Abstract
Background
Chronic low back pain (cLBP) is widespread, costly, and burdensome to patients and health systems. Little is known about non-pharmacological treatments for the secondary prevention of cLBP. There is some evidence that treatments addressing psychosocial factors in higher risk patients are more effective than usual care. However, most clinical trials on acute and subacute LBP have evaluated interventions irrespective of prognosis.
Methods
We have designed a phase 3 randomized trial with a 2 × 2 factorial design. The study is also a Hybrid type 1 trial with focus on intervention effectiveness while simultaneously considering plausible implementation strategies. Adults (n = 1000) with acute/subacute LBP at moderate to high risk of chronicity based on the STarT Back screening tool will be randomized in to 1 of 4 interventions lasting up to 8 weeks: supported self-management (SSM), spinal manipulation therapy (SMT), both SSM and SMT, or medical care. The primary objective is to assess intervention effectiveness; the secondary objective is to assess barriers and facilitators impacting future implementation. Primary effectiveness outcome measures are: (1) average pain intensity over 12 months post-randomization (pain, numerical rating scale); (2) average low back disability over 12 months post-randomization (Roland-Morris Disability Questionnaire); (3) prevention of cLBP that is impactful at 10–12 months follow-up (LBP impact from the PROMIS-29 Profile v2.0). Secondary outcomes include: recovery, PROMIS-29 Profile v2.0 measures to assess pain interference, physical function, anxiety, depression, fatigue, sleep disturbance, and ability to participate in social roles and activities. Other patient-reported measures include LBP frequency, medication use, healthcare utilization, productivity loss, STarT Back screening tool status, patient satisfaction, prevention of chronicity, adverse events, and dissemination measures. Objective measures include the Quebec Task Force Classification, Timed Up & Go Test, the Sit to Stand Test, and the Sock Test assessed by clinicians blinded to the patients’ intervention assignment.
Discussion
By targeting those subjects at higher risk this trial aims to fill an important gap in the scientific literature regarding the effectiveness of promising non-pharmacological treatments compared to medical care for the management of patients with an acute episode of LBP and the prevention of progression to a severe chronic back problem.
Trial registration
ClinicalTrials.gov Identifier: NCT03581123.
Similar content being viewed by others
Background
The United States is in the midst of an unprecedented pain management crisis [1] with annual costs estimated at $560 to $635 billion per year [2]. Low back pain (LBP) is the most common chronic pain condition in adults and one of the leading causes of disability worldwide [3, 4]. Approximately 20% of acute cases will become chronic, [5] with roughly 40% of those with chronic LBP (cLBP) experiencing high-impact pain that significantly interferes with work, social activities, and daily life [6,7,8]. Given the socio-economic consequences of high-impact cLBP, research focusing on its prevention has become a national imperative [7,8,9,10,11].
It is now widely recognized that LBP is a complex condition influenced by several interrelated physical, psychological, and social factors [12]. However, most treatments still focus entirely on symptom management using a ‘one size fits all’ approach that fails to address the biopsychosocial (BPS) needs of those affected [13,14,15,16,17]. Treatment is frequently characterized by the persistent use of marginally effective and potentially harmful therapies that largely ignore the psychosocial aspects of LBP. For example, the use of epidural injections, opioid prescriptions, and spinal surgeries for LBP has increased at accelerating rates over the past few decades with little positive impact on patient outcomes [18, 19]. Of particular concern is the overreliance on opioids, which are used by an estimated 30% of chronic LBP patients [20] despite LBP clinical guidelines suggesting other pharmacologic and nonpharmacologic treatment options [21] and mounting recognition of opioid misuse, addiction, and fatal overdose [1].
There is growing evidence that physical and psychosocial risk factors can predict whether or not acute LBP progresses to become chronic [5, 22]. Such evidence has led to recommendations for clinical trials to focus on participants at higher risk of chronicity, limiting the testing of interventions to those most in need [23]. There is also evidence that treatments addressing psychosocial risk factors in patients at risk for chronicity are more effective than treatment with usual care [24]. However, most clinical trials to date on acute and subacute LBP populations have tested interventions irrespective of prognosis, limiting the ability to make confident conclusions about their effectiveness in terms of secondary prevention among high-risk subjects [23]. Consequently, there is a need for research that can more rigorously assess the potential of promising interventions to prevent acute and sub-acute LBP from progressing to more persistent severe cLBP by appropriately targeting those at higher risk.
To reduce cLBP burden, patients should have greater access to front-line care addressing both their physical and psychosocial needs. To accomplish this, there has been increased interest in studies of multi-modal interventions that are better suited to meet patients’ whole person needs [25]. Such approaches are designed to integrate psychosocial interventions with traditional biologically based pain management approaches [26]. Physical therapists (PTs) and chiropractors (DCs) are the most common providers of non-pharmacologic treatment for LBP in the United States, with approximately 39% of LBP patients seeking treatment from DCs and 34% from PTs [27]. Both PTs and DCs help patients manage symptoms and aid in the restoration of movement and functional ability. Therefore, they are well suited to integrate psychosocial and biophysical strategies, [26, 28] and play an essential role in the frontline management of patients with LBP [29, 30].
Our long-term objective is to reduce overall LBP burden and prevent acute and sub-acute LBP from progressing to a severe chronic back problem, by targeting those at higher risk. We will assess the effectiveness of first-line non-pharmacologic strategies that address patients’ biopsychosocial needs compared to front-line medical care that consists of primarily pharmacological management.
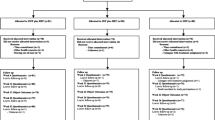
Methods/design
The purpose of this manuscript is to describe the design and methods of the PACBACK (Preventing Acute to Chronic Back Pain) trial (GRANT # UG3AT008769 and UH3AT008769) in accordance with the SPIRIT and CONSERVE guidance [31, 32]. The trial is a two-site, prospective, parallel group, phase 3 randomized type I hybrid effectiveness-implementation trial with a 2 × 2 factorial design. Adults with acute or subacute LBP and who are at moderate to high risk of chronicity, will be randomized to one of 4 interventions: supported self-management (SSM), spinal manipulation therapy (SMT), both SSM and SMT (SSM + SMT), or medical care (MC). Treatment duration will be up to 8 weeks. The trial is being conducted at University of Minnesota (UM) and University of Pittsburgh (UP) affiliated research clinics, with the UP serving as the central IRB (Institutional Review Board).
The first phase of PACBACK took place from September 2017 to November 2019 and included activities such as securing regulatory approvals, performing cross-site training of study staff and providers, developing study protocols and manuals of operations, and establishing data safety and monitoring and study accrual and retention plans. Additionally, the initial phase included an enrollment and randomization of 92 participants to assess performance milestones related to recruitment strategies, enrollment rates, intervention adherence and fidelity, and data collection. Upon satisfactory attainment of the performance milestones, a transition was made to the second phase, which started in November 2019 and is currently in active enrollment. The total sample size goal is 1,000 participants including the 92 participants from the initial phase.
Trial objectives
The primary objective of the trial is to determine intervention effectiveness by assessing average low back pain and disability over 12 months post-randomization, and prevention of progression to severe cLBP at 10–12 months follow-up. Our hypotheses are informed by our prior research [33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50]. We hypothesize that SMT and SSM will both be effective relative to MC, and that SSM + SMT will have a partially additive effect and therefore be more effective than either SMT or SSM alone.
Secondary objectives are to explore implementation related factors by gathering data from participants, clinicians and other stakeholders to inform future implementation, including the novel SSM intervention, if warranted by the trial results [51].
Roles and responsibilities
UM and UP serve as the Clinical Coordinating Centers (CCC) which oversee recruitment, screening and treatment in Minneapolis/St. Paul and Pittsburgh. The University of Washington (UW) serves as the Data Coordinating Center (DCC) and oversees auditing of trial conduct. Central IRB approval has been granted through UP (PRO18010414). An independent Data Safety and Monitoring Board (DSMB) and the funder, National Center for Complementary and Integrative Health (NCCIH), monitors project progress and reviews and approves all significant protocol amendments prior to implementation.
Tools and framework
The PRECIS-2 (Pragmatic Explanatory Continuum Indicator Summary) tool has been used to guide the study design and provide clarity regarding the pragmatic and explanatory features of the study (Fig. 1) [52]. The project is also informed by the RE-AIM framework which has provided guidance to addressing critical contextual factors related to Reach, Effectiveness, Adoption, Implementation and Maintenance, that can affect long-term implementation of the interventions [51].
