Abstract
Purpose
Total knee arthroplasty (TKA) in patients with osteoarthritis (OA) are considered to be a successful procedure, but with little being known about outcomes in patients with rheumatoid arthritis (RA). The aim of this study was to compare the outcomes of TKA in patients with RA versus OA.
Methods
Data were obtained from PubMed, Cochrane Library, EBSCO and Scopus for all available studies comparing the outcomes of THA in RA and OA patients (From January 1, 2000 to October 15, 2022). Outcomes of interest included infection, revision, venous thromboembolism (VTE), mortality, periprosthetic fractures, prosthetic loosening, length of stay, and satisfaction. Two reviewers independently assessed each study for quality and extracted data. The quality of the studies was scored using the Newcastle-Ottawa scale (NOS).
Results
Twenty-four articles with a total 8,033,554 patients were included in this review. The results found strong evidence for increased risk of overall infection (OR = 1.61, 95% CI, 1.24–2.07; P = 0.0003), deep infection (OR = 2.06, 95% CI, 1.37–3.09; P = 0.0005), VTE (OR = 0.76, 95% CI, 0.61–0.93; P = 0.008), pulmonary embolism (PE) (OR = 0.84, 95% CI, 0.78–0.90; P<0.00001), periprosthetic fractures (OR = 1.87, 95% CI, 1.60–2.17; P<0.00001); and reasonable evidence for increased risk of deep venous thrombosis (DVT) (OR = 0.74, 95% CI, 0.54–0.99; P = 0.05), and length of stay (OR = 0.07, 95% CI, 0.01–0.14; P = 0.03) after TKA in patients with RA versus OA. There were no significant differences in superficial site infection (OR = 0.84,95% CI, 0.47–1.52; P = 0.57), revision (OR = 1.33,95% CI, 0.79–2.23; P = 0.28), mortality (OR = 1.16,95% CI, 0.87–1.55; P = 0.32), and prosthetic loosening (OR = 1.75, 95% CI, 0.56–5.48; P = 0.34) between the groups.
Conclusion
Our study demonstrated that patients with RA have a higher risk of postoperative infection, VTE, periprosthetic fracture, and lengths of stay, but did not increase revision rate, prosthetic loosening and mortality compared to patients with OA following TKA. In conclusion, despite RA increased incidence of postoperative complications, TKA should continue to be presented as an effective surgical procedure for patients whose conditions are intractable to conservative and medical management of RA.
Similar content being viewed by others
Introduction
Total knee arthroplasty (TKA) is considered one of the most utilized and successful procedures available to resolve end-stage knee disease and can significantly improve patients’ quality of life and knee function postoperatively [1, 2]. With progressive global aging, the annual worldwide rate of TKA has increased steadily over the past two decades [3]. The main cause of end-stage arthritis is osteoarthritis (OA), which accounts for 90–97% of the primary indication for TKA, followed by rheumatoid arthritis (RA) [4, 5]. Outcomes following TKA are generally unexceptionable, with low complication rate and high satisfaction [6]. However, some complications including infection, revision, venous thromboembolism (VTE), even death have been troubling surgeons significantly [7,8,9].
RA is a chronic, symmetrical, progressive, inflammatory autoimmune disease that primarily affects the joints and is characterized by symmetrical, multi-articular, invasive joint inflammation of the joints of the whole body, especially the hands and feet, leads to the degeneration of cartilage and destruction of bones and joint structure eventually [10]. Patients with RA reported can be significantly improved in pain and function after TKA, yet major outcomes such as infection, revision, and readmission are reported to be higher for patients with RA compared to patients with OA [11, 12]. Since the utilization of disease modifying antirheumatic drugs (DMARDs), biologic agents and Janus kinase (JAK) inhibitors widely have been dramatically enhanced the quality of life for patients with RA, so the number of TKA has been declining in recent years [13, 14]. However, patients with RA may develop osteoporosis and ligament relaxation with joint deformity and disability from controlled RA unsatisfactorily, therefore, operation is still a crucial option for RA treatment [15].
While most TKA operations are performed in patients with OA, which is the most common form of arthritis, they are also efficient in treating progressive joint destruction of the knee in patients with RA [6, 16]. Therefore, numerous previous studies with respect to outcomes of TKA is relied on the experience in patients with OA [3, 17]. Both patients with RA and OA can be treated with TKA, but RA is essentially different from OA in terms of pathogenesis, prognosis, and medical therapy, so conceivable differences in TKA outcomes would be expected [6, 18].
However, few studies have inquired whether there are differences in outcomes for patients with RA versus OA. Some previous meta-analyses have demonstrated that TKA can improve the outcomes of patients with RA, but compared with OA, patients with RA are at higher risk of complications after TKA [6, 19]. However, these studies analyzed a few outcomes, and some literatures were older, and some literatures did not set control group, so the results obtained are controversial. Therefore, our current study was designed to compared outcomes following TKA for RA Versus for OA by pooling data from previous comparative studies (From January 1, 2000 to October 15, 2022). It was hypothesized that the incidence of infection, revision, VTE, mortality, periprosthetic fractures, prosthetic loosening and length of stay all would be higher, and lower satisfaction after TKA in patients with RA than in patients with OA.
Materials and methods
Data and literature sources
This systematic review and meta-analysis adhered to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines. We performed a systematic search of various electronic databases (i.e.: PubMed, Cochrane Library, EBSCO and Scopus), including reports published from January 1, 2000 to October 15, 2022 (to more closely reflect current clinical practice) that described studies of primary TKA and contained information on outcomes in RA and OA patients, without language and date restrictions. Broad MeSH terms and Boolean operators were selected for each database search; the following search terms were used: (Total knee arthroplasty OR TKA OR total knee replacement OR TKR) and (rheumatoid arthritis OR RA) and (Osteoarthritis OR OA). All obtained by searching titles and abstracts were carefully evaluated, and then full texts were read to determine the included articles.
