Abstract
Background
Allergies against implant materials are still not fully understood. Despite controversies about its relevance, some patients need treatment with hypoallergenic implants. This study compared coated and standard total knee arthroplasty (TKA) regarding inflammatory response and patient-reported outcome measures (PROMs).
Methods
76 patients without self-reported allergies against implant materials were included in a RCT and received a coated or standard TKA of the same cemented posterior-stabilized knee system. 73 patients completed the 3-year follow-up. Two patients died and there was one revision surgery. Serum levels of cytokines with a possible role in implant allergy were measured in patient`s serum (IL-1beta, IL-5, IL-6, IL-8, IL-10, IFN γ, TNF α) prior to, one and three years after surgery. Furthermore, PROMs including knee function (Oxford Knee Score, Knee Society Score) and health-related quality of life (QoL, EuroQuol questionnaire) were assessed. Additionally, 8 patients with patch-test proven skin allergy against implant materials who received the coated implant were assessed similarly and compared to a matched-pair group receiving the same implant.
Results
There were no differences in function and QoL between the assessed groups at any follow-up. The majority of patients demonstrated no elevation of the measured blood cytokines. Cytokine patterns showed no differences between study groups at any follow-up. The allergy patients demonstrated slower functional improvement and minor differences in cytokine pattern. Yet these results were not significant. There were no differences in the matched-pair analysis.
Conclusion
We observed no relevant increase in serum cytokine levels in any group. The inflammatory response measured seems limited, even in allergy patients. Furthermore, there were no differences between coated and standard TKA in non-allergy patients in the 3-year Follow-Up period.
Trial registration
The study protocol was registered in the US National Institutes of Health’s database (http://www.clinicaltrials.gov) registry under NCT03424174 on 03/17/2016.
Similar content being viewed by others
Background
Algorithms for the diagnostic approach of painful TKA have been introduced wherein hypersensitivity against implant materials is a possible cause of unexplained symptoms [1,2,3]. Yet, the relevance of allergies against implant materials is being discussed controversially [4,5,6,7]. There are reports of successful standard implants in patients with allergies, [8] but a growing number of reports has linked insufficient functional outcome, clinical symptoms and persistent pain to metal hypersensitivity [9,10,11].
Metal implants release ions due to wear and corrosion forming metallo-organic protein complexes which can be identified as agent by the immune system [12]. To address this issue hypoallergenic implants with surface modifications have been developed. Unfortunately, these implants show higher revision rates [13,14,15,16]. In a retrieval study hypoallergenic TKA demonstrated delamination potentially affecting the performance of the coating [14]. Therefore, a seven-layer zirconium nitride coating system (Advanced Surface - AS, B.Braun Aesculap, Tuttlingen, Germany) has been developed to improve coating quality [17]. This coating system demonstrated good long-term results [16, 18, 19].
Additionally several mediators have been reported to play a role in inflammatory reaction after TKA [20]. While some studies found increased IL-8 and IL-10 levels in standard TKA compared to coated TKA others did not find significant differences in blood cytokine patterns [21]. Cassuto et al. investigated inflammatory mediators, matrix proteins and bone regulating factors over a 20-year period after hip arthroplasty. He described response patterns and linked these to the phases of the healing process [22]. Generally, there is a lack of studies focusing on the inflammatory response after arthroplasty.
The underlying study was initiated to investigate the inflammatory response and patient-reported outcome measures (PROMs) in coated and standard TKA. It was hypothesized, that there would be a higher inflammatory response in standard TKA but no differences in PROMs compared to coated TKA.
Methods
After institutional review board approval (IRB 00001473, IORG 00001076, registered at Office for Human Research Protection, EK 101,032,016) a randomized-controlled trial was conducted. The study protocol was registered prior to enrollment in the US National Institutes of Health’s database (http://www.clinicaltrials.gov) registry under NCT03424174 on 06/02/2018. The CONSORT reporting guidelines were used [23].
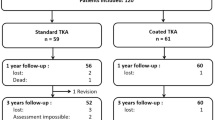
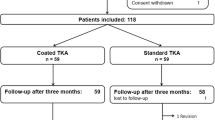
Patients scheduled for an unconstrained TKA without self-reported hypersensitivities against implant materials and without any existing metal implants were eligible to participate. After informed consent, a total of 80 patients were randomized using a software algorithm to receive a standard or coated TKA (Vega or Vega AS, B.Braun Aesculap, Tuttlingen, Germany). In one patient surgery was delayed due to medical problems and three patients needed a higher constraint during surgery. Therefore, a total of 76 patients was included in this study. An additional group including all patients with diagnosed allergies against one or more implant metals who presented during the study enrollment period was established (n = 8). Three patients of the allergy group were allergic to nickel only. Four patients had an allergy against nickel and cobalt and one patient presented with an allergy against nickel and palladium. These patients received the coated implant due to local guidelines (Fig. 1). In order to allow for comparability of the results we conducted a matched-pair analysis with non-allergic patients receiving a coated TKA. Matching criteria included age, sex, BMI and ASA-Score.
Both implants consisted of a CoCrMo-alloy (ISO 5832-4). The coated TKA had an additional multilayer coating system (Advanced Surface, AS) which was applied on the CoCrMo knee implants using a physical vapour deposition method in 7 layers with a gradient change in stiffness between the TKA body and the final layer with a total thickness of about 4 μm [19, 24]. All surgeries were performed by one of three experienced arthroplasty surgeons using a medial parapatellar approach and a tourniquet. All implants were posterior stabilized, cemented and no patellar resurfacing was performed.
Patients were seen by a study nurse prior to, 3 months, 1 year and 3 years after surgery. Knee Function (Oxford Knee Score, [OKS], Knee Society Score, [KSS]) and health-related quality of life (EQ-5D)) was assessed [25,26,27,28]. Furthermore, patients were asked about their overall satisfaction with the outcome of the TKA on a visual analogue scale (VAS) ranging from 0 (not satisfied) to 10 (very satisfied). 73 patients completed the 3year follow-up (FU) (Fig. 1).
