracy for both femoral and tibial implant component size prediction. A potential benefit is an ability to predict final component sizes of the prosthetic without reliance on digital or manual templating, therefore being faster than traditional methods. Also, showing good performance across different TKA component manufacturers, streamlining component selection processes, improving inventory control, and reducing shipping costs [35, 62].
Regarding prediction models for allogenic blood transfusion, the highest AUC score was reported by the RF and SVM-based models [25]. With a slightly lower difference of 0.038 in the AUC score, the ANN-based model was still significantly higher than the classic prediction models [38]. Overall, these results show how the implementation of various ML-based models can result in an improvement of peri-operative complications predictions, ensuring that the identified population at risk, for blood transfusion, receives proper care while also optimizing the operative process and reducing the risk of prolonged LOS, caused by complications, such as blood transfusion, during TKA.
A further finding is the already established importance of LR models when used in healthcare settings, which can lead to the development of patient-specific care and peri-operative planning. The most successful result of LR (AUC 0.88) was achieved by its implementation, together with DenseNet, in identifying a population at higher risk of TKA within 5 years, particularly at less advanced stages of OA [47]; although, in the more recent study published by Crawford et al. in 2023, compared to other models such as SGB and RF (AUC: 0.83), EPLR scored a lower performance in identification of population at risk of TKA [16]. Additionally, implementing LR with other models, like the ML-based remote patient monitoring system, can reduce the need for TKA revision, while acquiring continuous data for patients undergoing TKA, in terms of mobility and rehabilitation compliance. This patient monitoring system proved to be reliable, low-maintenance, and a well-received platform for the patient recovering from TKA [42]. Implementing LR models would result in higher objectivity, cost-effectiveness, and ability to acquire continuous data, together with higher accuracy in identifying at-risk population, overall increasing the success rate for TKA.
Financial aspects are to be considered when proposing a treatment plan to patients, as complications can arise during the surgery and recovery, drastically changing the cost expected beforehand. Although it was shown to be an important element to consider when planning peri-operative care during TKA, the cost-prediction outcome was only analysed in one article. Demonstrating high accuracy when used in clinical medicine, the DenseNet model [31, 63] can optimize and provide a cost-efficient organization of resources that can benefit the medical staff by reducing their workload and improving the quality of the arrangement of resources. Simultaneously, this method can identify populations at risk for complications, a benefit that would help reduce the higher cost of the procedure after TKA, making it possible to implement patient-specific payment plans benefitting both patients and healthcare providers.
Going over the performances of the GBM model analysed in different articles, we can observe how this algortihm is simple and efficient, it has been validated to improve both short- and long-term prognoses of TKA patients. Ko et al. successfully used this AI model for the prediction of the development of postoperative AKI after TKA, which can not only increase LOS but also be life-threatening [34]; while TreeNet GBM proved to be the most successful method when applied for predictors regarding patient satisfaction [18]. Additionally, GBM showed great results when predicting the disposition of patients at discharge [38], therefore the model’s implementation could improve the overall patient satisfaction and recovery rate post-TKA, while also assuring patient-specific peri-operative care is applied to prevent and manage possible complications.
Looking at more novel models less implemented up until recently in the healthcare settings, the following AI/ML models: DL-TL-MT, SVM, Deep Surv, and Cox-PH, proved to be of great use to individuate the population of patients at risk and develop patient-specific care. The DL-TL-MT model successfully predicted the risk of OA progression based on knee radiographs in patients that previously underwent TKA [36]. Presenting the same AUC level (of 0.87), the methods SVM, Deep Surv, and Cox-PH were successfully employed to predict the risk and time of TKA of an OA knee [27]. The implementation methods prove to be indispensable in predicting the progression of OA, even at an early stage. This ML-based model has great potential as a diagnostic tool for physicians when determining the prognosis for patient at all stages of OA, allowing for early intervention through TKA where needed, therefore reducing the risk of complications and of TKA revision.