Study selection
Inclusion and exclusion criteria
Two authors independently selected abstracts as well as full-text articles from the above listed databases using the aforementioned search strategies, and a third author adjudicated discrepancies.
The inclusion criteria were listed as follows
(1) Case-control studies (CCS), retrospective cohort studies (RCS), or prospective cohort studies (PCS) comparing outcomes in patients with RA and OA undergoing primary TKA were included; (2) at least one of the following outcome measures was reported: infection (periprosthetic joint infection or wound infection), revision, VTE (deep venous thrombosis (DVT) or pulmonary embolism (PE)), mortality, periprosthetic fractures, prosthetic loosening, length of stay, and satisfaction; (3) without restrictions on age and sex were imposed; and (4) without limitations on language and race were imposed.
The following exclusion criteria were used
(1) Non-peer reviewed publications; (2) certain study designs (non-human trials, observational studies, case reports, case series, review articles, letters to the editor); (3) the inclusion and exclusion criteria for the study were not clear or reasonable; and (4) the full text cannot be obtained or the original data are incomplete.
Data extraction
The following data were extracted: (1) demographic and clinical information of the studies(including author, year of publication, country, study type, study period, sample size, follow-up); (2) outcome measures: including infection, revision, VTE, DVT, PE, mortality, periprosthetic fractures, prosthetic loosening, length of stay and satisfaction. Pertinent data were extracted by two reviewers independently from all eligible studies in patients with RA in comparison to patients with OA using a standardized data collection form, and any disagreement was resolved by a third reviewer.
Assessment of methodological quality
For each included study, the methodological quality was evaluated using Newcastle-Ottawa scale (NOS) [20] by two independent reviewers. NOS Scale is a tool for quality assessment of case-control studies and cohort studies. The domains included case definition (selection of study cohorts, comparability of the cohorts, and outcome ascertainment). The total scores were 9, it had high quality when NOS scores ≥ 6.
Statistical analysis
All analyses were conducted using the RevMan software (RevMan version 5.3.5, The Nordic Cochrane Centre, The Cochrane Collaboration 2014, Copenhagen, Denmark). For continuous variables, this software estimates the weighted mean differences (WMD). The Mantel-Haenszel model and odds ratios (ORs) with 95% confidence intervals (CIs) for outcomes of interest were used to compare dichotomous variables. The effect size (ES) was used for analysis when the units of odds ratios (ORs) with 95% confidence intervals (CIs) were consistent. A P-value less than 0.05 was considered statistically significant. We calculated the I2 coefficient to assess heterogeneity with the following predetermined limits: low < 50%, moderate 50–74%, and high > 75%; and P ≥ 0.05 and I2 < 50% indicating no statistical heterogeneity between studies. A random-effects model was applied in circumstances of moderate or high heterogeneity; otherwise, a fixed-effects model was employed. If there was significant heterogeneity in the included studies, such data were considered unsuitable. Sensitivity analysis was conducted to assess the stability of the results if necessary. If there have other available data, subgroup analysis was also conducted to get more specific conclusions. Moreover, using the forest plots to depict the results of each study and evaluate pooled estimates respectively, and the funnel plots were used to evaluate publication bias.
Results
Study selection and quality of included studies
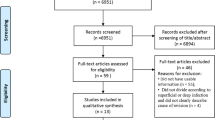
The search strategy previously described produced 6581 results (736 in PubMed, 935 in EBSCO, 1162 in Scopus and 3748 in Cochrane Library). 2802 duplicates were deleted by citation management software and manual review of records. After reviewed the titles and abstracts by two independent authors, 3615 irrelevant citations were removed. The remaining 164 full text papers were then retrieved for a more detailed analysis, of which 140 papers were excluded for several reasons, such as revision TKA (n = 4), data of TKA and THA cannot be distinguished (n = 31), outcomes does not meet the request (n = 79), data available cannot calculate the outcomes (n = 26). Finally, 24 studies were included in our study and could be quantitatively synthesized and the remaining two were qualitatively analyzed. The article selection process is illustrated in Fig. 1.
All studies [7,8,9, 11, 18, 21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39] including 15 RCS, 7 PCS, and 2 CCS had high quality with NOS scores ≥ 6 involved 7,786,321 patients in the OA group and 247,233 patients in the RA group. The quality evaluation and the basic characteristics of the selected trials are shown in Table 1. Outcomes after primary TKA in patients with RA versus patients with OA are shown in Table 2. Funnel plots were assessed for the potential publication bias, according to the funnel plot (Fig. 2), the influence of publication bias on the results could be ignored.
Outcomes of the meta-analysis
Infection
Sixteen [7,8,9, 11, 18, 22,23,24,25, 29,30,31, 34,35,36, 38] studies compared the rate of postoperative infection in patients with RA versus OA who underwent primary TKA. Meta-analysis of these 16 studies showed that the rate of postoperative infection was significantly higher in RA group than that in OA group (1230/241,888 vs. 30,951/7,697,924; OR 1.61, [95% CI 1.24–2.07]; I2 = 88%; P = 0.0003) (Fig. 3A). We performed a subgroup analysis based on deep and superficial infections. Subgroup analysis of 13 studies [7,8,9, 11, 18, 22,23,24,25, 29, 30, 34, 35] reported that the rate of deep infection after TKA was significantly higher in RA group than that in OA group (299/20,058 vs. 7039/741,273; OR 2.06, [95% CI 1.37–3.09]; I2 = 83%; P = 0.0005) (Fig. 3B). However, subgroup analysis of 4 studies [8, 24, 25, 31] reported that the rate of superficial infection were similar in RA and OA groups (15/396 vs. 78/2908; OR 0.84, [95% CI 0.47–1.52]; I2 = 49%; P = 0.57) (Fig. 3C).