Recruitment
We are using a multi-faceted recruitment campaign to reach potential participants from the general public. Strategies include: social media (e.g., Facebook, Instagram, Twitter) and direct mail distribution to zip codes with racial and ethnically diverse populations; mass transit billboard advertising; digital advertising (e.g., Google and local media focused on reaching Persons of Color); community engagement activities (e.g., volunteering, providing health related presentations, tabling at local events, participating in local radio shows); distribution of study flyers; electronic advertisements in University affiliated health clinics; ResearchMatch and University research registries; and other routine university communication platforms (e.g., podcasts and newsletters).
Screening and eligibility
Participants are initially screened by internet-based survey or phone, followed by a more in-depth phone screen with a clinician (PT, DC, nurse practitioner or physician assistant) trained in the study protocols. Willing and eligible individuals are then scheduled for a baseline screening appointment that includes informed consent and a health history and physical examination conducted by a clinician. Screening is followed immediately by a review of findings by the clinician with a study investigator (by phone) who together determine the eligibility of the participant based on the inclusion and exclusion criteria (see Table 1). The examining clinician follows a standardized algorithmic interview process in determining participant study eligibility. To qualify, participants must have experienced a new episode or aggravation of ongoing LBP in the past 12 weeks that lasted at least 2 weeks with a low back pain rating of three or more on average in the week before the baseline appointment. In addition, participants experiencing an aggravation of their LBP must rate their LBP in the month prior to the aggravation as mild or moderate, but not severe. Further, individuals with an aggravation of their LBP must agree that it is a worsening of their condition that is difficult to tolerate and generally impacts their usual activities and/or emotions. This operational definition is informed by an international consensus project that incorporated both patient and expert views for defining an aggravation or ‘flare’ of LBP [53].
Randomization
The DCC administers and maintains the centralized randomization system. Randomization is stratified by site and baseline STarT Back screening tool risk status (medium risk, or high risk of cLBP) [22, 54]. Within strata block randomization is applied using variable block sizes of 8 or 12 participants. Allocation is concealed from all CCC investigators and staff by centralized randomization, and the number of participants previously randomized to each group is concealed from the study personnel involved in eligibility determination.
Blinding
Blinding of clinicians and participants is not feasible due to the nature of the interventions. To minimize potential bias, all study personnel involved in screening and enrollment are masked to group assignments. Further, study personnel performing objective assessments are independent of intervention delivery and will remain blinded to study assignment until the end of data collection. One DCC staff member is unblinded and can access group assignment for closed DSMB reports. Additionally, all participants are queried in self-report questionnaires regarding any perceived attempts to influence their responses.
Interventions
Eligible participants are randomly assigned to one of four interventions: Spinal Manipulation Therapy (SMT), Supported Self-Management (SSM), a combination of SSM and SMT (SSM + SMT), and Medical Care (MC). The intervention design has been informed by previous research, [33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50] qualitative work of patients’ perspectives, [55, 56] and discussions with clinical providers and researchers. The interventions are provided face to face at outpatient research clinics affiliated with UP and UM or via video-conferencing technology using HIPAA-compliant Zoom. Descriptions of the interventions following the Template for Intervention Description and Replication (TIDieR) are provided in Table 2 and summarized below. The following are common across the intervention groups:
-
The intervention period can last up to 8 weeks.
-
Interventions are provided by licensed clinicians with a minimum of three years of clinical practice experience; all clinicians receive study specific training (see Table 2) and are provided a manual of operations.
-
Patients in all 4 groups receive standardized evidence-based information using a Back In Action booklet that describes the generally favorable prognosis of acute and sub-acute LBP, and simple strategies for remaining active and managing pain. All providers promote self-care practices consistent with the information in this booklet.
-
Patients can continue with their routine self-administrated over-the-counter pain management medications and self-care activities (which is measured monthly along with other healthcare use as described under Outcome Measures).
-
Only participants randomized to the medical care group received prescription medication. However, patients who experience a significant worsening of LBP symptoms during the 8-week intervention period in the SMT and SSM groups that requires additional management are referred to one of the trial medical providers for a short course of ‘rescue medications’, using a protocol from previous studies by the investigators [36, 37].
-
During the 12-month follow-up period participants who experience a recurrence of an acute LBP episode (if still meeting the study inclusion/exclusion criteria) are given the option to receive a short course of additional care in the group they were originally assigned.
Prohibited interventions
Participants are asked to limit treatment to their assigned intervention for the length of the initial 8-week intervention period; similarly, providers have been trained to refrain from delivering interventions that fall outside the scope of the study protocols. Participants retain the right to discontinue care at any time.
Supported self-management (SSM)
SSM is provided by licensed PTs (n = 4) and DCs (n = 4). The design of the SSM intervention was guided by the Behavior Change Wheel model (BCW), coupled with the dynamic biopsychosocial model of pain that acknowledges the complex and reciprocal interactions between the evolving biopsychological or “whole” person and their external, social environment [57,58,59]. SSM aims to provide patients the opportunities and resources to develop their capacity and motivation to self-manage their LBP [59]. It entails 4–8, 60-minute sessions. The main intervention elements include a biopsychosocial oriented needs assessment; individualized treatment plan; education and training in physical and mind-body exercises and strategies; empowerment and support; and persuasion. Additional details related to the SSM intervention are provided in Table 2; the design and development of SSM will also be described in a separate manuscript.
Spinal Manipulation Therapy (SMT). The SMT intervention is provided by licensed PTs and DCs. It is comprised of a minimum of two, 15–20 min visits and includes manipulative techniques with sufficient flexibility to be representative of the professions most commonly delivering SMT. The primary goal of the SMT intervention is to address the biophysical aspects of LBP with a focus on restoring spinal movement and functional ability. SMT includes a biophysical oriented needs assessment and development of an individualized treatment plan. The number of visits, spinal levels treated, and choice of SMT and supportive modalities are determined by provider according to patient needs and tolerance. SMT, includes grades 1–4, mobilization (low velocity, low-high amplitude passive movements) and/or manipulation (high velocity, low amplitude thrust) to the spine between the fifth thoracic and fifth lumbar vertebrae, and the sacroiliac joints. The chosen SMT techniques are based on those used in the UK BEAM Trial [60] and agreed upon by PT and DC professional groups. Supportive modalities including soft tissue techniques (cross-fiber stretch, longitudinal stretch, direct pressure, and deep friction massage), lumbar neural mobilization, and up to 10 min of heat.
Supported Self-Management (SSM) plus Spinal Manipulation Therapy (SMT). The SSM + SMT intervention is provided by licensed PTs and DCs. It involves a minimum of four, 75–80 min visits and includes the modalities and strategies described for SSM and SMT above.
Medical Care (MC). The MC intervention is provided by licensed physicians or advanced practice providers and entails a minimum of 2, 15–30 min visits during which patients receive guideline-based medication management, [21] which is a standard first-line approach for LBP in primary care. The primary goal of the MC intervention is to manage patient pain symptoms and restore daily function. MC includes review of symptoms, health history, and concomitant medications; medication choices are made based on clinician judgement and patient preferences. The first visit occurs in person or via videoconference; subsequent visits occur in person, via videoconference, or by phone as is standard in clinical practice. Decisions regarding visit frequency are made collaboratively by the provider and patient. Prescribed medications, include nonsteroidal anti-inflammatory drugs (NSAIDs, oral or topical) and skeletal muscle relaxants as a first-line approach. Patients who are unresponsive or unable to tolerate first-line medications may be prescribed acetaminophen, lidocaine patches, opioids, benzodiazepines, antiseizure medications, antidepressants, and selective serotonin reuptake Inhibitors and/or serotonin norepinephrine reuptake inhibitors. If deemed necessary providers have the option to recommend the use of heat, massage, or acupuncture; however, no formal referrals will be made.
Data management and data collection
The DCC supports an https-secured web page (https://pacback.org) that provides a centralized location for public information about the project for potential participants, investigators, and institutional agencies. The web page contains a link to the project portal. Study personnel log on to the private portal on the study web page with individual Shibboleth-based usernames and passwords to securely perform study data management activities. Data collection is conducted using web- and text-based delivery platforms for self-report questionnaires; these are administered free of provider and investigator influence and are overseen and managed by the DCC. Table 3 summarizes the data collection schedule.