Valid blood cytokine patterns were available from 74 patients from cryopreserved serum samples, which were stored at -20 °C. The blinded samples were assessed for the presence and concentration of 7cytokines by a multiplex cytometric bead assay (CBA; BD Biosciences, Heidelberg, Germany) via flow cytometry using a FACS canto (BD Biosciences, Heidelberg, Germany) [29]. The panel of cytokines included inflammatory (IL-1beta, IL-5, IL-6, IFN γ, TNF α), chemoattractant (IL-8,)) and immune regulating (IL-10) factors. The respective detection limit was < 0.01 pg/ml. The results were additionally evaluated by double assessment of 10 randomly selected blood samples.
Statistical analysis
Sample size calculation was performed using data from a cross-sectional study with the same coating system [30]. To detect a difference of 1pg/ml in IL-8 or IL-10 between coated and standard implants with a power of 80% with a significance level of p < 0.05, a minimum of 31 patients per group were necessary. Accounting for loss-to-follow-up, 40 patients per group were included. Since most serum cytokine levels range between 5 and 40 pg/ml in healthy subjects the detection limit of 1pg/ml was chosen to ensure a sufficient level of sensitivity [31].
Data description was based on means and standard deviation (SD) for continuous values and absolute and relative frequencies for categorical values. Comparisons between treatment groups were done by Mann-Whitney-U-Test for continuous values and chi-square test for categorical values. Differences between normal and elevated cytokine levels with regard to PROMs were also analyzed by Mann-Whitney-U-Test. Significance level was set at p < 0.05. The software SPSS (release 26 for Windows) was used for data analysis.
Results
While the non-allergy groups were not different regarding pre- and perioperative data, such as gender, age, body mass index (BMI), surgery time and co-morbidities we found the allergy patients to be significantly younger. Moreover, the gender was not distributed equally in this group since all allergy patients were female. Also, the cut-sew time in this group was significantly longer. In order to address this issue and allow for comparability we established a matched-pair analysis (Table 1). One patient was not available for 3year FU due to medical reasons. Two patients in the coated group deceased as result of preexisting comorbidities. There was one revision after 2 years in the coated TKA group due to osteonecrosis of the medial tibial plateau and subsequent loosening of the tibial component. The revision was delayed due to further medical conditions and serious third body wear from bone cement occurred. Even in this catastrophic wear situation about 50% of the coating was still intact.
The blood cytokine patterns prior to surgery, one and three years after TKA demonstrated for most parameters no differences between the non-allergy groups (Table 2). Only for IFN γ there was a difference between the standard and coated groups at the 1year FU. However, this difference had already been present prior to surgery. At the 3year FU IFN γ levels had decreased and no differences were detected. In nearly all patients there were measurable levels of IL-8 (98%). IL-6 was measurable in 66% of the patients. Furthermore, few patients had measurable levels of IL-10 (12%), IL-5 (2%), IL1-ß (3%), IFN γ (2%) and TNF α (3%) at the 3year FU. Cytokine levels did not have an influence upon PROMs.
In contrast to these findings, we observed no elevated IL-6 levels prior to surgery within the allergy group. This is significant compared to the non-allergy groups (p = 0.022). Within the matched-pair analysis, however, this was not evident. All allergy patients presented with measurable levels of IFN γ prior to surgery (p = < 0.001). IFN γ levels dropped at the 1year FU but were still significantly higher compared to the standard, coated and matched-pair group. Similarly, to the non-allergy cohorts IFN γ was not detectable at the 3year FU anymore. The further cytokines displayed no differences between the groups.
Within the non-allergy groups, we found no relevant differences in PROMs and ROM at any FU (Table 3). At the 1year FU the KSS Function score was significantly better in the coated group (p = 0.016). This resolved at the 3year FU.
Compared to these groups we observed a slower improvement within the allergy cohort. At the 1year FU the patient-reported scores in the allergy group were lower compared to the non-allergy groups. Yet these lower results were not significant.
Satisfaction was high in all groups, with a mean of 8.3 (± 2.0) in coated, 8.5 (± 1.7) in standard, 8.3 (± 1.5) in the allergy and 8.1 ± 2.7 in the matched-pair cohort.
Discussion
This study demonstrated no relevant differences in inflammatory response, cytokine expression patterns and PROMs between coated and standard TKA in patients without allergies during mid-term FU.
Regarding the inflammatory response there were no differences between the standard and coated treatment group. In comparison to the allergy cohort, we found differences especially for the expression of IL-6 and IFN γ.
IL-5, a messenger for type-1 allergic reactions was detectable prior to surgery as well as at the 1year FU. This was unexpected since IL-5 is involved in acute reactions of the immune system modulating the change of antibody class in lymphocytes to IgE causing mast cell degranulation. This however is not a mechanism for metal hypersensitivity [4]. The measurable serum levels of IL-5 remain therefore not fully understood but resolved at the 3year FU.
IL-6 is a cytokine involved in the regulation of acute-phase proteins and immune response towards acute inflammation [32]. We detected increased IL-6 levels for standard and coated TKA patients at all timepoints. Interestingly within the allergy group and similar non-allergy patients (matched pairs) IL-6 was not detectable prior to surgery. The rise of IL-6 may be a hint towards a local tissue reaction or chronic inflammatory process after TKA. Previous studies have suggested that IL-6 production could also be an indicator for periprosthetic joint infection (PJI) [33]. However, with more than 60% of the patients producing measurable Il-6 levels and no PJI diagnosed, this association seems – at least for the underlying study - unlikely. Other authors suggested that high preoperative levels of IL-6 might be associated with pain severity or ongoing osteoarthritis while persisting high levels of IL-6 after TKA might be a predictor of insufficient pain relief [34, 35].