Revision
We performed a meta-analysis comparing the revision rate in patients with RA versus OA including twelve studies [9, 11, 18, 21, 23, 27, 30, 31, 34, 35, 37, 39]. The rate of revision after primary TKA was no statistical difference between RA patients and OA groups (857/22,539 vs. 22,267/729,357; OR 1.33, [95% CI 0.79–2.23]; I2 = 96%; P = 0.28) (Fig. 4).
VTE
A total of eight studies [8, 23, 28, 31, 32, 34, 36, 38] provided data comparing the VTE rate in patients with RA versus OA group. The pooled results showed that the VTE rate of patients with RA was significantly higher than those patients with OA (1839/225,741 vs. 64,060/7,029,089; OR 0.76, [95% CI 0.61–0.93]; I2 = 69%; P = 0.008) (Fig. 5A). We also performed a subgroup analysis based on DVT and PE. Subgroup analysis of six studies [8, 28, 31, 32, 36, 38]reported that the rate of DVT after TKA was slightly higher in RA group than that in OA group (1073/222,410 vs. 36,018/6,959,203; OR 0.74, [95% CI 0.54–0.99]; I2 = 78%; P = 0.05) (Fig. 5B), and PE rate in patients with RA were significantly higher than OA patients (727/222,410 vs. 27,001/6,959,203; OR 0.84, [95% CI 0.78–0.90]; I2 = 0%; P<0.00001) (Fig. 5C).
Mortality
Eleven studies [8, 9, 11, 18, 22, 23, 31, 33, 34, 36, 38] reported mortality between RA patients and OA patients. The pooled results showed that there were no statistically significant differences in rate of mortality between the two groups (633/230,637 vs. 42,882/7,404,550; OR 1.16, [95% CI 0.87–1.55]; I2 = 72%; P = 0.32) (Fig. 6).
Periprosthetic fractures
A total of six studies [8, 11, 31, 34,35,36] provided data comparing the periprosthetic fractures rate in patients with RA versus OA group. The meta-analysis revealed that patients with RA dramatically increased risk of periprosthetic fractures compared to patients with OA (179/217,457 vs. 2916/6,713,443; OR 1.87, [95% CI 1.60–2.17]; I2 = 0%; P<0.00001) (Fig. 7).
Prosthetic loosening and length of stay
Six studies [8, 9, 11, 21, 30, 35] provided data comparing the prosthetic loosening rate in patients with RA versus OA group. The meta-analysis demonstrated that patients with RA were no statistically significant difference compared to OA (258/12,383 vs. 5477/571,389; OR 1.75, [95% CI 0.56–5.48]; I2 = 95%; P = 0.34) (Fig. 8). And five studies [7, 21, 26, 31, 38] reported length of stay between RA patients and OA patients. The pooled results showed that patients with RA had long length of stay compared with OA patients (WMD 0.07, [95% CI 0.01–0.14]; I2 = 65%; P = 0.03) (Fig. 9).
Discussion
In this review of 24 studies, we found strong evidence for increased risk of overall infection, deep infection, VTE, PE and periprosthetic fractures, and reasonable evidence for increased risk of DVT and length of stay after TKA in patients with RA versus OA. Meanwhile, the results demonstrated that no evidence to support any differences in superficial site infection (SSI), revision rate, mortality and prosthetic loosening following TKA in patients with RA versus OA. However, it surprised that in patients with RA achieve higher satisfaction compared to patients with OA, which was conversed as we hypothesized. According to a previous meta-analysis by Ravi et al [19], patients with RA have a higher risk of infection after TKA than those with OA, but they observed no differences regarding revision, mortality, or VTE. Our meta-analysis included a larger simple size, more recent data, and more outcome measures, The current study reported that studies published from January 1, 2000 to October 15, 2022 (to more closely reflect current clinical practice) of primary TKA and contained information on outcomes in RA and OA patients. We systematically collected relevant clinical trials of patients with RA and OA undergoing TKA and performed a meta-analysis and systematic review in this study.
Our present study revealed that patients with RA with the similar rate in superficial site infection, but much higher rate in deep and overall infection rate compared to OA patients. TKA is used to alleviate pain and improve mobility extensively in patients who develop severe destructive changes of their knee joints due to inflammatory or degenerative musculoskeletal diseases [16]. Of all the complications, prosthetic joint infection (PJI) is the most devastating in elective orthopaedic surgery, the incidence of PJI after TKA has been reported to be approximately 1–2% [40, 41]. The patient occurred PJI will usually be removal or exchange of the prosthesis associated with poor functional, long hospital stay, prolonged use of antibiotics and higher resource consumption burdens [40]. Previous studies have reported conflicting results concerning the risk of PJI after TKA for RA and OA [7, 11, 23, 31]. Two previous meta-analysis have revealed that compared with OA, patients with RA are at higher risk of infections after TKA [19, 42], in line with those studies, we also found an increased risk of postoperative infection among patients with RA, but similar rate in superficial site infection. The Guidelines revealed that the risk of postoperative infection complications after total joint arthroplasty (TJA) was increased in patients with RA nearly 2-fold, and deep infection complications increased by 1.5-fold [43]. Yeganeh et al. demonstrated that PJI among patients with RA following TKA is 1.6-fold greater than in patients for OA [44]. A cohort study with 71,793 patients reported that RA are at higher risk of infection after TKA relative to those with OA (1.26%, compared with 0.84% for recipients with OA [34]. Furthermore, a retrospective study with large samples also proved that revision for infection was significantly higher in the RA (HR = 1.37 (1.11–1.69), P = 0.003) compared to OA [9]. On the contrary, Chung et al. found no significant difference in acute TKA surgical site infection risk between RA and OA patients when controlling for potential confounders [7], in line with our study, similar rate surgical site infection may due to standard antirheumatic therapy and welled perioperative management. While da Cunha et al. also considered RA was not identified as a risk factor for perioperative infections in TKA [25]. This higher risk may be due to the immunosuppressive therapies for RA patients including disease-modifying antirheumatic drugs (DMARDs), corticosteroids [45,46,47], 46% of RA patients were receiving biologic DMARDs, 67% were receiving nonbiologic DMARDs, and 25% were receiving glucocorticoids [43]. On the other hand, RA patients are more susceptible to postoperative anemia and are more likely to require a blood transfusion because of bone marrow suppression with chronic disease or medication use, while blood transfusions may increase the risk of infection [7]. In addition, vulnerable soft tissue envelope around the knee joint could make the TKA in RA patients more susceptible to infection [37]. Therefore, preoperative management of those patients is well prepared and perioperative adverse events may decrease and during the perioperative period, anti-rheumatic therapy should be more standard to avoid infection in RA patients [10, 43].