To provide incentive for complying with follow-up questionnaires, participants receive a small monetary compensation for each of the monthly questionnaires completed. If participants choose to drop out of the trial or discontinue completing the required questionnaires, attempts are made to reach an agreement with participants to fill out at least monthly questionnaires at two-, six-, and 12-months post-randomization.
Baseline Measures include demographic, occupational, and clinical data, measured using the adapted acute/subacute version of the National Institutes of Health’s Research Task Force (NIH RTF) minimum dataset [9]. The Quebec Task Force’s classification system for spinal disorders is used for diagnostic classification [62]. Baseline duration of LBP (acute: <6 weeks vs. subacute: 6–12 weeks) and risk of cLBP (STarT Back screening tool status medium vs. high risk) are collected for pre-specified subgroup analyses (described below).
Adverse Events and Serious Adverse Events (AEs/SAEs) are identified during the intervention phase at intervention visits; during the study follow-up phase using monthly self-report questionnaires and by participants reporting to study staff. Events are followed until resolution or stabilization, whichever occurs first; resolution and stabilization are determined by the PI with input from a study clinician when appropriate. SAEs potentially related to treatment are brought to the attention of the IRB and the DSMB in writing. As part of the Data Safety and Monitoring Plan (DSMP) the DCC performs continuous and interim analysis of accruing safety data. Potentially treatment-related SAEs are monitored throughout the course of the study. The following guidelines are used when considering halting the trial for safety: [1] > 5% of participants experience an unexpected, related, moderate or greater adverse event; and [2] ≥ 2% SAE overall that are unexpected and related to the intervention. The DSMB considers this guidance when making recommendations regarding trial continuation.
Effectiveness measures
The trial has three primary outcome measures: 1) average pain intensity over 12 months post-randomization (0–10 numerical rating scale (NRS)); [63,64,65] [2] average disability over 12 months post-randomization (0–24 scale, Roland Morris Disability Questionnaire (RMD));[66, 67] and [3] prevention of cLBP that is impactful at 10–12 months follow-up (8–50, LBP Impact scale using mean from months 10–12). The LBP impact scale includes measures of pain intensity, pain interference, and physical function from the PROMIS-29 Profile v2.0) [9].
Secondary outcomes include recovery at 6 months (binary composite outcome defined as pain NRS = 0 and RMD < = 2.) and PROMIS-29 Profile v2.0 measures to assess pain interference, physical function, anxiety, depression, fatigue, sleep disturbance and the ability to participate in social roles and activities [68]. Other secondary outcomes include LBP frequency, over-the-counter and prescription medication use (including class and frequency); healthcare utilization (e.g., MRIs, injections, hospitalizations, surgery, integrative and complementary treatments); productivity loss (e.g., missed work, reduced productivity while at work); [69] STarT Back screening tool status; [54] patient satisfaction; [70] global improvement; [71] chronic LBP status; [9] frequency of LBP interference with daily activities; self-reported LBP trajectory; [72] adverse events and COVID-19 impact. Objective measures included as secondary outcomes are the Quebec Task Force Classification, Timed Up & Go Test, the Sit to Stand Test, and the Sock Test. All objective measures will be assessed by clinicians blinded to the patients’ intervention assignment [62, 73,74,75].
Psychosocial Mediator Measures that are likely to change as a result of treatment, and potentially affect the primary outcomes are also collected. These include self-efficacy (Chronic Pain Self-Efficacy Scale); [76] coping (Coping Strategies Questionnaire); [77] kinesiophobia (Tampa Scale for Kinesiophobia-11); [78] and catastrophizing (Pain Catastrophizing Scale) [79]. All of these measures have been used in clinical research with diverse population including LBP.
Implementation Measures are addressed to explore factors that could assess results interpretation and affect future implementation of the experimental interventions. as guided by the Reach, Effectiveness, Adoption, Implementation, and Maintenance framework (RE- AIM) [52, 80]. The RE-AIM framework was developed to help balance the focus of internal and external validity and is an ideal complement to hybrid trial designs. It advocates mixed-methods data collection to gather a diverse range of contextual data from multiple levels of stakeholders. In this trial, quantitative and open-ended survey questions, individual interviews, activity and process tracking are used to assess participants and non-participants (e.g. those not enrolled in the trial), providers and other stakeholders to gather information on demographics, processes, and views related to barriers and facilitators. These measures are summarized in Table 4 and will be addressed in further detail in a separate publication.
The timing and frequency of data collection for outcomes, mediating measures, and participant-level implementation measures are detailed in Table 3. Provider and other stakeholder implementation data is collected throughout the trial’s life-cycle (see Table 4).
Sample size and power
The trial focuses on two separate effectiveness research questions with different time frames. First, we consider the overall time-averaged patient status for both pain and disability over the full 12 months of follow-up. We recognize the importance of considering multiple comparisons when evaluating both average pain and average function over one year, and we therefore adopt a multiple comparison correction for these two outcomes. Second, we consider the long-term impact of LBP using an assessment of impact over months 10–12. However, given the different focus of the major questions and the timing of assessments further adjustment for multiple comparisons across the two questions is not indicated. Sample size and power for the trial is based on these two effectiveness research questions. To characterize the power of our primary analyses (an overall ANOVA F-test) a summary table is provided that considers potential standardized mean differences comparing the individual intervention arms to medical care (see Table 5). For a small effect size (Cohen’s d of 0.2) and an additive effect we have greater than 88% power to reject the null. However, additivity may not hold so we also consider sub-additive scenarios, in addition to scenarios where only one intervention is effective. For small to moderate effect size differences (0.25–0.35) we have > 80% power for all scenarios for LBP impact and most scenarios for pain and disability. We assume n = 1000 enrolled with 90% follow-up that yields 900 evaluated participants. Power is based on 5000 simulations per scenario with adjustment for period (4-arm, 2-arm) and site, and accounting for the group imbalance that results from our temporary restriction to 2-arm randomization (see trial modifications due to Covid page 11).
Statistical analysis plan
Effectiveness analyses
Primary outcomes analyses
Average pain over 12 months post-randomization, average low back disability over 12 months post-randomization and prevention of cLBP that is impactful (based on averaged LBP Impact scores from the PROMIS-29 Profile v2.0 over months 10–12), will be analyzed using a single ANOVA with an omnibus test for the equality of means across the four treatment groups. Linear regression with adjustment for site, baseline risk group, and study period (4-arm, 2-arm) will be computed separately for each outcome. Because pain and disability are measured within the same timeframe, we will apply multiple comparison correction for the two outcomes (using the Holm-Bonferroni method as detailed in FDA guidance); primary estimation contrasts are SMT versus MC, SSM versus MC, and SSM + SMT versus MC using Fisher’s least significant difference. All group comparisons of the primary outcomes will be presented as mean differences with 95% confidence intervals. A secondary evaluation will consider whether the effects of SMT and SSM are potentially synergistic or antagonistic and will be done using a formal test for interaction.
Additional analyses of primary outcomes
Responder analyses. We will conduct responder analyses by assessing the proportion of patients experiencing ≥ 50% improvement in pain or disability from baseline to six months, and from baseline to twelve months. We will also evaluate the proportion experiencing ≥ 30% improvement, and conduct a comprehensive responder analysis that looks at the cumulative percentage of participants achieving a range of improvement [86].
Longitudinal analyses. We will use the monthly measures of disability and weekly measures of pain to conduct longitudinal analysis that characterizes the mean profile over time for each intervention group. Formal comparison of profiles will be based on linear mixed models or generalized estimating equations (GEE). We will also report on changes from baseline to post treatment at 2 months and at 6 and 12 month follow-ups. These analyses will evaluate the magnitude of short, medium, and long-term effects of treatment, which are traditionally used in systematic reviews and meta-analyses. Furthermore, we will conduct exploratory analyses that assume latent classes with associated trajectories, and we can evaluate whether these groups differ across the intervention arms [87, 88].
Missing data
Missing data may include missing covariate information, study dropout, or missed and/or mistimed participant visits. While the protocol includes procedures to ensure the most “complete” follow-up data on every enrolled participant, it is likely that some participants will have incomplete data. We will determine reasons for missingness and classify each missingness pattern as missing completely at random (MCAR), missing at random (MAR), or missing not at random (MNAR). The MCAR mechanism occurs when the probability of response is independent of both the observed data and the unobserved data [89]. In longitudinal analyses, likelihood-based analyses of complete-case data for the linear mixed-effects model, the generalized linear mixed-effects model, and the nonlinear mixed-effects model lead to valid inference under MCAR and MAR mechanisms, whereas the GEE analyses lead to valid inference only in the presence of MCAR mechanisms [89, 90]. Statistical tests to assess the validity of the MCAR assumption in certain circumstances are available, but they are model-dependent and non-robust [91,92,93]. In general, we will advocate the use of multiple imputation (MI) [94] both to assess the sensitivity of results and to correct for potential bias from missing covariates. We will consider the missing data mechanism, analysis approach, and plausibility of the congeniality assumption [95, 96].