The SVM predictor model showed also a very promising results when applied in the different settings, and especially for the segmentation of the TUG test and extraction of information from each subtask perioperative to TKA, solving the problems regarding subjectiveness and other biases [24, 64]. The benefits that come with the usage of this AI model would be a more precise segmentation and therefore data extraction, which results in further understanding and classification of improvements in patients, leading to the employment of patient-specific treatments and rehabilitation models.
Looking at the results of the different articles involved in the review, the emergence of ML models in the medical setting becomes an evident matter: most data corroborates the idea that novel AI models present better results and predictive powers, compared to traditional models, when identifying predictors of TKA and analyzing multiple outcomes simultaneously. In the prediction of complications after primary TKA, Devana et al. prove the superiority of AP, compared to traditional models, regarding the discriminative ability and the capability to suggest nonlinear relationships between variables in the outcomes of TKA. Consequently, AP can be a versatile tool that may be utilized for the identification of crucial patient characteristics when predicting outcomes across a variety of datasets, thereby improving the patient outcomes [17]. Additionally, Harris et al. demonstrated how AI can produce preoperative prediction models for one-year improvement in pain and functioning after TKA; and how the GBM model, which performs well in important interactions, and the QDA model, which performs better in nonlinear association, can be applied to produce an easy-to-use predictive model able to achieve similar or better accuracy with far fewer inputs in respect to traditional predictive models [22].
Lastly, the NLPM model presents great potential as a newly emerging algorithm, in particular when applied in clinical settings for the interpretation of a text, which has been applied in different studies for the classification of patient satisfaction [14], knee revisions after TKA due to preoperative opioid use [12], and for the processing of clinical free text from electronic health records [46]. The strength of this ML-based model relies on its ability to automate the extraction of embedded information in perioperative notes and patient-centered surveys, decreasing the need for costly manual chart reviewing and improving data quality while being less time-consuming. The use of this model would improve patient feedback and perioperative notes to better patient-specific risk-based care resulting in higher patient satisfaction and a reduction in costs for the healthcare system due to possible lawsuits [65], together with the reduction of the cost due to manual chart reviews [46].
Like both the Hinterwimmer et al. 2021, and the Lee et al. 2022 review, this systematic review confirms the great potential of AI/ML methods and their application in orthopedics for cost predictions, diagnostic applications, and identification of risk factors, while also clearing the doubts regarding the inaccuracy and lack of sufficient evaluation of these models. In comparison, this review analyzed 49 articles, including the publications already examined in previous reviews. This more extensive research concluded that not only is it possible to implement these models in the prediction of TKA perioperative care, disease progression of OA, and distinct outcomes applying specific data, but also the prediction of more complex outcomes is now feasible through the application of more novel AI/ML algorithms [13, 17, 21, 22, 27, 30]. Although, as mentioned in several studies, further research may enhance the reliability of AI/ML models and allow for their use in patient preoperative and perioperative care [8, 11, 19, 21, 43, 50].
Limitations
The main limitation of this review derives from the possible bias of information regarding the performance of the different AI models, which, as highlighted by the MINORS table, results as the most at-risk parameter due to the omission by several articles of either AUC score or Accuracy score for the different predictive models examined. Moreover, many of the studies included in this review are retrospective studies obtaining the data, regarding the patients for the testing of the AI/ML prediction models, from national databases and electronic health recordings; limitations by the lack of detailed clinical information, potential misclassification of data, and in many cases a small cohort of patients presenting limited characteristics from which to derive input and compare outputs, which may lead to the results not being generalizable to all patient populations [11, 19, 21]. Validation of analyzed predictive models on larger populations of patients is needed. Lastly, due to the heterogeneity between data, it was not possible to perform a meta-analysis.