IL-8 is a proinflammatory cytokine which induces chemotaxis in neutrophils and other granulocytes causing migration to the site of immune reaction [36]. We observed measurable levels for most patients prior to surgery. This could be a sign of chronic osteoarthritis and its local inflammation [37]. After TKA the levels of IL-8 decreased by about 1/3 in both non-allergy groups suggesting a reduction of the inflammatory process. However, at the 3year FU IL-8 was detectable again in most patients. Since IL-8 can be triggered by various conditions (i.e. liver fibrosis) there might be no connection to the implanted prosthesis [38, 39]. On the other hand, this could be a sign of an immune reaction in which the body is dealing with the implant materials as other authors have shown an association between IL-8 expression and metal exposure [40, 41]. Thomas et al. showed an increase of IL-8 after standard TKA compared to coated TKA while Lützner et al. showed a correlation between worse functional outcome and elevated IL-8 levels [30, 42]. This, however, does not match our results. The reasons for the different results remain unclear. Yet there are studies suggesting a timely fluctuation of cytokine levels in the peripheral blood [22].
IFN γ has multiple functions. Among these, it works as a proinflammatory cytokine modulating the immune system during infection [43]. While we found no differences between standard and coated TKA, the IFN γ levels in the allergy group were significantly higher at the 1year FU. This was also true comparing the allergy patients with non-allergy matched pairs. This may suggest an ongoing effort of the body adapting to the implant material before reaching a tolerance. However, there might be a relevant bias, since IFN γ levels were elevated already prior to surgical treatment. At the 3year FU IFN γ had decreased in all cohorts.
IL-10 has a regulatory function. It acts anti-inflammatory and inhibits the expression of other cytokines. Within our study we found measurable levels of IL-10 prior to and during the 1year FU in every patient. At the 3year FU only few patients presented with measurable blood levels. A possible function might be to counteract the inflammatory processes mediated by IL-8 and IFN γ as discussed above. The elevated levels of IL-8 and IL-10 are in contrast to previously published results [42]. The specific reasons for elevation remain unclear.
The allergy group demonstrated several interesting findings. All patients were allergic to nickel and four concomitantly to cobalt. Metal allergy as delayed type hypersensitivity (DTH) is characterized by predominant Th1-type inflammation with IFN γ being a marker-signaling factor. Correspondingly, detectable IFN γ in blood characterizes the “DTH favouring status” – being visible already in the preoperative blood samples. What affects IFN γ in the serum to finally drop under the detection limit at the 3year FU is difficult to decipher. A transition to counteracting cytokine patterns – at least to Th2 or immune dampening - is not evident from our data, since levels of IL-5 are unchanged and IL-10 is even decreasing. Are (internal) sensitizing allergen and particle contacts decreased thus reducing the generation of new Th1 effector cells? This constellation may be given using surface coated implants and by the disappearance of initial periimplant saw blade/cutting guide derived particles [44, 45]. Other factors such as reduced external contact to problem eliciting nickel containing items by the patients or further yet unknown anti-inflammatory properties of surface coated implants may contribute.
The elevated serum levels for IL-5, -6, -8 and -10 prior to surgery might resemble the ongoing inflammatory reactions of the underlying osteoarthritis [37]. However, there is also the possibility that the elevation is due to confounding factors (i.e. comorbidities, smoking, etc.). Further cytokines such as IL1-ß and TNF-α presented without relevant measurable changes. The serum cytokine levels did not seem to have an effect upon the PROMs evaluated. Possible confounding factors biasing the blood testing were addressed by standardized storage of the samples and temporary exclusion of patients during periods of minor infections or other invasive measures (i.e. dental treatment). Furthermore, repeated controls yielded similar results accounting for a valid examination technique.
While hypersensitivities against implant materials have been known for a long time there is an ongoing debate about its importance in TKA. Especially, whether or not hypoallergenic implants are necessary is being discussed controversially [4,5,6,7, 46]. Some authors state that there is no evidence for the use of hypoallergenic implants [5, 8]. Others advise the use for patients with self-reported history of metal hypersensitivity [47,48,49]. Within these patients skin patch testing is the widely accepted diagnostic standard [50, 51]. This is despite the fact, that skin testing can – depending upon the evaluated substance – have a lower sensitivity than the more complex lymphocyte transformation test [LTT] [52, 53]. Yet both tests might not lead to a conclusion regarding joint tissue reaction [4, 54]. While the reported prevalence for metal hypersensitivity using skin patch testing is up to 32% [55], a recent study using LTT revealed a prevalence of only 3% [54]. Due to the limited specificity of the tests, general assessment of TKA patients without self-reported allergies is not recommended.
Regardless of these debates, there are patients with a diagnosed metal allergy asking for hypoallergenic implants. Guidelines and legal regulations differ between countries, making either the use of hypoallergenic implants or extensive informed consent a requirement to use standard implants. It is known that these patients are at higher risk for early revision and less satisfactory results. Therefore, refusing them a hypoallergenic implant is often difficult [16, 52, 54, 56, 57].