Subgroup/Moderator Analyses: We will perform two pre-specified subgroup analyses to look at treatment effects within: risk of cLBP based on the STarT Back (medium vs. high); and participants stratified based on their duration of LBP (acute vs. sub-acute). Subgroup analyses will use linear regression among restricted subsets to quantify specific treatment effects, and formal evaluation of differences in treatment effects across subgroups will be conducted using treatment by subgroup interactions.
Mediation analysis for psychosocial factors
Formal mediation analysis [97,98,99] will focus on characterizing the degree to which self-efficacy, coping, kinesiophobia, and pain catastrophizing measured at 8 weeks can explain treatment effects at 6 months, and whether these measures obtained at 6 months explain long-term treatment effects (1 year). We will quantify the percent of the treatment effect that is explained by changes in each scale individually, and in totality when included in a multivariate model for the outcome [100]. We will analyze mediation for LBP impact, pain NRS, and RMD measured at 6 months and 1 year.
Secondary outcomes analyses
All secondary outcomes, except the recovery outcome, will be assessed for each of the three intervention groups (SMT, SSM and SSM + SMT) relative to MC. We will use linear mixed models or GEE for longitudinal analysis [101]. We will also report on changes from baseline to post treatment at 2 months and at 6 and 12 months follow- up. These time-point analyses will evaluate the magnitude of short, medium, and long-term effects of treatment, for use in systematic reviews and meta-analyses.
The main secondary outcome of recovery at 6 months (a binary composite outcome derived from pain NRS = 0 AND RMD < = 2.) will be analyzed using a test on the overall (marginal) effect of SMT and a test of the overall (marginal) effect of SSM. These tests are obtained using logistic regression including the interaction between SMT and SSM and then overall effects are computed as a linear contrast of the two stratum-specific comparisons (i.e., the overall SMT effect is defined as the average of the SMT effect when SSM = 0 and the SMT effect when SSM = 1). We will assess the impact of alternative definitions of recovery (e.g., NRS < 3 and RMD < 4). We will also consider the time-until-recovery based on measurements taken every 4 weeks during the twelve months of follow-up. Specifically, we can define the time-until-recovery as the assessment month in which the subject is first observed to achieve an NRS = 0 and RMD < = 2. We will use discrete time (monthly data) cumulative incidence curves to show the percent of subjects in each treatment group who have achieved a first recovery by each follow-up time period. Because recovery may not be maintained, and subjects may subsequently relapse, we will display plots showing the percent of subjects who are currently in the recovered state as a function of time. Formal comparison of cumulative incidence curves can be obtained using the log rank test since in this situation the cumulative incidence is simply 1-survival as would be computed using Kaplan-Meier curves. In addition, we will use a model-based survival analysis.
The robustness of NIH RTF case definition of chronic LBP will be assessed using measures of pain frequency and LBP-related burden (pain, disability, productivity loss, healthcare utilization) by assessing differences between participants meeting the case definition and those who do not.
Implementation analyses
Quantitative data will be analyzed using descriptive statistics, independent t-tests (for means) and z-tests (for proportions) to assess group differences as appropriate. For the qualitative analysis, teams of 2–3 will perform rapid deductive, directed content analytic methods; the coding structure and operational definitions will be guided by the study’s conceptual models to provide insights into barriers and facilitators to future implementation [51, 58, 59]. Directed content analyses will also allow for inductive gathering of important themes that might fall outside of our chosen models and frameworks [102, 103]. Rapid approaches have been advocated for implementation research as they balance rigor with efficiency, yielding timely and meaningful evaluation of stakeholder needs and perspectives that can be more quickly matched to solutions [102, 104].
Additional Statistical Considerations: A priori criteria were established for combining study data from the UG3 and UH3 phases for the statistical analysis. NCCIH, and the DSMB reviewed and approved combining data for the UG3 and UH3 phases at the end of the UG3 phase.
Dissemination
A Publications Committee with representation from the CCC and the DCC facilitates timely dissemination of study findings, maintains high scientific standards for published material, prioritizes the order of publication and presentations, and ensures equitable investigator participation and attribution of authorship. The committee ensures publications are well-aligned with the trial’s research objectives and are not redundant. The committee also reviews and guides all data analysis plans, as well as research abstracts, presentations, and manuscripts before submission. The committee reviews proposals for ancillary studies and ensures all publications meet the NIH Open Access criteria including deposit in PubMed Central.
Protocol modifications and impact on the trial
All significant protocol modifications were reviewed and approved by NIH, the DSMB, and IRB.
Change in Eligibility Criteria. Our definition for an acute/sub-acute episode of LBP was re-examined and changed during the UG3 phase of the trial due to difficulty recruiting patients. Our initial definition required a one-month period without bothersome LBP prior to the start of the episode. In addition, participants were required to report LBP interfered with their regular daily activities on less than half of the days over the past six months. We encountered many individuals with ongoing back problems that had experienced a recent worsening or aggravation for which they were seeking care but were ineligible. In addition, many patients had difficulty interpreting what constituted “bothersome LBP symptoms” and recalling LBP symptoms accurately over the past six months. As a result, we updated the definition to include aggravations of LBP using a recommended definition from an international group of LBP experts [53]. To qualify, participants must experience a new episode or aggravation of ongoing LBP in the past 12 weeks that lasted at least 2 weeks. Further, individuals with an aggravation of their LBP must agree that it is a worsening of their condition that is difficult to tolerate and generally impacts their usual activities and/or emotions. We also updated the requirements in the month prior to the new episode/aggravation from “no bothersome LBP symptoms” to “less than severe LBP on average” and dropped the requirement based on symptom recall in the past 6 months. This change enabled a more accurate identification of eligible participants without biasing the trial.
Change in primary outcome for preventing chronic LBP
Our original primary outcome for preventing chronic LBP used the NIH RTF items based on participant recall for the frequency of LBP in the past six months with LBP on half the days or more considered chronic. The NIH RTF chronicity outcome measure has several recognized limitations. The measure is based on a 6-month recall period and was not intended to be used as an outcome measure by the NIH RTF (April 2021 personal communication with RTF member and co-investigator of this trial, Dennis Turk and chairman of the RTF, Rick Deyo). Further, it is a dichotomous outcome that does not consider the severity of pain or the amount of interference with daily activities and therefore does not define the degree of overall impact. After the trial began, new information highlighting the importance of measuring the impact of chronic pain was published [6,7,8, 10, 11]. This recent body of literature prompted us to reevaluate the adequacy of our primary chronicity outcome measure. We decided to adopt the chronicity measure that was already being collected at a monthly basis, prevention of impactful cLBP (based on averaged LBP Impact scores from the PROMIS-29 Profile v2.0 over months 10–12) [9].
By transitioning from a dichotomous to a quantitative primary outcome, we gained statistical power to evaluate the LBP impact/chronicity aim. Based on our updated power calculations, the impact of imbalance in the randomization allocation due to restricted randomization was minimal. By reducing the original sample size of 1180 based on the recovery outcome to 1000 participants we retain excellent power for determining clinically meaningful group differences on all primary outcomes with effect size differences of at least 0.30.