Conclusion
Regarding the implementation of AI/ML models in TKA, the articles in this review mostly consider these predictive models to be helpful and suggest that their application in medical settings for perioperative TKA clinical decision-making and prediction of the progression of OA into TKA may result in an improvement of patient satisfaction, risk managing, and cost efficiency. Among the best qualities, for which the AI/ML models outperform the traditional prediction models, frequently reported higher accuracy, cost efficiency, simple application, lack of subjectiveness, and overall reduction of time consumption thanks to the automation of tasks. Therefore, it is possible to conclude that, although the results of the reviewed articles should be further validated by their testing on larger cohorts of patients, the findings of these articles highlight the great potentials that derive from the inclusion of AI/ML predictive models in a further branch of medicine.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- AdaBoost:
-
Adaptive Boosting
- AI:
-
Artificial Intelligence
- AKI:
-
Acute Kidney Infection
- ALBT:
-
Allogenic Blood Transfusion
- ANN:
-
Artificial Neural Network
- ANN-TL-MT:
-
Artificial Neural Network – Transfer Learning – multitask
- AP:
-
AutoPrognosis
- APR:
-
All patient refined
- ASA-PS:
-
American society of anesthesiologists’ physical status
- ASA:
-
America Society of Anesthesiologists
- AUC-ROC:
-
Area Under the Receiver Operating Characteristic Curve
- BMI:
-
Body mass index
- BP:
-
Blood pressure
- BT:
-
Blood transfusion
- CBC:
-
Cartilage and bone classification
- CCS:
-
Charlson comorbidity score
- CMS:
-
Centers for Medicare & Medicaids Services
- CoxPH:
-
Cox proportional hazards
- CPS:
-
Cohort pilot study
- CS:
-
Comparative study
- DCNN:
-
Deep Convolutional Neural Network
- DD:
-
Discharge disposition
- DenseNet:
-
Densely Connected Convolutional Network
- DL:
-
Deep Learning
- DP:
-
Disposition of patient
- DS:
-
DeepSurv
- DT:
-
Decision tree
- EPLR:
-
Elastic-net Penalized Logistic Regression
- ESSKA:
-
European Society of Sports Traumatology, Knee Surgery and Arthroscopy
- GBM:
-
Gradient Boosting Machine
- Hb:
-
Heamoglobin
- HEP:
-
Home exercise program
- HKA:
-
Hip-knee-angle
- IC:
-
Impatient charges/costs
- KA:
-
Knee Artrhoplasty
- KNN:
-
K-Nearest Neighbors
- KOOS:
-
Knee Injury and Osteoarthritis Outcome Score
- KOOS-JR:
-
Knee injury and Osteoarthritis Outcome Score for Joint Replacement
- KSS-F:
-
Knee society score function
- KSS:
-
Knee society score
- LASSO:
-
Least Absolute Shrinkage and Selection Operator
- LDA:
-
Linear Discriminant Analysis
- LOS:
-
Length of stay
- LR:
-
Logistic Regression
- MCBC:
-
Muscle, cartilage, bone classification
- MCIDs:
-
Minimally clinically important differences
- MCRS:
-
Multi center retrospective study
- MCS:
-
Mechanical circulatory support
- MINORS:
-
Methodological Index for Non-Randomized Studies
- ML:
-
Machine Learning
- MLP:
-
Multilayer perceptron
- MSAENET:
-
Multi-Step Adaptive Elastic NETwork
- MTBCC:
-
Muscle, tendon, bone, cartilage classification
- MTLR:
-
Multi-Task Logistic Regression
- NB:
-
Näive-Bayes
- NHS:
-
National Health Service
- NIS:
-
National (Nationwide) Inpatient Sample database
- NLPM:
-
Natural Language Processing Method
- NN:
-
Neural Network
- Non-Linear-GMDH:
-
Non-Linear-Group Method of Data Handling
- NSAID:
-
Non-steroidal anti-inflammatory drugs
- NSQIP:
-
National Surgical Quality Improvement Program
- OA:
-
Osteoarthritis
- OAI:
-