Our meta-analysis demonstrated that there were no significant differences in the revision rate between RA patients and OA patients. It is reported that lots of revision TKA procedures continue increasing at a high rate with the number of TKA rising, while infection and aseptic loosening were the two most common reasons for revision in both OA and RA following TKA [48, 49]. Theoretically, RA patients have a higher infection rate, so the revision rate should be higher. The McMaster Arthroplasty Collaborative (MAC) found that 1.41% of individuals experienced revision TKA for PJI in 2022 [50]. Because less number of TKA for RA compared to OA, so a small part of these revision procedures are performed in RA patients. Revision TKA in patients with RA will be very challenging due to medical comorbidities, poor bone stock and soft tissue, substantially increases the risk of postoperative complications. Several previous studies have compared revision rate after TKA in patients with RA and OA patients. A Prospective, Population-Based Study with 24,293 patients (2,462 knees in the RA 21,832 knees in OA) proved that RA patients had a higher risk of revision(RR 5.4, 95% CI 1.9–16; P = 0.002), while had a 1.6 times higher risk of revision for infection after TKA(RR 4.1, 95%CI 1.6–11; P = 0.004) versus OA patients [37]. Another retrospective study revealed that patients with RA had a significantly increased risk of overall revision TKA(RR 1.6, 95% CI 1.5–1.6; P<0.0001) compared with patients with OA. On the contrary, Burn et al. stated that there was no significant in the incidence of revision over the 10 years following TKA among individuals with RA compared to those with OA [23]. Meanwhile, by using Kaplan–Meier survivorship analysis, Abram et al. estimated the revision rate without statistical difference in RA patients compared with OA patients during long-term follow-up [51]. Interestingly, a large national database proved that the rate of revision after TKA in RA patients is lower than those with OA [9]. Consequently, the higher revision rate in RA patients may be related to their younger age at the time of surgery or high risk of infection, while similar or lower revision rate due to comorbidities and weak bone stocks of RA that surgeons may preferentially conservative treatment, such as conducted knee infections with antibiotics or debridement instead of revision, and decreased wear of the prosthesis due to lower physical activity in patients with RA.
VTE including DVT and PE are the most dreadful and potentially life threatening complications after TKA and other orthopedic surgical procedures, because of its closely linked with mortality and health care costs [32]. Previous studies have demonstrated that patients who have significantly higher risk of VTE after lower extremity surgery, especially RA patients following TKA [34, 36]. However, although the implementation of greatly enhanced antithrombotic prevention, the incidence of VTE after TKA remains high [8]. But whether RA is a potential candidate predisposing patients to postoperative VTE have demonstrated highly variable outcomes. Several studies have reported on differences in perioperative outcomes between RA and OA patients performing TKA. A retrospective study using National Surgical Quality Improvement Program (NSQIP) database by Jauregui et al. revealed that no significant difference was found in the incidence of PE (P = 0.99), and DVT (P = 0.72) [52]. Another study contains 355 patients (238 knees in the RA, 169 knees in OA) demonstrated that the incidence of DVT after TKA was significantly higher in OA patients than in those with RA, interestingly, when the patients were adjusted for age and anti-inflammatory drugs (NSAIDs) use, the incidence of DVT was similar in the two groups [32]. Therefore, RA may not represent a predictor for VTE according to their research. But some studies have demonstrated a higher rate of VTE in patients with RA undergoing TKA compared to OA patients [8, 31], and hypercoagulability with reduced fibrinolysis owing to raised levels of autoantibodies and vascular endothelium is easily damaged might illustrate these findings. Furthermore, frequent use of NSAIDs with the resulting antiplatelet activity and RA patients along with younger age distribution, and lower body mass index (BMI), which advocating a lower thrombotic risk [32]. We also speculated that the lower hemoglobin and blood dilution may also be the reason for the similar or less incidence of thrombosis in RA patients. In our present study, RA patients was associated with significant differences in the risk for VTE following TKA, so surgeons should pay more close attention to the thromboprophylaxis use of anticoagulant therapies and perioperative drug management after TKA in patients with RA.