Change in statistical analysis plan and effectiveness objectives to control for multiplicity
The trial initially had three main effectiveness objectives: (1) prevention of chronic LBP at twelve months; (2) recovery from acute/sub-acute LBP at six months; (3) Average of pain and disability over twelve months. In 2021, the NIH statistician overseeing the trial raised the question of the adequacy of the planned adjustment for multiplicity given the trial’s three main effectiveness objectives and accompanying four co-primary outcome measures. In response to this concern, the lead investigators recommended the recovery objective be changed to a key secondary outcome. Early in the conduct of the trial it was decided and approved as a protocol change to include patients that had an acute aggravation of ongoing LBP, if the ongoing pain was not rated as severe in the month prior to the aggravation. This protocol change substantially lowers the proportion of patients that can be expected to recover according to our criteria (pain severity = 0 and RMD score ≤ 2). Given this change, the recovery outcome was less appropriate as a primary effectiveness objective and demoting it to a secondary outcome mitigates the concern of cross-objective control. To further mitigate concerns of cross-objective control for multiplicity, the investigative team developed a publication plan that addresses two separate research questions that would normally be the focus of two separate trials. The first manuscript will focus on the primary outcomes listed for the cumulative LBP and disability experience over twelve-months post-randomization. This paper would present recovery at six months as the key pre-specified secondary outcome. The second manuscript will focus on chronic impact at twelve months using the pre-specified primary outcome of LBP Impact averaged over months 10–12. The focus of the second manuscript will have a different objective from the first and would not warrant adjustment for multiple comparisons as the separate endpoint, distinct twelve-month outcome time period, and separate publication all imply no correction for other analyses.
Extenuating circumstances and impact on the Trial
COVID-19 Pandemic: In March 2020, the COVID-19 global pandemic resulted in a temporary suspension of the trial, including recruitment, enrollment, intervention delivery, and the collection of objective secondary outcomes. In response to increased severity of the COVID-19 pandemic, including increased rates of community spread, hospitalization and death rates and a rapidly changing environment, we made important modifications to the trial protocol. Two of the trial arms (SMT and SSM + SMT) required face-to-face contact with study participants. In order to avoid physical interaction, we updated the protocol to allow for remote assessments and interventions in a partial 2 group randomization period during which participants were randomized only to MC or SSM, delivered using HIPAA-compliant videoconferencing technology. In order to accomplish this, several modifications were made including: transitioning to an electronic consent process; updating study protocols and training staff to assess eligibility criteria, deliver SSM and MC, and objective measures via telehealth; addition of secondary outcome measures regarding COVID-19 impact and telehealth usability; modifications to randomization scheme for the partial 2-group randomization period; and implementation of active COVID-19 monitoring at participating sites. The partial 2-group randomization period began in December 2020. In November 2021, when conditions were met to safely return to in-person activities, we returned to full 4 group treatment allocation. Clinic activities that were suspended at UM Epidemiology Clinical Research Center due to COVID-19 were moved to the Berman Center for Outcomes and Clinical Research. In order to account for potential period-effects, we updated our statistical analysis plan to include an adjustment for partial randomization time periods in all analyses. Since November 2021, all trial procedures have been compliant with Covid mitigation rules (masking, distancing, and sanitation of surfaces) established by the Universities of Minnesota and Pittsburgh. All modifications were planned by the principal investigative team, reviewed and approved by the DSMB and the funding agency, and reported within the trial registration at ClinicalTrials.gov.
George Floyd Murder & Social Unrest in the Twin Cities (Minneapolis-St Paul). In May 2020, George Floyd was killed by a uniformed Minneapolis police officer only a short distance from where study activities normally took place. Mr. Floyd’s murder resulted in considerable trauma and social unrest in the Twin Cities, including riots, shootings, vandalism and looting. The impacts of this continue to affect communities of color. The original recruitment plan included planned events and initiatives to recruit diverse populations. However, the study team was advised by community partners that participation in research during these troubling times simply wasn’t a priority, and we were encouraged to be sensitive to the communities’ needs. As a result, the study team made important modifications to the original recruitment plan at the advice of community study consultants.
Discussion
This trial aims to fill an important gap in the scientific literature regarding the effectiveness of promising non-pharmacological treatments compared to medical care in the management patients with an acute episode of LBP and the prevention of progression to severe cLBP, by targeting those at higher risk [23].
The trial has several strengths that will facilitate the advancement of LBP research and practice. First, the hybrid effectiveness implementation design was informed by the PRECIS tool, [52] which maximizes pragmaticism while including several explanatory elements to ensure internal validity. Noteworthy pragmatic design features include outcomes that span biopsychosocial domains relevant to patients with pain and a high degree of intervention flexibility (see Fig. 2) including tailoring and individualization to meet patient needs (see Table 2). Further, while type 1 hybrid designs prioritize effectiveness outcomes, the trial also uses the RE-AIM translational framework to consider key contextual factors and processes from different stakeholders that can facilitate or impede eventual intervention implementation [51, 105]. While such approaches have gained traction in other health fields [106,107,108] for addressing the translation of effective interventions to practice, there have been few such studies in the LBP arena [109].
This trial seeks to overcome some of the limitations of existing research, especially regarding self-management. This includes heterogenous content and format, [110, 111] poor methodological quality, lack of long-term follow-up, inattention to intervention fidelity and absence of theoretical rationales aligning patient needs and risk factors [112]. We have used the TIDieR checklist to guide the reporting of the interventions to facilitate future results interpretation, as well as dissemination and replication [61]. Additionally, in accordance with reporting standards, we have described protocol modifications with their potential impacts, including those due to extenuating circumstances which were beyond the research team’s control [32].
Another strength of this research is the application of theoretical frameworks to the experimental intervention design (SSM and SSM + SMT). We used the well-established behavior change wheel (BCW) model, coupled with the dynamic biopsychosocial model of pain that acknowledges the complex and reciprocal interactions between the evolving biopsychological or “whole” person and their external, social environment [57,58,59]. While widely applied to other health conditions, the BCW has rarely been applied in LBP research. An advantage of the BCW is that it represents a synthesis of 19 behavioral theoretical frameworks and thus is more comprehensive in addressing the complexity of human behavior versus a single theory driven model. The application of the BCW in the intervention design, as well provider training, will be addressed in depth in a subsequent publication [59, 113].
Also noteworthy is the pragmatic advantage to having the biopsychosocial elements of care delivered by a single practitioner in the SSM and SSM + SMT interventions. This has the potential to improve patient access to harmonized, multi-modal care and decrease patient burden and associated costs [114]. PTs and DCs are the most common providers of non-pharmacologic treatment for back pain conditions in the United States [27]. This makes them optimally positioned for delivering integrated psychosocial strategies to complement biological/physical approaches, [26, 28] and play a critical role in the frontline non-drug management of LBP [29, 30]. Indeed, there have already been shifts in both the PT and DC fields to integrate more psychosocial aspects into their care models to better support patient self-management [26, 28, 115, 116].
Data availability
Study materials are available from the corresponding author by reasonable request.
Abbreviations
- AE:
-
Adverse Event
- BCW:
-
Behavior Change Wheel
- BPS:
-
Biopsychosocial
- CCC:
-
Clinical Coordinating Centers
- cLBP:
-
Chronic Low Back Pain
- DCC:
-
DC Chiropractor
- DSMB:
-
Data Safety and Monitoring Board
- DSMP:
-
Data Safety and Monitoring Plan
- GEE:
-
Generalized Estimating Equations
- HIPAA:
-
Health Insurance Portability and Accountability Act
- IRB:
-
Institutional Review Board
- LBP:
-
Low Back Pain
- MAR:
-
Missing at Random
- MC:
-
Medical Care
- MCAR:
-
Missing Completely at Random
- MNAR:
-
Missing Not at Random
- NCCIH:
-
National Center for Complementary and Integrative Health
- NIH:
-
RTF National Institute of Health’s Research Task Force
- NRS:
-
Numerical Rating Scale
- NSAIDs:
-
Nonsteroidal Anti-inflammatory Drugs
- PACBACK:
-
Preventing Acute to Chronic Back Pain
- PRECIS:
-
Pragmatic Explanatory Continuum Indicator Summary
- PT:
-
Physical Therapist
- RE-AIM:
-
Reach, Effectiveness, Adoption, Implementation, and Maintenance
- RMD:
-
Roland Morris Disability Questionnaire
- SAE:
-
Serious Adverse Event
- SMT:
-
Spinal Manipulation Therapy
- SSM:
-
Supported Self-Management
- TIDieR:
-
Template for Intervention Description and Replication
- UM:
-
University of Minnesota
- UP:
-
University of Pittsburgh
- UW:
-
University of Washington
References
Dowell D, Ragan KR, Jones CM, Baldwin GT, Chou R. CDC Clinical Practice Guideline for Prescribing Opioids for Pain - United States, 2022. MMWR Recomm Rep. 2022;71(3):1–95.
Medicine Io. Relieving Pain in America: a blueprint for transforming Prevention, Care, Education, and Research. Washington, DC: The National Academies Press; 2011. p. 382.