Even though coated implants have proven a better resistance against wear in vitro and the AS coating used in this study has demonstrated excellent long-term results, data of arthroplasty registries have suggested higher overall revision rates for hypoallergenic implants [17, 58, 59]. It needs to be considered that these implants are being used in patients with diagnosed or presumed hypersensitivity against implant materials. The coating itself is therefore very likely not the primary reason leading to revision. Generally, patients with metal hypersensitivity have shown worse outcome in TKA and total hip arthroplasty regardless of the implant used [56, 57, 60, 61]. The reasons are not fully understood. A connection between allergies, depression and anxiety disorders has been suggested since patients with a high level of psychological distress and anxiety tend to evaluate the outcome of TKA worse [62,63,64]. This occurs, even though the self-reported improvement after TKA (delta) is similar to psychologically healthy patients [62]. Since women are affected more often by anxiety disorders and allergies alike, this might explain for at least a certain percentage of differences [65,66,67,68]. Further reasons for the worse outcome need to be elucidated. In order to compare homogenous patient collectives and due to local guidelines, in the present study patients with self-reported metal allergy were excluded from randomization and observed as an additional group. This allows to compare the results of the standard implants directly to the coated implants eliminating biasing factors due to patient-specific characteristics. Regarding demographic parameters we found no differences between the standard and coated group. The allergy patients however were significantly younger. This may be a hint towards an earlier pain onset. Furthermore, all patients of this group were female. This is in line with the aforementioned higher percentage of women being affected by metal hypersensitivities. In order to address this demographic imbalance, we conducted a matched-pair analysis. Comparing the allergy cohort to similar non-allergy patients we found no differences in knee function and PROMs. This is consistent with previous findings in literature. While there are only few studies comparing coated to standard TKA, all of these demonstrated similar results [15, 69,70,71,72,73]. Comparing coated TKA to the allergy cohort we found a trend towards a slower rehabilitation within the allergy patients. While they reached similar scores at the 3year FU it seemed as though they needed longer. Yet these results were not significant and can therefore only be described as a trend. The reasons for this trend remain unclear.
To our knowledge, the present study is the first randomized controlled trial reporting longitudinal cytokine patterns after coated and standard TKA. It has, however, some limitations. This is mainly the exclusion of patients with a history of metal allergy and therefore the target population for coated implants. As explained, this was inevitable according to local guidelines. Yet, the allergy group demonstrated similar results, although this group included only a limited number of patients. Excluding patients with any kind of metal implants was necessary to avoid bias through these implants. This may have resulted in a study population with lower musculoskeletal comorbidities, potentially influencing the PROMs. Furthermore, a 3year FU is relatively short for patients following TKA. There might be changes between the groups at a later stage. Another limitation concerns the intake of anti-inflammatory drugs which may influence the serum cytokine levels. The duration and total amount of NSAR intake, however, was not evaluated during the FU. Also, it needs to be mentioned that the expression patterns of cytokines and other inflammatory mediators are fluctuating and not fully understood.
Conclusion
There were no differences in blood cytokine patterns and PROMs between standard and coated TKA during mid-term follow up. The inflammatory response measured with several cytokines was low in all assessed groups. The use of the investigated coating system did not lead to higher complication rates or a worse outcome. It therefore seems to be a safe treatment option in patients who need a hypoallergenic implant.
Data Availability
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- BMI:
-
Body mass index
- CoCrMo:
-
Cobalt chrome mylobdenum
- DTH:
-
Delayed type hypersensitivity
- EQ-5D:
-
European Quality of Life 5 Dimensions 3 Level Version
- FACS:
-
Fluorescence activated cell sorting
- FU:
-
Follow up
- IFN:
-
Interferon
- IgE:
-
Immunoglobulin E
- IL:
-
Interleukin
- KSS:
-
Knee Society Score
- LTT:
-
Lymphocyte transformation test
- OKS:
-
Oxford Knee Score
- PJI:
-
Periprosthetic joint infection
- PROM:
-
Patient reported outcome
- ROM:
-
Range of motion
- RT:
-
Randomized trial
- SD:
-
Standard deviation
- Th:
-
T-helper (cells)
- TKA:
-
Total knee arthroplasty
- VAS:
-
Visual analogue scale
References
Mitchelson AJ, Wilson CJ, Mihalko WM, Grupp TM, Manning BT, Dennis DA et al. Biomaterial hypersensitivity: is it real? Supportive evidence and approach considerations for metal allergic patients following total knee arthroplasty. Biomed Res Int [Internet]. 2015 [cited 2022 Jul 7];2015. Available from: https://pubmed.ncbi.nlm.nih.gov/25883940/.
Thiele K, Fussi J, Perka C, Pfitzner T. [The Berlin diagnostic algorithm for painful knee TKA]. Orthopade [Internet]. 2016 Jan 1 [cited 2022 Jul 7];45(1):38–46. Available from: https://pubmed.ncbi.nlm.nih.gov/26679494/.
Röhner E, Heinecke M, Matziolis G. Diagnostic algorithm in aseptic TKA failure - What is evidence-based? J Orthop [Internet]. 2021 Mar 1 [cited 2022 Jul 5];24:248. Available from: /pmc/articles/PMC8039505/.
Middleton S, Toms A. Allergy in total knee arthroplasty. https://doi.org/101302/0301-620X98B436767 [Internet]. 2016 Apr 1 [cited 2022 Jul 5];98B(4):437–41. Available from: https://online.boneandjoint.org.uk/doi/abs/10.1302/0301-620X.98B4.36767.
Bravo D, Wagner ER, Larson DR, Davis MP, Pagnano MW, Sierra RJ. No Increased Risk of Knee Arthroplasty Failure in Patients With Positive Skin Patch Testing for Metal Hypersensitivity: A Matched Cohort Study. J Arthroplasty [Internet]. 2016 Aug 1 [cited 2022 Jul 7];31(8):1717–21. Available from: https://pubmed.ncbi.nlm.nih.gov/26869063/.
Guenther D, Thomas P, Kendoff D, Omar M, Gehrke T, Haasper C. Allergic reactions in arthroplasty: myth or serious problem? Int Orthop [Internet]. 2016 Feb 1 [cited 2022 Jul 7];40(2):239–44. Available from: https://pubmed.ncbi.nlm.nih.gov/26526701/.
Münch HJ, Jacobsen SS, Olesen JT, Menné T, Soballe K, Johansen JD, et al. The association between metal allergy, total knee arthroplasty, and revision. Acta Orthop. 2015;86(3):378–83.
Razak A, Ebinesan AD, Charalambous CP. Metal allergy screening prior to joint arthroplasty and its influence on implant choice: a delphi consensus study amongst orthopaedic arthroplasty surgeons. Knee Surg Relat Res [Internet]. 2013 [cited 2022 Jul 6];25(4):186–93. Available from: https://pubmed.ncbi.nlm.nih.gov/24368996/.