Osteoarthritis initiative
- OME:
-
Orthopedic Minimal Data Set database
- OSHPD:
-
Office of Statewide Health Planning and Development
- PASE:
-
Physical activity scale for the Elderly
- PCL:
-
Posterior cruciate ligament
- PCS:
-
Physical component summary
- PHRS:
-
Preoperative patient-reported health state
- PICO:
-
Population Intervention Comparison Outcome
- PJI:
-
Prosthetic Joint Infection
- PRISMA:
-
Preferred Reporting Items for Systematic reviews and Meta-Analysis
- PROMs:
-
Patient reported outcome measures
- PROs:
-
Prediction of patient-reported outcomes
- PS:
-
Pilot study
- QDA:
-
Quadrant Discriminant Analysis
- QOL:
-
Quality of Life
- RCS:
-
Retrospective cohort study
- RCT:
-
Randomized control trial
- RF:
-
Random Forest
- RP:
-
Recursive Partitioning
- RPM:
-
Remote Patient Monitoring
- SAFs:
-
Standard analytical files
- SF-36 PCS:
-
Short form – physical component summary
- SGB:
-
Stochastic Gradient Boosting
- SHC:
-
Stochastic Hill Climbing
- SORG-MLA:
-
Skeletal Oncology Research Group Machine Learning Algorithm
- SPARCS:
-
State-wide Planning and Research Cooperative System
- SVM:
-
Support Vector Machines
- TKA:
-
Total Knee Arthroplasty
- TUG:
-
Timed Up-and-Go
- UCLA:
-
University of California Los Angeles
- UKA:
-
Unicompartmental Knee Arthroplasty
- VA:
-
Veteran’s affairs
- VAS:
-
Visual analog scale
- WHO:
-
World Health Organization
- WOMAC:
-
Western Ontario and McMaster Universities Osteoarthritis Index
- XGBoost:
-
EXtreme Gradient Boosting
References
Cabitza F, Locoro A, Banfi G. Machine learning in orthopedics: a literature review. Front Bioeng Biotechnol. 2018;6:75.
Lee LS, Chan PK, Wen C, Fung WC, Cheung A, Chan VWK, Cheung MH, Fu H, Yan CH, Chiu KY. Artificial intelligence in diagnosis of knee osteoarthritis and prediction of arthroplasty outcomes: a review. Arthroplasty. 2022;4(1):16.
Martín Noguerol T, Paulano-Godino F, Martín-Valdivia MT, Menias CO, Luna A. Strengths, weaknesses, opportunities, and threats analysis of artificial intelligence and machine learning applications in radiology. J Am Coll Radiol. 2019;16(9 Pt B):1239–47.
Shohat N, Goswami K, Tan TL, Yayac M, Soriano A, Sousa R, Wouthuyzen-Bakker M, Parvizi J. (NINJA) ESGoIAIEatNINoJA: 2020 Frank Stinchfield award: identifying who will fail following irrigation and debridement for prosthetic joint infection. Bone Joint J. 2020;102-B(7_Supple_B):11–9.
Hinterwimmer F, Lazic I, Suren C, Hirschmann MT, Pohlig F, Rueckert D, Burgkart R, von Eisenhart-Rothe R. Machine learning in knee arthroplasty: specific data are key-a systematic review. Knee Surg Sports Traumatol Arthrosc. 2022;30(2):376–88.
Kunze KN, Polce EM, Sadauskas AJ, Levine BR. Development of machine learning algorithms to predict patient dissatisfaction after primary total knee arthroplasty. J Arthroplasty. 2020;35(11):3117–22.
Pua YH, Kang H, Thumboo J, Clark RA, Chew ES, Poon CL, Chong HC, Yeo SJ. Machine learning methods are comparable to logistic regression techniques in predicting severe walking limitation following total knee arthroplasty. Knee Surg Sports Traumatol Arthrosc. 2020;28(10):3207–16.
Jo C, Ko S, Shin WC, Han HS, Lee MC, Ko T, Ro DH. Transfusion after total knee arthroplasty can be predicted using the machine learning algorithm. Knee Surg Sports Traumatol Arthrosc. 2020;28(6):1757–64.
Ogink PT, Groot OQ, Karhade AV, Bongers MER, Oner FC, Verlaan JJ, Schwab JH. Wide range of applications for machine-learning prediction models in orthopedic surgical outcome: a systematic review. Acta Orthop. 2021;92(5):526–31.