Johannes CB, Le TK, Zhou X, Johnston JA, Dworkin RH. The prevalence of chronic pain in United States adults: results of an internet-based survey. J pain: official J Am Pain Soc. 2010;11(11):1230–9.
Wu A, March L, Zheng X, Huang J, Wang X, Zhao J, et al. Global low back pain prevalence and years lived with disability from 1990 to 2017: estimates from the global burden of Disease Study 2017. Annals of Translational Medicine. 2020;8(6):299.
Chou R, Shekelle P. Will this patient develop persistent disabling low back pain? JAMA. 2010;303(13):1295–302.
Von Korff M, Scher AI, Helmick C, Carter-Pokras O, Dodick DW, Goulet J, et al. United States National Pain Strategy for Population Research: concepts, definitions, and Pilot Data. J pain: official J Am Pain Soc. 2016;17(10):1068–80.
Herman PM, Broten N, Lavelle TA, Sorbero ME, Coulter ID. Health Care costs and opioid Use Associated with High-impact chronic spinal Pain in the United States. Spine. 2019;44(16):1154–61.
Dahlhamer J, Lucas J, Zelaya C, Nahin R, Mackey S, DeBar L, et al. Prevalence of Chronic Pain and High-Impact Chronic Pain among adults - United States, 2016. MMWR Morb Mortal Wkly Rep. 2018;67(36):1001–6.
Deyo RA, Dworkin SF, Amtmann D, Andersson G, Borenstein D, Carragee E, et al. Report of the NIH Task Force on research standards for chronic low back pain. J pain: official J Am Pain Soc. 2014;15(6):569–85.
Von Korff M, DeBar LL, Krebs EE, Kerns RD, Deyo RA, Keefe FJ. Graded chronic pain scale revised: mild, bothersome, and high-impact chronic pain. Pain. 2020;161(3):651–61.
Pitcher MH, Von Korff M, Bushnell MC, Porter L. Prevalence and profile of high Impact Chronic Pain in the United States. J Pain. 2018.
Hartvigsen J, Hancock MJ, Kongsted A, Louw Q, Ferreira ML, Genevay S, et al. What low back pain is and why we need to pay attention. Lancet. 2018;391(10137):2356–67.
Carey TS, Freburger JK, Holmes GM, Castel L, Darter J, Agans R, et al. A long way to go: practice patterns and evidence in chronic low back pain care. Spine. 2009;34(7):718–24.
Mardian AS, Hanson ER, Villarroel L, Karnik AD, Sollenberger JG, Okvat HA et al. Flipping the Pain Care Model: a Sociopsychobiological Approach to High-Value Chronic Pain Care. Pain Med. 2020.
Foster NE, Anema JR, Cherkin D, Chou R, Cohen SP, Gross DP, et al. Prevention and treatment of low back pain: evidence, challenges, and promising directions. Lancet. 2018;391(10137):2368–83.
Pincus T, Kent P, Bronfort G, Loisel P, Pransky G, Hartvigsen J. Twenty-five years with the biopsychosocial model of low back pain-is it time to celebrate? A report from the twelfth international forum for primary care research on low back pain. Spine. 2013;38(24):2118–23.
Engel GL. The clinical application of the biopsychosocial model. Am J Psychiatry. 1980;137(5):535–44.
Deyo RA, Mirza SK, Turner JA, Martin BI. Overtreating chronic back pain: time to back off? J Am Board Family Medicine: JABFM. 2009;22(1):62–8.
Reuben DB, AA HA, Ashikaga T, Bogat GA, Callahan CM, Ruffing V et al. National Institutes of Health Pathways to Prevention Workshop: the role of Opioids in the treatment of Chronic Pain. Ann Intern Med. 2015.
Lee SW, Patel J, Kim SY, Miranda-Comas G, Herrera J, Bartels MN. Use of opioid analgesics in patients with chronic low back Pain and knee osteoarthritis. Am J Phys Med Rehabil. 2019;98(8):e97–e8.
Qaseem A, Wilt TJ, McLean RM, Forciea MA. Clinical Guidelines Committee of the American College of P. Noninvasive treatments for Acute, Subacute, and chronic low back Pain: a clinical practice Guideline from the American College of Physicians. Ann Intern Med. 2017;166(7):514–30.
Von Korff M, Shortreed SM, Saunders KW, LeResche L, Berlin JA, Stang P, et al. Comparison of back pain prognostic risk stratification item sets. J pain: official J Am Pain Soc. 2014;15(1):81–9.
Gewandter JS, Dworkin RH, Turk DC, Farrar JT, Fillingim RB, Gilron I, et al. Research design considerations for chronic pain prevention clinical trials: IMMPACT recommendations. Pain. 2015;156(7):1184–97.
Hill JC, Whitehurst DG, Lewis M, Bryan S, Dunn KM, Foster NE, et al. Comparison of stratified primary care management for low back pain with current best practice (STarT back): a randomised controlled trial. Lancet. 2011;378(9802):1560–71.
Hush JM. Low back pain: it is time to embrace complexity. Pain. 2020;161(10):2248–51.
Foster NE, Delitto A. Embedding psychosocial perspectives within clinical management of low back pain: integration of psychosocially informed management principles into physical therapist practice–challenges and opportunities. Phys Ther. 2011;91(5):790–803.
Ivanova JI, Birnbaum HG, Schiller M, Kantor E, Johnstone BM, Swindle RW. Real-world practice patterns, health-care utilization, and costs in patients with low back pain: the long road to guideline-concordant care. Spine J. 2011;11(7):622–32.
Brunner E, De Herdt A, Minguet P, Baldew SS, Probst M. Can cognitive behavioural therapy based strategies be integrated into physiotherapy for the prevention of chronic low back pain? A systematic review. Disabil Rehabil. 2013;35(1):1–10.
Hartvigsen J, Foster NE, Croft PR. We need to rethink front line care for back pain. BMJ. 2011;342:d3260.
Murphy DR, Justice BD, Paskowski IC, Perle SM, Schneider MJ. The establishment of a primary spine care practitioner and its benefits to health care reform in the United States. Chiropr Man Therap. 2011;19(1):17.
Chan AW, Tetzlaff JM, Altman DG, Laupacis A, Gøtzsche PC, Krleža-Jerić K, et al. SPIRIT 2013 statement: defining standard protocol items for clinical trials. Ann Intern Med. 2013;158(3):200–7.
Orkin AM, Gill PJ, Ghersi D, Campbell L, Sugarman J, Emsley R, et al. Guidelines for reporting trial protocols and completed trials modified due to the COVID-19 pandemic and other Extenuating Circumstances: the CONSERVE 2021 Statement. JAMA. 2021;326(3):257–65.
Chou R, Deyo R, Friedly J, Skelly A, Hashimoto R, Weimer M et al. Noninvasive treatments for low back Pain. Rockville MD2016 Feb.
Chou R. Nonpharmacologic therapies for Acute and Chronic Low Back Pain: a review of the evidence for an american Pain Society/American College of Physicians Clinical Practice Guideline. Ann Intern Med. 2007;147(7):492.
Bronfort G, Goldsmith CH, Nelson CF, Boline PD, Anderson AV. Trunk exercise combined with spinal manipulative or NSAID therapy for chronic low back pain: a randomized, observer-blinded clinical trial. J Manip Physiol Ther. 1996;19(9):570–82.
Bronfort G, Hondras MA, Schulz CA, Evans RL, Long CR, Grimm R. Spinal manipulation and home exercise with advice for subacute and chronic back-related leg pain: a trial with adaptive allocation. Ann Intern Med. 2014;161(6):381–91.
Bronfort G, Maiers MJ, Evans RL, Schulz CA, Bracha Y, Svendsen KH, et al. Supervised exercise, spinal manipulation, and home exercise for chronic low back pain: a randomized clinical trial. Spine J. 2011;11(7):585–98.
Evans R, Haas M, Schulz C, Leininger B, Hanson L, Bronfort G. Spinal manipulation and exercise for low back pain in adolescents: a randomized trial. Pain. 2018;159(7):1297–307.
Schulz C, Evans R, Maiers M, Schulz K, Leininger B, Bronfort G. Spinal manipulative therapy and exercise for older adults with chronic low back pain: a randomized clinical trial. Chiropr Man Ther. 2019;27(1):21.
Delitto A, Patterson CG, Stevans JM, Freburger JK, Khoja SS, Schneider MJ et al. Stratified care to prevent chronic low back pain in high-risk patients: the TARGET trial. A multi-site pragmatic cluster randomized trial. EClinicalMedicine. 2021;34.