Bergschmidt P, Bader R, Mittelmeier W. Metal hypersensitivity in total knee arthroplasty: revision surgery using a ceramic femoral component - a case report. Knee [Internet]. 2012 Mar [cited 2022 Oct 25];19(2):144–7. Available from: https://pubmed.ncbi.nlm.nih.gov/21292491/.
Stathopoulos IP, Andrianopoulos N, Paschaloglou D, Tsarouchas I. Revision total knee arthroplasty due to bone cement and metal hypersensitivity. Arch Orthop Trauma Surg [Internet]. 2017 Feb 1 [cited 2022 Oct 25];137(2):267–71. Available from: https://pubmed.ncbi.nlm.nih.gov/28070650/.
Whiteside LA. Clinical Results of Revision TKA in Patients With Presumed Metal and Cement Allergy. J Arthroplasty [Internet]. 2022 Jun 1 [cited 2022 Oct 25];37(6S):S250–7. Available from: https://pubmed.ncbi.nlm.nih.gov/35196568/.
Merritt K, Brown SA. Metal sensitivity reactions to orthopedic implants. Int J Dermatol. 1981;20:89.
Galetz MC, Fleischmann EW, Konrad CH, Schuetz A, Glatzel U. Abrasion resistance of oxidized zirconium in comparison with CoCrMo and titanium nitride coatings for artificial knee joints. J Biomed Mater Res B Appl Biomater [Internet]. 2010 Apr [cited 2022 Jul 7];93(1):244–51. Available from: https://pubmed.ncbi.nlm.nih.gov/20162723/.
Herbster M, Döring J, Nohava J, Lohmann CH, Halle T, Bertrand J. Retrieval study of commercially available knee implant coatings TiN, TiNbN and ZrN on TiAl6V4 and CoCr28Mo6. J Mech Behav Biomed Mater [Internet]. 2020 Dec 1 [cited 2022 Jul 7];112. Available from: https://pubmed.ncbi.nlm.nih.gov/32871541/.
van Hove RP, Sierevelt IN, van Royen BJ, Nolte PA. Titanium-Nitride Coating of Orthopaedic Implants: A Review of the Literature. Biomed Res Int [Internet]. 2015 [cited 2022 Jul 7];2015. Available from: https://pubmed.ncbi.nlm.nih.gov/26583113/.
Grimberg AW, Grupp TM, Elliott J, Melsheimer O, Jansson V, Steinbrück A. Ceramic Coating in Cemented Primary Total Knee Arthroplasty is Not Associated With Decreased Risk of Revision due to Early Prosthetic Joint Infection. J Arthroplasty [Internet]. 2021 Mar 1 [cited 2022 Jul 7];36(3):991–7. Available from: https://pubmed.ncbi.nlm.nih.gov/33012599/.
Reich J, Hovy L, Lindenmaier HL, Zeller R, Schwiesau J, Thomas P et al. [Preclinical evaluation of coated knee implants for allergic patients]. Orthopade [Internet]. 2010 [cited 2022 Jul 7];39(5):495–502. Available from: https://pubmed.ncbi.nlm.nih.gov/20091294/.
Lützner J, Beyer F, Lützner C, Tille E, Postler AE. A Novel Multilayer-Coating for Total Knee Arthroplasty Implants is Safe – 10-Year Results From a Randomized-Controlled Trial. J Arthroplasty [Internet]. 2022 Jul [cited 2022 Aug 25]; Available from: https://pubmed.ncbi.nlm.nih.gov/35921997/.
Lützner J, Altermann B, Laura Puente Reyna A, Grupp M. T. Modern Coatings in Knee Arthroplasty. In: Arthroplasty - Advanced Techniques and Future Perspectives [Working Title] [Internet]. IntechOpen; 2022. p. 13. Available from: https://www.intechopen.com/online-first/82486.
Summer B, Paul C, Mazoochian F, Rau C, Thomsen M, Banke I et al. Nickel (Ni) allergic patients with complications to Ni containing joint replacement show preferential IL-17 type reactivity to Ni. Contact Dermatitis [Internet]. 2010 [cited 2022 Jul 7];63(1):15–22. Available from: https://pubmed.ncbi.nlm.nih.gov/20597929/.
Lützner J, Hartmann A, Dinnebier G, Spornraft-Ragaller P, Hamann C, Kirschner S. Metal hypersensitivity and metal ion levels in patients with coated or uncoated total knee arthroplasty: a randomised controlled study. Int Orthop [Internet]. 2013 Oct [cited 2022 Jul 7];37(10):1925–31. Available from: https://pubmed.ncbi.nlm.nih.gov/23860793/.
Cassuto J, Folestad A, Göthlin J, Malchau H, Kärrholm J. The key role of proinflammatory cytokines, matrix proteins, RANKL/OPG and Wnt/β-catenin in bone healing of hip arthroplasty patients. Bone [Internet]. 2018 Feb 1 [cited 2022 Oct 25];107:66–77. Available from: https://pubmed.ncbi.nlm.nih.gov/29129760/.
Schulz KF, Altman DG, Moher D. CONSORT 2010 statement: updated guidelines for reporting parallel group randomised trials. BMJ [Internet]. 2010 Mar 27 [cited 2023 Jun 3];340(7748):698–702. Available from: https://pubmed.ncbi.nlm.nih.gov/20332509/.
Reich J, Hovy L, Lindenmaier HL, Zeller R, Schwiesau J, Thomas P et al. [Preclinical evaluation of coated knee implants for allergic patients]. Orthopade [Internet]. 2010 [cited 2022 Nov 9];39(5):495–502. Available from: https://pubmed.ncbi.nlm.nih.gov/20091294/.