Bonakdari H, Pelletier JP, Martel-Pelletier J. A reliable time-series method for predicting arthritic disease outcomes: new step from regression toward a nonlinear artificial intelligence method. Comput Methods Programs Biomed. 2020;189:105315.
Hyer JM, White S, Cloyd J, Dillhoff M, Tsung A, Pawlik TM, Ejaz A. Can we improve prediction of adverse surgical outcomes? Development of a surgical complexity score using a novel machine learning technique. J Am Coll Surg. 2020;230(1):43–52.e41.
Ben-Ari A, Chansky H, Rozet I. Preoperative opioid use is associated with early revision after total knee arthroplasty: a study of male patients treated in the veterans affairs system. J Bone Joint Surg Am. 2017;99(1):1–9.
Bloomfield RA, Williams HA, Broberg JS, Lanting BA, McIsaac KA, Teeter MG. Machine learning groups patients by early functional improvement likelihood based on wearable sensor instrumented preoperative timed-up-and-go tests. J Arthroplasty. 2019;34(10):2267–71.
Bovonratwet P, Shen TS, Islam W, Ast MP, Haas SB, Su EP. Natural language processing of patient-experience comments after primary total knee arthroplasty. J Arthroplasty. 2021;36(3):927–34.
Chan B, Rudan JF, Mousavi P, Kunz M. Intraoperative integration of structured light scanning for automatic tissue classification: a feasibility study. Int J Comput Assist Radiol Surg. 2020;15(4):641–9.
Crawford AM, Karhade AV, Agaronnik ND, Lightsey HM, Xiong GX, Schwab JH, Schoenfeld AJ, Simpson AK. Development of a machine learning algorithm to identify surgical candidates for hip and knee arthroplasty without in-person evaluation. Arch Orthop Trauma Surg. 2023;143(9):5985–92.
Devana SK, Shah AA, Lee C, Roney AR, van der Schaar M, SooHoo NF. A novel, potentially universal machine learning algorithm to predict complications in total knee arthroplasty. Arthroplast Today. 2021;10:135–43.
Farooq H, Deckard ER, Ziemba-Davis M, Madsen A, Meneghini RM. Predictors of patient satisfaction following primary total knee arthroplasty: results from a traditional statistical model and a machine learning algorithm. J Arthroplasty. 2020;35(11):3123–30.
Farooq H, Deckard ER, Arnold NR, Meneghini RM. Machine learning algorithms identify optimal sagittal component position in total knee arthroplasty. J Arthroplasty. 2021;36(7S):S242–9.
Fontana MA, Lyman S, Sarker GK, Padgett DE, MacLean CH. Can machine learning algorithms predict which patients will achieve minimally clinically important differences from total joint arthroplasty? Clin Orthop Relat Res. 2019;477(6):1267–79.
Harris AHS, Kuo AC, Weng Y, Trickey AW, Bowe T, Giori NJ. Can machine learning methods produce accurate and easy-to-use prediction models of 30-day complications and mortality after knee or hip arthroplasty? Clin Orthop Relat Res. 2019;477(2):452–60.
Harris AHS, Kuo AC, Bowe TR, Manfredi L, Lalani NF, Giori NJ. Can machine learning methods produce accurate and easy-to-use preoperative prediction models of one-year improvements in pain and functioning after knee arthroplasty? J Arthroplasty. 2021;36(1):112–117.e116.
Heisinger S, Hitzl W, Hobusch GM, Windhager R, Cotofana S. Predicting total knee replacement from symptomology and radiographic structural change using artificial neural networks-data from the osteoarthritis initiative (OAI). J Clin Med. 2020;9(5):1298.
Hsieh CY, Huang HY, Liu KC, Chen KH, Hsu SJ, Chan CT. Subtask segmentation of timed up and go test for mobility assessment of perioperative total knee arthroplasty. Sensors (Basel). 2020;20(21):6302.
Huang Z, Huang C, Xie J, Ma J, Cao G, Huang Q, Shen B, Byers Kraus V, Pei F. Analysis of a large data set to identify predictors of blood transfusion in primary total hip and knee arthroplasty. Transfusion. 2018;58(8):1855–62.