Childs JD, Fritz JM, Flynn TW, Irrgang JJ, Johnson KK, Majkowski GR, et al. A clinical prediction rule to identify patients with low back pain most likely to benefit from spinal manipulation: a validation study. Ann Intern Med. 2004;141(12):920–8.
Erhard RE, Delitto A, Cibulka MT. Relative effectiveness of an extension program and a combined program of manipulation and flexion and extension exercises in patients with acute low back syndrome. Phys Ther. 1994;74(12):1093–100.
Fritz JM, Delitto A, Erhard RE. Comparison of classification-based physical therapy with therapy based on clinical practice guidelines for patients with acute low back pain: a randomized clinical trial. Spine. 2003;28(13):1363–71. discussion 72.
George SZ, Childs JD, Teyhen DS, Wu SS, Wright AC, Dugan JL, et al. Brief psychosocial education, not core stabilization, reduced incidence of low back pain: results from the Prevention of Low Back Pain in the military (POLM) cluster randomized trial. BMC Med. 2011;9:128.
George SZ, Fritz JM, Bialosky JE, Donald DA. The effect of a fear-avoidance-based physical therapy intervention for patients with acute low back pain: results of a randomized clinical trial. Spine. 2003;28(23):2551–60.
George SZ, Zeppieri G Jr, Cere AL, Cere MR, Borut MS, Hodges MJ, et al. A randomized trial of behavioral physical therapy interventions for acute and sub-acute low back pain (NCT00373867). Pain. 2008;140(1):145–57.
Licciardone JC, Minotti DE, Gatchel RJ, Kearns CM, Singh KP. Osteopathic manual treatment and ultrasound therapy for chronic low back pain: a randomized controlled trial. Ann Fam Med. 2013;11(2):122–9.
Licciardone JC, Stoll ST, Fulda KG, Russo DP, Siu J, Winn W, et al. Osteopathic manipulative treatment for chronic low back pain: a randomized controlled trial. Spine. 2003;28(13):1355–62.
Schneider M, Haas M, Glick R, Stevans J, Landsittel D. Comparison of spinal manipulation methods and usual medical care for acute and subacute low back pain: a randomized clinical trial. Spine. 2015;40(4):209–17.
McDonough SM, Tully MA, O’Connor SR, Boyd A, Kerr DP, O’Neill SM, et al. The back 2 activity trial: education and advice versus education and advice plus a structured walking programme for chronic low back pain. BMC Musculoskelet Disord. 2010;11:163.
Holtrop JS, Estabrooks PA, Gaglio B, Harden SM, Kessler RS, King DK, et al. Understanding and applying the RE-AIM framework: clarifications and resources. J Clin Translational Sci. 2021;5(1):e126.
Loudon K, Treweek S, Sullivan F, Donnan P, Thorpe KE, Zwarenstein M. The PRECIS-2 tool: designing trials that are fit for purpose. BMJ. 2015;350:h2147.
Costa N, Ferreira ML, Setchell J, Makovey J, Dekroo T, Downie A, et al. A definition of “Flare” in Low Back Pain: a multiphase process involving perspectives of individuals with Low Back Pain and Expert Consensus. J pain: official J Am Pain Soc. 2019;20(11):1267–75.
Hill JC, Dunn KM, Lewis M, Mullis R, Main CJ, Foster NE, et al. A primary care back pain screening tool: identifying patient subgroups for initial treatment. Arthritis Rheum. 2008;59(5):632–41.
Haanstra TM, Hanson L, Evans R, van Nes FA, De Vet HC, Cuijpers P, et al. How do low back pain patients conceptualize their expectations regarding treatment? Content analysis of interviews. Eur Spine J. 2013;22(9):1986–95.
Evans R, Bronfort G, Maiers M, Schulz C, Hartvigsen J. I know it’s changed”: a mixed-methods study of the meaning of global Perceived Effect in chronic neck pain patients. Eur Spine J. 2014;23(4):888–97.
Sturgeon JA, Zautra AJ. Social pain and physical pain: shared paths to resilience. Pain Manag. 2016;6(1):63–74.
Lehman BJ, David DM, Gruber JA. Rethinking the biopsychosocial model of health: understanding health as a dynamic system. Soc Pers Psychol Compass. 2017;11:8.
Michie SAL, West R. The Behaviour Change Wheel: a Guide to Designing Interventions. London: Silverback Publishing; 2014.
Harvey E, Burton AK, Moffett JK, Breen A. Spinal manipulation for low-back pain: a treatment package agreed to by the UK chiropractic, osteopathy and physiotherapy professional associations. Man Ther. 2003;8(1):46–51.
Hoffmann TC, Glasziou PP, Boutron I, Milne R, Perera R, Moher D, et al. Better reporting of interventions: template for intervention description and replication (TIDieR) checklist and guide. BMJ. 2014;348(mar07 3):g1687–g.
Spitzer WOLF, Dupuis M. Scientific approach to the assessment and management of activity-related spinal disorders. A monograph for clinicians. Report of the Quebec Task Force on spinal Disorders. Spine. 1987;12(7 Suppl):1–59.
Jaeschke R, Singer J, Guyatt GH. A comparison of seven-point and visual analogue scales. Data from a randomized trial. Control Clin Trials. 1990;11(1):43–51.
Jensen MP, Karoly P, Braver S. The measurement of clinical pain intensity: a comparison of six methods. Pain. 1986;27(1):117–26.
Carlsson AM. Assessment of chronic pain. I. Aspects of the reliability and validity of the visual analogue scale. Pain. 1983;16(1):87–101.
Roland M, Morris R. A study of the natural history of back pain. Part I: development of a reliable and sensitive measure of disability in low-back pain. Spine. 1983;8(2):141–4.
Roland M, Morris R. A study of the natural history of low-back pain. Part II: development of guidelines for trials of treatment in primary care. Spine. 1983;8(2):145–50.
Cook KF, Jensen SE, Schalet BD, Beaumont JL, Amtmann D, Czajkowski S, et al. PROMIS measures of pain, fatigue, negative affect, physical function, and social function demonstrated clinical validity across a range of chronic conditions. J Clin Epidemiol. 2016;73:89–102.
Reilly MC, Gooch KL, Wong RL, Kupper H, van der Heijde D. Validity, reliability and responsiveness of the Work Productivity and Activity Impairment Questionnaire in ankylosing spondylitis. Rheumatology (Oxford). 2010;49(4):812–9.
Turk DC, Dworkin RH, Allen RR, Bellamy N, Brandenburg N, Carr DB, et al. Core outcome domains for chronic pain clinical trials: IMMPACT recommendations. Pain. 2003;106(3):337–45.
Kamper SJ, Maher CG, Mackay G. Global rating of change scales: a review of strengths and weaknesses and considerations for design. J Man Manip Ther. 2009;17(3):163–70.
Dunn KM, Campbell P, Jordan KP. Validity of the visual Trajectories Questionnaire for Pain. J pain: official J Am Pain Soc. 2017;18(12):1451–8.
Denteneer L, Van Daele U, Truijen S, De Hertogh W, Meirte J, Stassijns G. Reliability of physical functioning tests in patients with low back pain: a systematic review. Spine J. 2018;18(1):190–207.
Jakobsson M, Gutke A, Mokkink LB, Smeets R, Lundberg M. Level of evidence for reliability, validity, and responsiveness of physical capacity tasks designed to assess functioning in patients with Low Back Pain: a systematic review using the COSMIN Standards. Phys Ther. 2019;99(4):457–77.
Andersson EI, Lin CC, Smeets RJ. Performance tests in people with chronic low back pain: responsiveness and minimal clinically important change. Spine. 2010;35(26):E1559–63.
Anderson KO, Dowds BN, Pelletz RE, Edwards WT, Peeters-Asdourian C. Development and initial validation of a scale to measure self-efficacy beliefs in patients with chronic pain. Pain. 1995;63(1):77–84.
Carver CS. You want to measure coping but your protocol’s too long: consider the brief COPE. Int J Behav Med. 1997;4(1):92–100.
Woby SR, Roach NK, Urmston M, Watson PJ. Psychometric properties of the TSK-11: a shortened version of the Tampa Scale for Kinesiophobia. Pain. 2005;117(1–2):137–44.
Sullivan MJ, Bishop SR, Pivik J. The Pain Catastrophizing Scale: development and validation. Psychol Assess. 1995(7):524–32.