Our meta-analysis, however, found no significant difference in rate of mortality between RA and OA patients. It is generally considered that patients suffering from RA were shown to have higher rates of mortality after TKA due to higher rates of infection, cardiac morbidity, VTE and pulmonary disease [48]. Most surgeons emphasize 30-day and 90-day mortality for RA patients following TKA, because the increased mortality rates in years 1–10 suggest disease-specific rather than surgery-induced mortality [9, 38]. Surprisingly, most of the literatures available on mortality of patients undergoing TKA for RA patients support that there is no significant difference in mortality compared to OA patients. For example, Domsic et al. revealed that RA patients had an OR of 0.771 (95%CI 0.570–1.041, P = 0.09) for mortality with TKA versus OA patients at the final follow-up(10years) by using data from the Nationwide Inpatient Sample (1993–2006) [53]. Similarly, a present study including more than 7 million patients undergoing different types of surgery including TKA found no differences in the incidence of mortality among patients with a diagnosis of RA compared to those without [54]. But a study by Baek et al. demonstrated that the overall mortality rates in the RA and OA groups were 15.8% (9/57) and 4.4% (5/114) at the final follow-up(10years), respectively (P < 0.05) [11], and Michaud et al. also revealed that RA was associated with a significantly higher long-term mortality [55]. Mortality rates following TKA are reported to be high in patients with RA, which may be due to surgical techniques, prostheses, non-standard anti-rheumatoid therapy, and improper perioperative management. So, orthopedic surgeons take greater perioperative management and long-term postoperative follow-up when operating on RA patients will improve the survival of RA patients.
The interesting finding of this study was that TKA in patients with RA showed higher rates of post-operative periprosthetic fractures when compared to OA. To our knowledge, there is no relevant meta-analysis to analyze and summarize periprosthetic fractures. However, with increasing percentages of TKA worldwide, it is likely that the absolute burden of periprosthetic fractures will continue to grow, which along with worse functional outcomes and higher medical costs [56]. Schnaser et al. reported that patients with RA had significantly more inpatient post-operative periprosthetic fractures when compared to OA (P<0.01) [36], while Abram et al. also reported that there were more periprosthetic fractures in the RA patients than OA patients and all occurred more than 5 years after TKA [51]. RA patients usually are more prone to osteoporosis due to chronic steroid treatment and physical impairment, which all can result in osteopenia and related fractures. Therefore, surgeons should be aware that RA patients are possibly to be at risk of periprosthetic fracture. Another finding of our meta-analysis was that RA patients had similar incidence of loosening after TKA compared with OA patients. Loosening is the major cause of revision among various complications, and it tends to occur many years after the initial surgery, survival of primary TKA is substantially diminished with each consecutive revision [57, 58]. Therefore, the early detection of loosening in patients with TKA has become a dominating importance and interest in the orthopedic field. Most of previous literatures showed that patients with RA had no significant difference in loosening rate than patients with OA probably because decreased wear of the prosthesis due to lower physical activity in patients with RA [9, 11, 30]. In conclusion, rigorous control of RA activity by biological treatment not only controls local joint inflammation, which would improve bone quality and reduced rate of premature loosening of the implant.
In our present study RA patients had longer mean lengths of hospitalization with an OR of 0.07 (95%CI 0.01–0.14, P = 0.03) compared to OA patients, while several studies have also shown that RA patients would prolong hospitalization [21, 26, 38]. The hospitalization after TKA depends on a variety of factors, including the patient’s health status, ability to walk safely, overall pain control, and the physical activity in patients with RA. Longer hospitalization increased infection rates and health care costs, therefore, surgeons must pay more attention to perioperative management of patients with RA and shorten the length of stay. Currently, patient satisfaction gains attention as an important outcome measure, because there is a well-documented discrepancy between clinician and patient ratings of health status. Furthermore, better postoperative analgesia, ambulation, patient symptom, expectation, minimal drug consumption and complications all correlated with patient satisfaction. But satisfaction is evaluated in different ways in previous studies that cannot be pooled analysis, which RA patients have similar or more excellent outcomes after TKA compared with OA patients [18, 26, 30, 59]. This may be related to the fact that the preoperative symptoms of RA are more severe, and that patients with RA recovery faster with younger age and have lower expectations.
Some limitations must be taken into consideration when interpreting the results of this study. Firstly, there have no elaborate information on other potential confounders, such as length of surgery time, blood loss, medication use, disease activity or other non-measurable factors (e.g., the types of implants, surgical technique, surgical approach, etc.). Further research is necessary to elucidate for these findings, including anti-rheumatic medication use, implant choice, perioperative antibiotic prophylaxis, and method of rehabilitation following TKA. Secondly, the diagnostic criteria for RA are different, the timing of the diagnosis of PJI is different, deep or superficial infection is not clear in some studies, such a high rate of misclassification may threaten our study and we cannot analyze these risk factors or outcomes in this study. Thirdly, some of the studies more than 10 years ago, surgical techniques and medicine may be different from those currently used. As many outcomes were included in a small number of literatures, we did not perform subgroup analyses. More studies are needed to perform subgroup analyses of these outcomes. Finally, the studies included are mainly retrospective case-control studies and cohort studies, with no randomized controlled trials studies, so more prospective studies and confounders controlled are warranted to evaluate short-term and long-term clinical outcomes of TKA in patients with RA. These recognized limitations are inherent to all studies using this database design and could potentially be improved through prospective data collection.
To our knowledge, this is the first study that has compared outcomes after TKA comprehensively in patients with RA. Our study demonstrated that patients with RA are at higher risk of postoperative infection, VTE, periprosthetic fracture, and lengths of hospitalization compared to patients with OA following TKA, but there are no significant differences in revision rate, mortality and loosening. In addition, we also should be aware of the possibility that RA patients may have an increased risk for perioperative transfusions, an increased incidence of pneumonia and cardiac complications, surgeons must pay more attention to perioperative management of patients with RA and dramatically reduce the risk of complications. In conclusion, despite RA increased incidence of postoperative infection, VTE, periprosthetic fracture, and lengths of hospitalization, TKA should continue to be presented as an effective surgical procedure for patients whose conditions are intractable to conservative and medical management of RA.
Data Availability
All data generated or analyzed during this study are included in the Additional file.