Huber M, Kurz C, Leidl R. Predicting patient-reported outcomes following hip and knee replacement surgery using supervised machine learning. BMC Med Inform Decis Mak. 2019;19(1):3.
Jamshidi A, Pelletier JP, Labbe A, Abram F, Martel-Pelletier J, Droit A. Machine learning-based individualized survival prediction model for total knee replacement in osteoarthritis: data from the osteoarthritis initiative. Arthritis Care Res (Hoboken). 2021;73(10):1518–27.
Jayakumar P, Moore MG, Furlough KA, Uhler LM, Andrawis JP, Koenig KM, Aksan N, Rathouz PJ, Bozic KJ. Comparison of an artificial intelligence-enabled patient decision aid vs educational material on decision quality, shared decision-making, patient experience, and functional outcomes in adults with knee osteoarthritis: a randomized clinical trial. JAMA Netw Open. 2021;4(2):e2037107.
Johannesdottir KB, Kehlet H, Petersen PB, Aasvang EK, Sørensen HBD, Jørgensen CC, Group CfF-tHaKRC. Machine learning classifiers do not improve prediction of hospitalization > 2 days after fast-track hip and knee arthroplasty compared with a classical statistical risk model. Acta Orthop. 2022;93:117–23.
Jones GG, Kotti M, Wiik AV, Collins R, Brevadt MJ, Strachan RK, Cobb JP. Gait comparison of unicompartmental and total knee arthroplasties with healthy controls. Bone Joint J. 2016;98-B(10 Supple B):16–21.
Karnuta JM, Navarro SM, Haeberle HS, Helm JM, Kamath AF, Schaffer JL, Krebs VE, Ramkumar PN. Predicting inpatient payments prior to lower extremity arthroplasty using deep learning: which model architecture is best? J Arthroplasty. 2019;34(10):2235-2241.e2231.
Katakam A, Karhade AV, Schwab JH, Chen AF, Bedair HS. Development and validation of machine learning algorithms for postoperative opioid prescriptions after TKA. J Orthop. 2020;22:95–9.
Katakam A, Karhade AV, Collins A, Shin D, Bragdon C, Chen AF, Melnic CM, Schwab JH, Bedair HS. Development of machine learning algorithms to predict achievement of minimal clinically important difference for the KOOS-PS following total knee arthroplasty. J Orthop Res. 2022;40(4):808–15.
Ko S, Jo C, Chang CB, Lee YS, Moon YW, Youm JW, Han HS, Lee MC, Lee H, Ro DH. A web-based machine-learning algorithm predicting postoperative acute kidney injury after total knee arthroplasty. Knee Surg Sports Traumatol Arthrosc. 2022;30(2):545–54.
Kunze KN, Polce EM, Patel A, Courtney PM, Levine BR. Validation and performance of a machine-learning derived prediction guide for total knee arthroplasty component sizing. Arch Orthop Trauma Surg. 2021;141(12):2235–44.
Leung K, Zhang B, Tan J, Shen Y, Geras KJ, Babb JS, Cho K, Chang G, Deniz CM. Prediction of total knee replacement and diagnosis of osteoarthritis by using deep learning on knee radiographs: data from the osteoarthritis initiative. Radiology. 2020;296(3):584–93.
Li H, Jiao J, Zhang S, Tang H, Qu X, Yue B. Construction and comparison of predictive models for length of stay after total knee arthroplasty: regression model and machine learning analysis based on 1,826 cases in a single Singapore center. J Knee Surg. 2022;35(1):7–14.
Mohammed H, Huang Y, Memtsoudis S, Parks M, Ma Y. Utilization of machine learning methods for predicting surgical outcomes after total knee arthroplasty. PLoS One. 2022;17(3):e0263897.
Navarro SM, Wang EY, Haeberle HS, Mont MA, Krebs VE, Patterson BM, Ramkumar PN. Machine learning and primary total knee arthroplasty: patient forecasting for a patient-specific payment model. J Arthroplasty. 2018;33(12):3617–23.