Gaglio B, Phillips SM, Heurtin-Roberts S, Sanchez MA, Glasgow RE. How pragmatic is it? Lessons learned using PRECIS and RE-AIM for determining pragmatic characteristics of research. Implement Sci. 2014;9:96.
Greco CM, Yu L, Johnston KL, Dodds NE, Morone NE, Glick RM, et al. Measuring nonspecific factors in treatment: item banks that assess the healthcare experience and attitudes from the patient’s perspective. Qual Life Res. 2016;25(7):1625–34.
Coleman BC, Purcell N, Geda M, Luther SL, Peduzzi P, Kerns RD, et al. Assessing the impact of the COVID-19 pandemic on pragmatic clinical trial participants. Contemp Clin Trials. 2021;111:106619.
Parmanto B, Lewis AN Jr, Graham KM, Bertolet MH. Development of the Telehealth Usability Questionnaire (TUQ). Int J Telerehabil. 2016;8(1):3–10.
Anderson K, Francis T, Ibanez-Carrasco F, Globerman J. Physician’s perceptions of Telemedicine in HIV Care Provision: a cross-sectional web-based survey. JMIR Public Health Surveill. 2017;3(2):e31.
Ostelo RW, Stomp-van den Berg SG, Vlaeyen JW, Wolters PM, de Vet HC. Health care provider’s attitudes and beliefs towards chronic low back pain: the development of a questionnaire. Man Ther. 2003;8(4):214–22.
Farrar JT, Dworkin RH, Max MB. Use of the cumulative proportion of responders analysis graph to present pain data over a range of cut-off points: making clinical trial data more understandable. J Pain Symptom Manage. 2006;31(4):369–77.
Kongsted A, Kent P, Hestbaek L, Vach W. Patients with low back pain had distinct clinical course patterns that were typically neither complete recovery nor constant pain. A latent class analysis of longitudinal data. Spine J. 2015;15(5):885–94.
Deyo RA, Bryan M, Comstock BA, Turner JA, Heagerty P, Friedly J, et al. Trajectories of symptoms and function in older adults with low back disorders. Spine. 2015;40(17):1352–62.
Little RJA, Rubin DB. Statistical analysis with Missing Data. John Wiley and Sons Limited; 2016.
Fitzmaurice GM, Laird NM, Ware JH. Applied Longitudinal Analysis: Wiley; 2012.
Little RJA. A test of missing completely at Random for Multivariate Data with missing values. J Am Stat Assoc. 1988;83(404):1198–202.
Diggle PJ. Testing for random dropouts in repeated measurement data. Biometrics. 1989;45:1255–8.
Ridout MS. Testing for Random Dropouts in repeated Measurement Data. Biometrics. 1991;47(4):1617–9.
Schafer JL. Multiple imputation: a primer. Stat Methods Med Res. 1999;8(1):3–15.
Meng X-L. Multiple-imputation inferences with uncongenial sources of Input. Stat Sci. 1994;9(4):538–58.
Kim J, Yang S. A note on multiple imputation under complex sampling. Biometrika. 2017;104(1):221–8.
Imai K, Keele L, Tingley D. A general approach to causal mediation analysis. Psychol Methods. 2010;15(4):309–34.
Baron RM, Kenny DA. The moderator-mediator variable distinction in social psychological research: conceptual, strategic, and statistical considerations. J Personal Soc Psychol. 1986;51(6):1173–82.
Suri P, Pashova H, Heagerty PJ, Jarvik JG, Turner JA, Comstock BA, et al. Short-term improvements in disability mediate patient satisfaction after epidural corticosteroid injections for symptomatic lumbar spinal stenosis. Spine. 2015;40(17):1363–70.
Lin DY, Fleming TR, De Gruttola V. Estimating the proportion of treatment effect explained by a surrogate marker. Stat Med. 1997;16(13):1515–27.
Diggle P, Heagerty P, Liang K, Zeger S. Analysis of longitudinal data: Oxford University Press; 2002.
Nevedal AL, Reardon CM, Opra Widerquist MA, Jackson GL, Cutrona SL, White BS, et al. Rapid versus traditional qualitative analysis using the Consolidated Framework for implementation research (CFIR). Implement Sci. 2021;16(1):67.
Hsieh HF, Shannon SE. Three approaches to qualitative content analysis. Qual Health Res. 2005;15(9):1277–88.
Palinkas LA, Zatzick D. Rapid Assessment Procedure Informed Clinical Ethnography (RAPICE) in pragmatic clinical trials of Mental Health Services implementation: methods and Applied Case Study. Adm Policy Ment Health. 2019;46(2):255–70.
Landes SJ, McBain SA, Curran GM. Reprint of: an introduction to effectiveness-implementation hybrid designs. Psychiatry Res. 2020;283:112630.
Jang M, Chao A, Whittemore R. Evaluating intervention programs targeting parents to manage childhood overweight and obesity: a systematic review using the RE-AIM Framework. J Pediatr Nurs. 2015;30(6):877–87.
Ridgeway JL, LeBlanc A, Branda M, Harms RW, Morris MA, Nesbitt K, et al. Implementation of a new prenatal care model to reduce office visits and increase connectivity and continuity of care: protocol for a mixed-methods study. BMC Pregnancy Childbirth. 2015;15:323.
Petrescu-Prahova M, Belza B, Kohn M, Miyawaki C. Implementation and maintenance of a community-based older adult physical activity program. Gerontologist. 2015.
Irvine AB, Russell H, Manocchia M, Mino DE, Cox Glassen T, Morgan R, et al. Mobile-web app to self-manage low back pain: randomized controlled trial. J Med Internet Res. 2015;17(1):e1.
Du S, Hu L, Dong J, Xu G, Chen X, Jin S, et al. Self-management program for chronic low back pain: a systematic review and meta-analysis. Patient Educ Couns. 2017;100(1):37–49.
Lamb SE, Hansen Z, Lall R, Castelnuovo E, Withers EJ, Nichols V, et al. Group cognitive behavioural treatment for low-back pain in primary care: a randomised controlled trial and cost-effectiveness analysis. The Lancet. 2010;375(9718):916–23.
Mansell G, Hall A, Toomey E. Behaviour change and self-management interventions in persistent low back pain. Best Pract Res Clin Rheumatol. 2016;30(6):994–1002.
Michie S, Wood CE, Johnston M, Abraham C, Francis JJ, Hardeman W. Behaviour change techniques: the development and evaluation of a taxonomic method for reporting and describing behaviour change interventions (a suite of five studies involving consensus methods, randomised controlled trials and analysis of qualitative data). Health Technol Assess. 2015;19(99):1–188.
Bennell KL, Ahamed Y, Jull G, Bryant C, Hunt MA, Forbes AB, et al. Physical therapist-delivered Pain Coping Skills Training and Exercise for knee osteoarthritis: Randomized Controlled Trial. Arthritis Care Res (Hoboken). 2016;68(5):590–602.
Gliedt JA, Schneider MJ, Evans MW, King J, Eubanks JE. Jr. The biopsychosocial model and chiropractic: a commentary with recommendations for the chiropractic profession. Chiropr Man Therap. 2017;25:16.
George SZ, Teyhen DS, Wu SS, Wright AC, Dugan JL, Yang G, et al. Psychosocial education improves low back pain beliefs: results from a cluster randomized clinical trial (NCT00373009) in a primary prevention setting. Eur Spine J. 2009;18(7):1050–8.
Acknowledgements
The authors acknowledge the contributions of the dedicated research clinicians and staff that carried out this work.
Funding
Research reported in this publication was supported by the National Center For Complementary & Integrative Health of the National Institutes of Health under Award Numbers UG3AT008769 and UH3AT008769. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Author information
Authors and Affiliations
Contributions
GB, MS, PH, RE, CG, BL, CM, and LH drafted the manuscript text. All authors have contributed to the methods of the project and have read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
The research reported in this protocol has been performed in accordance with the Declaration of Helsinki. Ethical approval for the study was provided by the IRB at the University of Pittsburgh which served as the central IRB for the study (PRO18010414). All participants provided informed consent to participate.
Consent for publication
Not applicable.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Bronfort, G., Delitto, A., Schneider, M. et al. Effectiveness of spinal manipulation and biopsychosocial self-management compared to medical care for low back pain: a randomized trial study protocol. BMC Musculoskelet Disord 24, 415 (2023). https://doi.org/10.1186/s12891-023-06549-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12891-023-06549-w