Abbreviations
- TKA:
-
Total knee arthroplasty
- RA:
-
Rheumatoid arthritis
- OA:
-
Osteoarthritis
- OR:
-
Odds ratio
- M-H:
-
Mantel-Haenszel
- CI:
-
Confidence interval
- VTE:
-
Venous thromboembolism
- DVT:
-
Deep venous thrombosis
- PE:
-
Pulmonary embolism
- PPF:
-
Periprosthetic fractures
- PL:
-
Prosthetic loosening
- LOS:
-
Length of stay
- MO:
-
Mortality
- WMD:
-
Weighted mean difference
- IV:
-
Inverse variance
- NOS:
-
Newcastle-Ottawa Scale
- CCS:
-
Case-control studies
- RCS:
-
Retrospective cohort studies
- PCS:
-
Prospective cohort studies
References
Flury A, Weigelt L, Camenzind RS, Fritz B, Hasler J, Baumgaertner B, et al. Total and unicondylar knee arthroplasty are equivalent treatment options in end-stage spontaneous osteonecrosis of the knee, and the size of the lesion has no influence on the results. Knee Surg Sports Traumatol Arthrosc. 2021;29:3254–61.
Takeda R, Matsumoto T, Maenohara Y, Omata Y, Inui H, Nagase Y, et al. Increasing trend of radiographic features of knee osteoarthritis in rheumatoid arthritis patients before total knee arthroplasty. Sci Rep. 2022;12:10452.
Alrawashdeh W, Eschweiler J, Migliorini F, El Mansy Y, Tingart M, Rath B. Effectiveness of total knee arthroplasty rehabilitation programmes: a systematic review and meta-analysis. J Rehabil Med. 2021;53:jrm00200.
Fujimura K, Haraguchi A, Sakurai R, Kamura S, Sakuraba K, Miyahara H, et al. Have the radiographic characteristics of total knee arthroplasty recipients in rheumatoid arthritis changed after the induction of biologic disease modifying antirheumatic drugs? Mod Rheumatol. 2022;32:1047–53.
Zhang Y, Chu SS, Liu K, Huang Q, Wang Y. Outcomes in patients with rheumatoid versus osteoarthritis for total hip arthroplasty: a meta-analysis and systematic review. Semin Arthritis Rheum. 2022;56:152061.
Pan X, Wang J, Shi Z, Cheng H, Lin Z, Wu X, et al. Rheumatoid arthritis Versus Osteoarthritis in Patients receiving revision total knee arthroplasty in the United States: increased perioperative risks? A National Database-Based propensity score-matching study. J Am Acad Orthop Surg. 2021;29:e1176–83.
Chung HK, Wen SH, Chang WC, Liu KL. Acute surgical site infection after total knee arthroplasty in patients with rheumatoid arthritis versus osteoarthritis. Sci Rep. 2021;11:22704.
Li Z, Feng B, Du Y, Wang Y, Bian Y, Weng X. Complications of total knee arthroplasty in patients with haemophilia compared with osteoarthritis and rheumatoid arthritis: a 20-year single-surgeon cohort. Haemophilia. 2020;26:861–6.
Mooney L, Lewis PL, Campbell DG, Peng Y, Hatton A. Rates and outcomes of total knee replacement for rheumatoid arthritis compared to osteoarthritis. ANZ J Surg. 2019;89:184–90.
Goodman SM, Springer BD, Chen AF, Davis M, Fernandez DR, Figgie M, et al. 2022 American College of Rheumatology/American Association of hip and knee Surgeons Guideline for the Perioperative Management of Antirheumatic Medication in patients with rheumatic Diseases undergoing Elective Total hip or total knee arthroplasty. J Arthroplasty. 2022;37:1676–83.
Baek JH, Lee SC, Kim JW, Ahn HS, Nam CH. Inferior outcomes of primary total knee arthroplasty in patients with rheumatoid arthritis compared to patients with osteoarthritis. Knee Surg Sports Traumatol Arthrosc. 2022;30:2786–92.
Rodriguez-Merchan EC, Delgado-Martinez AD. (2022) Risk factors for Periprosthetic Joint infection after primary total knee arthroplasty. J Clin Med 11.
Fang YF, Liu JR, Chang SH, Kuo CF, See LC. Comparative safety of Janus kinase inhibitors and tumor necrosis factor inhibitors in patients undergoing treatment for rheumatoid arthritis. Int J Rheum Dis. 2022;25:1254–62.
Weitz JI, Szekanecz Z, Charles-Schoeman C, Vranic I, Sahin B, Paciga SA et al. (2022) Biomarkers to predict risk of venous thromboembolism in patients with rheumatoid arthritis receiving tofacitinib or tumour necrosis factor inhibitors. RMD Open 8.
Fujimaki H, Nakazawa A, Hirano M, Takeuchi T, Kadowaki A, Kusayama Y, et al. Status of fracture risk assessment and osteoporosis treatment in japanese patients with rheumatoid arthritis. Mod Rheumatol. 2021;31:987–91.
Meena A, Hoser C, Abermann E, Hepperger C, Raj A, Fink C. Total knee arthroplasty improves sports activity and the patient-reported functional outcome at mid-term follow-up. Knee Surg Sports Traumatol Arthrosc. 2022. https://doi.org/10.1007/s00167-022-07025-z.
Niemeläinen M, Moilanen T, Huhtala H, Eskelinen A. Outcome of knee arthroplasty in patients aged 65 years or less: a prospective study of 232 patients with 2-year follow-up. Scand J Surg. 2019;108:313–20.
Minator Sajjadi M, Keyhani S, Kazemi SM, Hanafizadeh B, Ebrahimpour A, Banasiri M. Patient satisfaction following total knee arthroplasty: comparison of short-term results in rheumatoid arthritis and osteoarthritis. Arch Bone Jt Surg. 2019;7:61–6.