Rajamohan HR, Wang T, Leung K, Chang G, Cho K, Kijowski R, Deniz CM. Prediction of total knee replacement using deep learning analysis of knee MRI. Sci Rep. 2023;13(1):6922.
Ramazanian T, Yan S, Rouzrokh P, Wyles CC, O Byrne TJ, Taunton MJ, Maradit Kremers H. Distribution and correlates of hip-knee-ankle angle in early osteoarthritis and preoperative total knee arthroplasty patients. J Arthroplasty. 2022;37(6S):S170–5.
Ramkumar PN, Haeberle HS, Ramanathan D, Cantrell WA, Navarro SM, Mont MA, Bloomfield M, Patterson BM. Remote patient monitoring using mobile health for total knee arthroplasty: validation of a wearable and machine learning-based surveillance platform. J Arthroplasty. 2019;34(10):2253–9.
Ramkumar PN, Karnuta JM, Navarro SM, Haeberle HS, Scuderi GR, Mont MA, Krebs VE, Patterson BM. Deep learning preoperatively predicts value metrics for primary total knee arthroplasty: development and validation of an artificial neural network model. J Arthroplasty. 2019;34(10):2220–2227.e2221.
Rexwinkle JT, Werner NC, Stoker AM, Salim M, Pfeiffer FM. Investigating the relationship between proteomic, compositional, and histologic biomarkers and cartilage biomechanics using artificial neural networks. J Biomech. 2018;80:136–43.
Sachau J, Otto JC, Kirchhofer V, Larsen JB, Kennes LN, Hüllemann P, Arendt-Nielsen L, Baron R. Development of a bedside tool-kit for assessing sensitization in patients with chronic osteoarthritis knee pain or chronic knee pain after total knee replacement. Pain. 2022;163(2):308–18.
Sagheb E, Ramazanian T, Tafti AP, Fu S, Kremers WK, Berry DJ, Lewallen DG, Sohn S, Maradit Kremers H. Use of natural language processing algorithms to identify common data elements in operative notes for knee arthroplasty. J Arthroplasty. 2021;36(3):922–6.
Tolpadi AA, Lee JJ, Pedoia V, Majumdar S. Deep learning predicts total knee replacement from magnetic resonance images. Sci Rep. 2020;10(1):6371.
Tsai CC, Huang CC, Lin CW, Ogink PT, Su CC, Chen SF, Yen MH, Verlaan JJ, Schwab JH, Wang CT, et al. The Skeletal Oncology Research Group Machine Learning Algorithm (SORG-MLA) for predicting prolonged postoperative opioid prescription after total knee arthroplasty: an international validation study using 3,495 patients from a Taiwanese cohort. BMC Musculoskelet Disord. 2023;24(1):553.
Verstraete MA, Moore RE, Roche M, Conditt MA. The application of machine learning to balance a total knee arthroplasty. Bone Jt Open. 2020;1(6):236–44.
Wei C, Quan T, Wang KY, Gu A, Fassihi SC, Kahlenberg CA, Malahias MA, Liu J, Thakkar S, Gonzalez Della Valle A, et al. Artificial neural network prediction of same-day discharge following primary total knee arthroplasty based on preoperative and intraoperative variables. Bone Joint J. 2021;103(8):1358–66.
Yeo I, Klemt C, Robinson MG, Esposito JG, Uzosike AC, Kwon YM. The use of artificial neural networks for the prediction of surgical site infection following TKA. J Knee Surg. 2023;36(6):637–43.
Yi PH, Wei J, Kim TK, Sair HI, Hui FK, Hager GD, Fritz J, Oni JK. Automated detection & classification of knee arthroplasty using deep learning. Knee. 2020;27(2):535–42.
Zhang S, Lau BPH, Ng YH, Wang X, Chua W. Machine learning algorithms do not outperform preoperative thresholds in predicting clinically meaningful improvements after total knee arthroplasty. Knee Surg Sports Traumatol Arthrosc. 2022;30(8):2624–30.