Scuderi GR, Bourne RB, Noble PC, Benjamin JB, Lonner JH, Scott WN. The new Knee Society Knee Scoring System. Clin Orthop Relat Res [Internet]. 2012 Jan 1 [cited 2023 Jun 3];470(1):3–19. Available from: https://pubmed.ncbi.nlm.nih.gov/22045067/.
Insall JN, Dorr LD, Scott RD, Scott WN. Rationale of the Knee Society clinical rating system. Clin Orthop Relat Res. 1989;(248):13–4.
Rabin R, De Charro F. EQ-5D: a measure of health status from the EuroQol Group. Ann Med [Internet]. 2001 [cited 2023 Jun 3];33(5):337–43. Available from: https://pubmed.ncbi.nlm.nih.gov/11491192/.
Dawson J, Fitzpatrick R, Murray D, Carr A. Questionnaire on the perceptions of patients about total knee replacement. J Bone Joint Surg Br [Internet]. 1998 Jan [cited 2023 Jun 3];80(1):63–9. Available from: https://pubmed.ncbi.nlm.nih.gov/9460955/.
Cossarizza A, Chang HD, Radbruch A, Acs A, Adam D, Adam-Klages S et al. Guidelines for the use of flow cytometry and cell sorting in immunological studies (second edition). Eur J Immunol [Internet]. 2019 Oct 1 [cited 2023 Jun 3];49(10):1457–973. Available from: https://pubmed.ncbi.nlm.nih.gov/31633216/.
Thomas P, Hisgen P, Kiefer H, Schmerwitz U, Ottersbach A, Albrecht D et al. Blood cytokine pattern and clinical outcome in knee arthroplasty patients: comparative analysis 5 years after standard versus ‘hypoallergenic’ surface coated prosthesis implantation. Acta Orthop [Internet]. 2018 Nov 2 [cited 2022 Jul 7];89(6):646–51. Available from: https://pubmed.ncbi.nlm.nih.gov/30372661/.
Kleiner G, Marcuzzi A, Zanin V, Monasta L, Zauli G. Cytokine levels in the serum of healthy subjects. Mediators Inflamm [Internet]. 2013 [cited 2023 Apr 17];2013. Available from: https://pubmed.ncbi.nlm.nih.gov/23533306/.
Tanaka T, Narazaki M, Kishimoto T. Interleukin (IL-6) Immunotherapy. Cold Spring Harb Perspect Biol [Internet]. 2018 Aug 1 [cited 2022 Jul 6];10(8). Available from: https://pubmed.ncbi.nlm.nih.gov/28778870/.
Gollwitzer H, Dombrowski Y, Prodinger PM, Peric M, Summer B, Hapfelmeier A et al. Antimicrobial peptides and proinflammatory cytokines in periprosthetic joint infection. J Bone Joint Surg Am [Internet]. 2013 Apr 3 [cited 2022 Jul 7];95(7):644–51. Available from: https://pubmed.ncbi.nlm.nih.gov/23553300/.
Gandhi R, Santone D, Takahashi M, Dessouki O, Mahomed NN. Inflammatory predictors of ongoing pain 2 years following knee replacement surgery. Knee [Internet]. 2013 Oct [cited 2022 Jul 8];20(5):316–8. Available from: https://pubmed.ncbi.nlm.nih.gov/23157967/.
Azim S, Nicholson J, Rebecchi MJ, Galbavy W, Feng T, Rizwan S et al. Interleukin-6 and leptin levels are associated with preoperative pain severity in patients with osteoarthritis but not with acute pain after total knee arthroplasty. Knee [Internet]. 2018 Jan 1 [cited 2022 Jul 8];25(1):25–33. Available from: http://www.thekneejournal.com/article/S0968016017303319/fulltext.
Baggiolini M, Clark-Lewis I. Interleukin-8, a chemotactic and inflammatory cytokine. FEBS Lett [Internet]. 1992 Jul 27 [cited 2022 Jul 7];307(1):97–101. Available from: https://onlinelibrary.wiley.com/doi/full/10.1016/0014-5793%2892%2980909-Z.
Kapoor M, Martel-Pelletier J, Lajeunesse D, Pelletier JP, Fahmi H. Role of proinflammatory cytokines in the pathophysiology of osteoarthritis. Nature Reviews Rheumatology 2010 7:1 [Internet]. 2010 Nov 30 [cited 2022 Jul 8];7(1):33–42. Available from: https://www.nature.com/articles/nrrheum.2010.196.
Signorelli SS, Valerio F, Malaponte G. Inflammation and peripheral arterial disease: the value of circulating biomarkers (Review). Int J Mol Med [Internet]. 2014 [cited 2022 Jul 7];33(4):777–83. Available from: https://pubmed.ncbi.nlm.nih.gov/24535646/.
Nobili V, Marcellini M, Giovannelli L, Girolami E, Muratori F, Giannone G et al. Association of serum interleukin-8 levels with the degree of fibrosis in infants with chronic liver disease. J Pediatr Gastroenterol Nutr [Internet]. 2004 [cited 2022 Jul 7];39(5):540–4. Available from: https://pubmed.ncbi.nlm.nih.gov/15572896/.
Lawrence H, Deehan D, Holland J, Kirby J, Tyson-Capper A. The immunobiology of cobalt: demonstration of a potential aetiology for inflammatory pseudotumours after metal-on-metal replacement of the hip. Bone Joint J [Internet]. 2014 Sep [cited 2022 Jul 7];96-B(9):1172–7. Available from: https://pubmed.ncbi.nlm.nih.gov/25183586/.
Ninomiya JT, Kuzma SA, Schnettler TJ, Krolikowski JG, Struve JA, Weihrauch D. Metal ions activate vascular endothelial cells and increase lymphocyte chemotaxis and binding. J Orthop Res [Internet]. 2013 Sep [cited 2022 Jul 7];31(9):1484–91. Available from: https://pubmed.ncbi.nlm.nih.gov/23629852/.