Ravi B, Escott B, Shah PS, Jenkinson R, Chahal J, Bogoch E, et al. A systematic review and meta-analysis comparing complications following total joint arthroplasty for rheumatoid arthritis versus for osteoarthritis. Arthritis Rheum. 2012;64:3839–49.
Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25:603–5.
Blevins JL, Chiu YF, Lyman S, Goodman SM, Mandl LA, Sculco PK, et al. Comparison of Expectations and Outcomes in Rheumatoid Arthritis Versus Osteoarthritis Patients undergoing total knee arthroplasty. J Arthroplasty. 2019;34:1946–52. e1942.
Böhm P, Holy T, Pietsch-Breitfeld B, Meisner C. Mortality after total knee arthroplasty in patients with osteoarthrosis and rheumatoid arthritis. Arch Orthop Trauma Surg. 2000;120:75–8.
Burn E, Edwards CJ, Murray DW, Silman A, Cooper C, Arden NK, et al. The impact of rheumatoid arthritis on the risk of adverse events following joint replacement: a real-world cohort study. Clin Epidemiol. 2018;10:697–704.
Chesney D, Sales J, Elton R, Brenkel IJ. Infection after knee arthroplasty a prospective study of 1509 cases. J Arthroplasty. 2008;23:355–9.
da Cunha BM, de Oliveira SB, Santos-Neto L. Incidence of infectious complications in hip and knee arthroplasties in rheumatoid arthritis and osteoarthritis patients. Rev Bras Reumatol. 2011;51:609–15.
Goodman SM, Johnson B, Zhang M, Huang WT, Zhu R, Figgie M, et al. Patients with rheumatoid arthritis have similar excellent outcomes after total knee replacement compared with patients with Osteoarthritis. J Rheumatol. 2016;43:46–53.
Himanen AK, Belt E, Nevalainen J, Hämäläinen M, Lehto MU. Survival of the AGC total knee arthroplasty is similar for arthrosis and rheumatoid arthritis. Finnish arthroplasty Register report on 8,467 operations carried out between 1985 and 1999. Acta Orthop. 2005;76:85–8.
Izumi M, Migita K, Nakamura M, Jiuchi Y, Sakai T, Yamaguchi T, et al. Risk of venous thromboembolism after total knee arthroplasty in patients with rheumatoid arthritis. J Rheumatol. 2015;42:928–34.
Jämsen E, Huhtala H, Puolakka T, Moilanen T. Risk factors for infection after knee arthroplasty. A register-based analysis of 43,149 cases. J Bone Joint Surg Am. 2009;91:38–47.
Kobayashi S, Niki Y, Harato K, Nagura T, Nakamura M, Matsumoto M. Rheumatoid arthritis patients achieve better satisfaction but lower functional activities as compared to Osteoarthritis Patients after total knee arthroplasty. J Arthroplasty. 2019;34:478–482e471.
LoVerde ZJ, Mandl LA, Johnson BK, Figgie MP, Boettner F, Lee YY, et al. Rheumatoid arthritis does not increase risk of short-term adverse events after total knee arthroplasty: a retrospective case-control study. J Rheumatol. 2015;42:1123–30.
Niki Y, Matsumoto H, Hakozaki A, Mochizuki T, Momohara S. Rheumatoid arthritis: a risk factor for deep venous thrombosis after total knee arthroplasty? Comparative study with osteoarthritis. J Orthop Sci. 2010;15:57–63.
Ohzawa S, Takahara Y, Furumatsu T, Inoue H. Patient survival after total knee arthroplasty. Acta Med Okayama. 2001;55:295–9.
Ravi B, Croxford R, Hollands S, Paterson JM, Bogoch E, Kreder H, et al. Increased risk of complications following total joint arthroplasty in patients with rheumatoid arthritis. Arthritis Rheumatol. 2014;66:254–63.
Robertsson O, Knutson K, Lewold S, Lidgren L. The swedish knee Arthroplasty Register 1975–1997: an update with special emphasis on 41,223 knees operated on in 1988–1997. Acta Orthop Scand. 2001;72:503–13.
Schnaser EA, Browne JA, Padgett DE, Figgie MP, D’Apuzzo MR. Perioperative Complications in patients with inflammatory arthropathy undergoing total knee arthroplasty. J Arthroplasty. 2015;30:76–80.
Schrama JC, Espehaug B, Hallan G, Engesaeter LB, Furnes O, Havelin LI, et al. Risk of revision for infection in primary total hip and knee arthroplasty in patients with rheumatoid arthritis compared with osteoarthritis: a prospective, population-based study on 108,786 hip and knee joint arthroplasties from the norwegian Arthroplasty Register. Arthritis Care Res (Hoboken). 2010;62:473–9.
Stundner O, Danninger T, Chiu YL, Sun X, Goodman SM, Russell LA, et al. Rheumatoid arthritis vs osteoarthritis in patients receiving total knee arthroplasty: perioperative outcomes. J Arthroplasty. 2014;29:308–13.
Tayton ER, Frampton C, Hooper GJ, Young SW. The impact of patient and surgical factors on the rate of infection after primary total knee arthroplasty: an analysis of 64,566 joints from the New Zealand Joint Registry. Bone Joint J. 2016;98–b:334–40.
Mur I, Jordán M, Rivera A, Pomar V, González JC, López-Contreras J et al. (2020) Do prosthetic joint infections worsen the functional ambulatory outcome of patients with joint replacements? A Retrospective Matched Cohort Study. Antibiot (Basel) 9.
Nabet A, Sax OC, Shanoada R, Conway JD, Mont MA, Delanois RE, et al. Survival and outcomes of 1.5-Stage vs 2-Stage Exchange total knee arthroplasty following prosthetic joint infection. J Arthroplasty. 2022;37:936–41.