Longo UG, De Salvatore S, Intermesoli G, Pirato F, Piergentili I, Becker R, Denaro V. Metaphyseal cones and sleeves are similar in improving short- and mid-term outcomes in total knee arthroplasty revisions. Knee Surg Sports Traumatol Arthrosc. 2023;31(3):861–82.
Hinterwimmer F, Lazic I, Langer S, Suren C, Charitou F, Hirschmann MT, Matziolis G, Seidl F, Pohlig F, Rueckert D, et al. Prediction of complications and surgery duration in primary TKA with high accuracy using machine learning with arthroplasty-specific data. Knee Surg Sports Traumatol Arthrosc. 2023;31(4):1323–33.
Longo UG, Maffulli N, Denaro V. Minimally invasive total knee arthroplasty. N Engl J Med. 2009;361(6):633–4 author reply 634.
Lambrechts A, Wirix-Speetjens R, Maes F, Van Huffel S. Artificial intelligence based patient-specific preoperative planning algorithm for total knee arthroplasty. Front Robot AI. 2022;9:840282.
Berton A, Longo UG, Candela V, Fioravanti S, Giannone L, Arcangeli V, Alciati V, Berton C, Facchinetti G, Marchetti A, et al. Virtual Reality, Augmented Reality, Gamification, and Telerehabilitation: Psychological Impact on Orthopedic Patients' Rehabilitation. J Clin Med. 2020;9(8).
Goplen CM, Kang SH, Randell JR, et al. Effect of preoperative long-term opioid therapy on patient outcomes after total knee arthroplasty: an analysis of multicentre population-based administrative data. Can J Surg. 2021;64(2):E135–43. https://doi.org/10.1503/cjs.007319.
Longo UG, Ciuffreda M, Mannering N, D’Andrea V, Cimmino M, Denaro V. Patellar resurfacing in total knee arthroplasty: systematic review and meta-analysis. J Arthroplasty. 2018;33(2):620–32.
Bravi M, Longo UG, Laurito A, Greco A, Marino M, Maselli M, Sterzi S, Santacaterina F. Supervised versus unsupervised rehabilitation following total knee arthroplasty: a systematic review and meta-analysis. Knee. 2023;40:71–89.
Longo UG, Silva S, Perdisa F, Salvatore G, Filardo G, Berton A, Piergentili I, Denaro V. Gender related results in total knee arthroplasty: a 15-year evaluation of the Italian population. Arch Orthop Trauma Surg. 2023;143(3):1185–92.
Longo UG, Loppini M, Trovato U, Rizzello G, Maffulli N, Denaro V. No difference between unicompartmental versus total knee arthroplasty for the management of medial osteoarthtritis of the knee in the same patient: a systematic review and pooling data analysis. Br Med Bull. 2015;114(1):65–73.
Longo UG, Ciuffreda M, Mannering N, D’Andrea V, Locher J, Salvatore G, Denaro V. Outcomes of posterior-stabilized compared with cruciate-retaining total knee arthroplasty. J Knee Surg. 2018;31(4):321–40.
Stelfox HT, Gandhi TK, Orav EJ, Gustafson ML. The relation of patient satisfaction with complaints against physicians and malpractice lawsuits. Am J Med. 2005;118(10):1126–33.
Acknowledgements
Not applicable.
Funding
This research received no external funding.
Author information
Authors and Affiliations
Contributions
Conceptualization, U.G.L., S.D.S.; methodology, U.G.L., F.V; software.B.V, K.S; validation, U.G.L., B.V.; formal analysis, M.V.C, K.S, F.V; resources, U.G.L., S.DS; data curation, F.V ; writing—original draft preparation M.V.C, B.V., K.S., F.V.; writing—review and editing, M.V.C, U.G.L., ; visualization, K.S., F.M ; supervision, U.G.L., All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Longo, U.G., De Salvatore, S., Valente, F. et al. Artificial intelligence in total and unicompartmental knee arthroplasty. BMC Musculoskelet Disord 25, 571 (2024). https://doi.org/10.1186/s12891-024-07516-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12891-024-07516-9