Lützner J, Beyer F, Lützner C, Thomas P, Summer B. Increased inflammatory response is associated with less favorable functional results 5 years after total knee arthroplasty. Knee Surgery, Sports Traumatology, Arthroscopy [Internet]. 2022 Feb 11 [cited 2022 Jul 8];1–7. Available from: https://link.springer.com/article/10.1007/s00167-021-06836-w.
Schroder K, Hertzog PJ, Ravasi T, Hume DA. Interferon-γ: an overview of signals, mechanisms and functions. J Leukoc Biol [Internet]. 2004 Feb 1 [cited 2022 Jul 8];75(2):163–89. Available from: https://onlinelibrary.wiley.com/doi/full/10.1189/jlb.0603252.
Samelko L, Caicedo MS, Jacobs J, Hallab NJ. Transition from metal-DTH resistance to susceptibility is facilitated by NLRP3 inflammasome signaling induced Th17 reactivity: implications for orthopedic implants. PLoS ONE. 2019;14(1).
Lawrie CM, Bartosiak KA, Barrack TN, Nunley RM, Wright RW, Barrack RL. James A. Rand Young Investigator’s award: questioning the Nickel Free total knee arthroplasty. J Arthroplasty. 2022;37(8):705–9.
Faschingbauer M, Renner L, Boettner F. Allergy in Total Knee Replacement. Does It Exist? Review Article. HSS Journal [Internet]. 2017 Feb 1 [cited 2022 Jul 5];13(1):12–9. Available from: https://link.springer.com/article/10.1007/s11420-016-9514-8.
Teo ZWW, Schalock PC. Hypersensitivity Reactions to Implanted Metal Devices: Facts and Fictions. J Investig Allergol Clin Immunol [Internet]. 2016 [cited 2022 Oct 25];26(5):279–94. Available from: https://pubmed.ncbi.nlm.nih.gov/27763855/.
Teo WZW, Schalock PC. Metal Hypersensitivity Reactions to Orthopedic Implants. Dermatol Ther (Heidelb) [Internet]. 2017 Mar 1 [cited 2022 Oct 25];7(1):53–64. Available from: https://pubmed.ncbi.nlm.nih.gov/27995484/.
Thyssen JP, Jakobsen SS, Engkilde K, Johansen JD, Søballe K, Menné T. The association between metal allergy, total hip arthroplasty, and revision. Acta Orthop [Internet]. 2009 Dec 23 [cited 2022 Oct 25];80(6):646–52. Available from: https://pubmed.ncbi.nlm.nih.gov/19995314/.
Schalock PC, Crawford G, Nedorost S, Scheinman PL, Atwater AR, Mowad C et al. Patch Testing for Evaluation of Hypersensitivity to Implanted Metal Devices: A Perspective From the American Contact Dermatitis Society. Dermatitis [Internet]. 2016 Sep 1 [cited 2022 Oct 25];27(5):241–7. Available from: https://pubmed.ncbi.nlm.nih.gov/27649347/.
Thyssen JP, Menné T. Metal allergy–a review on exposures, penetration, genetics, prevalence, and clinical implications. Chem Res Toxicol [Internet]. 2010 Feb 15 [cited 2022 Oct 25];23(2):309–18. Available from: https://pubmed.ncbi.nlm.nih.gov/19831422/.
Richards LJ, Streifel A, Rodrigues JM. Utility of Patch Testing and Lymphocyte Transformation Testing in the Evaluation of Metal Allergy in Patients with Orthopedic Implants. Cureus [Internet]. 2019 Sep 25 [cited 2022 Jul 18];11(9). Available from: https://pubmed.ncbi.nlm.nih.gov/31723520/.
Ständer S, Oppel E, Thomas P, Summer B. Evaluation of lymphocyte transformation tests as compared with patch tests in nickel allergy diagnosis. Contact Dermatitis [Internet]. 2017 Apr 1 [cited 2022 Aug 29];76(4):228–34. Available from: https://pubmed.ncbi.nlm.nih.gov/28176340/.
Boutefnouchet T, Vallières F, Delisle J, Benderdour M, Fernandes JC. Lymphocyte transformation test reveals low prevalence of true metal hypersensitivity among pre-operative total knee arthroplasty patients. Knee Surg Sports Traumatol Arthrosc [Internet]. 2022 [cited 2022 Jul 6]; Available from: https://pubmed.ncbi.nlm.nih.gov/35380240/.
Duarte I, Mendonça RF, Korkes KL, Lazzarini R, Hafner M. de FS. Nickel, chromium and cobalt: the relevant allergens in allergic contact dermatitis. Comparative study between two periods: 1995–2002 and 2003–2015. An Bras Dermatol [Internet]. 2018 Jan 1 [cited 2022 Jul 6];93(1):59–62. Available from: https://pubmed.ncbi.nlm.nih.gov/29641698/.
Hinarejos P, Ferrer T, Leal J, Torres-Claramunt R, Sánchez-Soler J, Monllau JC. Patient-reported allergies cause inferior outcomes after total knee arthroplasty. Knee Surg Sports Traumatol Arthrosc. 2015;24(10):3242–6.
Otero JE, Graves CM, Gao Y, Olson TS, Dickinson CC, Chalus RJ et al. Patient-Reported Allergies Predict Worse Outcomes After Hip and Knee Arthroplasty: Results From a Prospective Cohort Study. J Arthroplasty [Internet]. 2016 Dec 1 [cited 2022 Jul 5];31(12):2746–9. Available from: http://www.arthroplastyjournal.org/article/S0883540316304648/fulltext.
The German Arthroplasty Registry (EPRD). Available from: www.eprd.de.
Australian Orthopaedic Association National Joint Replacement Registry [Internet]. 2022. Available from: https://aoanjrr.sahmri.com/annual-reports-2022.
Graves CM, Otero JE, Gao Y. Patient reported allergies are a risk factor for poor outcomes in total hip and knee arthroplasty. J Arthroplasty. 2014;29:147.