Lee DK, Kim HJ, Cho IY, Lee DH. Infection and revision rates following primary total knee arthroplasty in patients with rheumatoid arthritis versus osteoarthritis: a meta-analysis. Knee Surg Sports Traumatol Arthrosc. 2017;25:3800–7.
Goodman SM, Springer B, Guyatt G, Abdel MP, Dasa V, George M, et al. 2017 American College of Rheumatology/American Association of hip and knee Surgeons Guideline for the Perioperative Management of Antirheumatic Medication in patients with rheumatic Diseases undergoing Elective Total hip or total knee arthroplasty. Arthritis Care Res (Hoboken). 2017;69:1111–24.
Yeganeh MH, Kheir MM, Shahi A, Parvizi J. Rheumatoid arthritis, Disease Modifying Agents, and Periprosthetic Joint infection: what does a joint surgeon need to know? J Arthroplasty. 2018;33:1258–64.
Cordtz R, Odgaard A, Kristensen LE, Overgaard S, Dreyer L. Risk of medical complications following total hip or knee arthroplasty in patients with rheumatoid arthritis: a register-based cohort study from Denmark. Semin Arthritis Rheum. 2020;50:30–5.
Kittle H, Ormseth A, Patetta MJ, Sood A, Gonzalez MH. Chronic corticosteroid use as a risk factor for Perioperative Complications in patients undergoing total joint arthroplasty. J Am Acad Orthop Surg Glob Res Rev. 2020;4:e2000001.
Salt E, Wiggins AT, Rayens MK, Morris BJ, Mannino D, Hoellein A, et al. Moderating effects of immunosuppressive medications and risk factors for post-operative joint infection following total joint arthroplasty in patients with rheumatoid arthritis or osteoarthritis. Semin Arthritis Rheum. 2017;46:423–9.
Baek JH, Lee SC, Jin H, Kim JW, Ahn HS, Nam CH. Poor outcomes of revision total knee arthroplasty in patients with septic loosening compared to patients with aseptic loosening. J Orthop Surg Res. 2021;16:624.
Kirschbaum S, Erhart S, Perka C, Hube R, Thiele K. (2022) Failure analysis in multiple TKA revisions-periprosthetic infections remain surgeons’ Nemesis. J Clin Med 11.
(2022) Incidence and predictors of prosthetic joint infection following primary total knee arthroplasty: a 15-Year Population-Based Cohort Study. J Arthroplasty 37:367–372e361.
Abram SG, Nicol F, Hullin MG, Spencer SJ. The long-term outcome of uncemented low contact stress total knee replacement in patients with rheumatoid arthritis: results at a mean of 22 years. Bone Joint J. 2013;95–b:1497–9.
Jauregui JJ, Kapadia BH, Dixit A, Naziri Q, Hip-Flores DJ, Harwin SF, et al. Thirty-day complications in rheumatoid patients following total knee arthroplasty. Clin Rheumatol. 2016;35:595–600.
Domsic RT, Lingala B, Krishnan E. Systemic lupus erythematosus, rheumatoid arthritis, and postarthroplasty mortality: a cross-sectional analysis from the nationwide inpatient sample. J Rheumatol. 2010;37:1467–72.
Yazdanyar A, Wasko MC, Kraemer KL, Ward MM. Perioperative all-cause mortality and cardiovascular events in patients with rheumatoid arthritis: comparison with unaffected controls and persons with diabetes mellitus. Arthritis Rheum. 2012;64:2429–37.
Michaud K, Fehringer EV, Garvin K, O’Dell JR, Mikuls TR. Rheumatoid arthritis patients are not at increased risk for 30-day cardiovascular events, infections, or mortality after total joint arthroplasty. Arthritis Res Ther. 2013;15:R195.
Grzelecki D, Marczak D, Kwolek K, Dudek P, Tyrakowski M, Olewnik Ł et al. (2021) Shaft fractures in patients requiring primary or revision total knee arthroplasty can be successfully treated with long-stemmed implants without additional fixation. J Clin Med 10.
Klasan A, Magill P, Frampton C, Zhu M, Young SW. Factors predicting repeat revision and outcome after aseptic revision total knee arthroplasty: results from the New Zealand Joint Registry. Knee Surg Sports Traumatol Arthrosc. 2021;29:579–85.
Lau LCM, Chui ECS, Man GCW, Xin Y, Ho KKW, Mak KKK, et al. A novel image-based machine learning model with superior accuracy and predictability for knee arthroplasty loosening detection and clinical decision making. J Orthop Translat. 2022;36:177–83.
Plantz MA, Sherman AE, Miller CH, Hardt KD, Lee YC. Outcomes of total knee arthroplasty in patients with rheumatoid arthritis. Orthopedics. 2021;44:e626–32.
Acknowledgements
The authors are grateful to all authors who provided information of their studies.
Funding
This study was funded by Youth Science and Technology Foundation of Gansu Province (No.: 20JR5RA588; 21JR7RA014), Key Research and Development Program of Gansu Province (No.:21YF5FA154).
Author information
Authors and Affiliations
Contributions
Yongjie Qiao, Feng Li, Lvdan Zhang and Shenghu Zhou conceived the study, participated in the study design, performed the statistical analysis, and drafted the manuscript. Yongjie Qiao, Feng Li and Haoqiang Zhang contributed to data collection and the statistical interpretation. Xiaoyang Song, Xinyuan Yu, Peng Liu and Shenghu Zhou participated in the study design, and oversaw the manuscript drafting process. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Ethical approval
Not applicable.
Informed consent
Not applicable.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Qiao, Y., Li, F., Zhang, L. et al. A systematic review and meta-analysis comparing outcomes following total knee arthroplasty for rheumatoid arthritis versus for osteoarthritis. BMC Musculoskelet Disord 24, 484 (2023). https://doi.org/10.1186/s12891-023-06601-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12891-023-06601-9