McLawhorn AS, Bjerke-Kroll BT, Blevins JL. Patient-reported allergies are associated with poorer patient satisfaction and outcomes after lower extremity arthroplasty: a retrospective cohort study. J Arthroplasty. 2015;30:1132.
Pérez-Prieto D, Gil-González S, Pelfort X, Leal-Blanquet J, Puig-Verdié L, Hinarejos P. Influence of depression on total knee arthroplasty outcomes. J Arthroplasty. 2014;29(1):44–7.
Peña P, Ortega MA, Buján J, de la Torre B. Influence of Psychological Distress in Patients with Hypoallergenic Total Knee Arthroplasty. Treatment Algorithm for Patients with Metal Allergy and Knee Osteoarthritis. Int J Environ Res Public Health [Internet]. 2021 Jun 1 [cited 2022 Jul 5];18(11). Available from: https://pubmed.ncbi.nlm.nih.gov/34204981/.
Ferrer T, Hinarejos P, Goicoechea N, Leal-Blanquet J, Sanchez-Soler J, Torres-Claramunt R et al. Anxiety is the cause of the worse outcomes of allergic patients after total knee arthroplasty. Knee Surg Sports Traumatol Arthrosc [Internet]. 2020 Oct 1 [cited 2022 Jul 7];28(10):3135–41. Available from: https://pubmed.ncbi.nlm.nih.gov/31722034/.
Pigott TA. Gender differences in the epidemiology and treatment of anxiety disorders. J Clin Psychiatry [Internet]. 1999 [cited 2023 Apr 17];60 Suppl 18(SUPPL. 18):4–15. Available from: https://pubmed.ncbi.nlm.nih.gov/10487250/.
Paller A, Jaworski JC, Simpson EL, Boguniewicz M, Russell JJ, Block JK et al. Major Comorbidities of Atopic Dermatitis: Beyond Allergic Disorders. Am J Clin Dermatol [Internet]. 2018 Dec 1 [cited 2023 Apr 17];19(6):821–38. Available from: https://pubmed.ncbi.nlm.nih.gov/30168085/.
Ahlström MG, Thyssen JP, Wennervaldt M, Menné T, Johansen JD. Nickel allergy and allergic contact dermatitis: A clinical review of immunology, epidemiology, exposure, and treatment. Contact Dermatitis [Internet]. 2019 Oct 1 [cited 2023 Apr 17];81(4):227–41. Available from: https://pubmed.ncbi.nlm.nih.gov/31140194/.
Lidén C. Metal allergy: Nickel. Metal Allergy: From Dermatitis to Implant and Device Failure [Internet]. 2018 Apr 13 [cited 2023 Apr 17];423–34. Available from: https://link.springer.com/chapter/10.1007/978-3-319-58503-1_32.
Göbel F, Ulbricht S, Hein W, Bernstein A. [Radiological mid-term results of total knee arthroplasty with femoral components of different materials]. Z Orthop Unfall [Internet]. 2008 [cited 2022 Jul 7];146(2):194–9. Available from: https://pubmed.ncbi.nlm.nih.gov/18404582/.
Postler A, Beyer F, Lützner C, Tille E, Lützner J. Similar outcome during short-term follow-up after coated and uncoated total knee arthroplasty: a randomized controlled study. Knee Surg Sports Traumatol Arthrosc [Internet]. 2018 Nov 1 [cited 2022 Jul 7];26(11):3459–67. Available from: https://pubmed.ncbi.nlm.nih.gov/29616285/.
Thienpont E. Titanium niobium nitride knee implants are not inferior to chrome cobalt components for primary total knee arthroplasty. Arch Orthop Trauma Surg [Internet]. 2015 Dec 1 [cited 2022 Jul 7];135(12):1749–54. Available from: https://pubmed.ncbi.nlm.nih.gov/26318754/.
Garrett SJNYPSAWD. Differences in metal ion release following cobalt-chromium and oxidized zirconium total knee arthroplasty., Jacobs S, Yates N, Smith P, Wood A. D. Differences in metal ion release following cobalt-chromium and oxidized zirconium total knee arthroplasty. Acta Orthop Belg. 2010;76(4):513–20.
Postler A, Beyer F, Lützner C, Tille E, Lützner J. The use of knee prostheses with a hypoallergenic coating is safe in the medium term: a randomized controlled study. Orthopade. 2021.
Acknowledgements
The authors thank Brit Brethfeld and Anne Schützer for her valuable assistance during follow-up visits and B.Braun Aesculap for funding this study.
Funding
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Contributions
ET, FB, CL, AP, PT, BS and JL have been involved in planning, execution of this study. ET, FB, CL and JL have been involved in analysis of the results. ET and JL have written the draft and all authors have corrected the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
After institutional review board evaluation approval for this study and all experimental protocols was given by the local ethics committee (Ethikkomission Technical University Dresden, IRB 00001473, IOGR 00001076 registered at Office of Human Research Protection) under EK 101032016. All methods were carried out in accordance with the Declaration of Helsinki and its relevant guidelines and regulations. Written informed consent was obtained from all participants prior to enrollment. The study protocol was registered in the US National Institutes of Health’s database (http://www.clinicaltrials.gov) registry under NCT03424174 on 03/17/2016.
Consent for publication
Not applicable.
Competing Interest and Funding
This study was supported by a research grant from B.Braun Aesculap. JL has received research grants outside the submitted work from Arthrosehilfe, B.Braun Aesculap, Innovationsfond, Link, Mathys, Smith&Nephew, ZimmerBiomet and honoraria from B.Braun Aesculap, Grünenthal, Mathys and Pfizer.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Tille, E., Beyer, F., Lützner, C. et al. No difference in patient reported outcome and inflammatory response after coated and uncoated total knee arthroplasty – a randomized controlled study. BMC Musculoskelet Disord 24, 968 (2023). https://doi.org/10.1186/s12891-023-07061-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12891-023-07061-x