Abstract
Background
To explore the effect and mechanism of action of miR-210 on postmenopausal osteoporosis (PMPO) in ovariectomized rats in vivo.
Methods
An ovariectomized (OVX) rat model was established by ovariectomy. Tail vein injection was performed to overexpress and knock down miR-210 in OVX rats, followed by the collection of blood and femoral tissues from each group of rats. And quantitative real-time polymerase chain reaction (qRT-PCR) was applied to assess the expression level of miR-210 in femoral tissues of each group. Micro computed tomography (Micro CT) was adopted to scan the microstructure of the femoral trabecula in each group to obtain relevant data like bone mineral density (BMD), bone mineral content (BMC), trabecular bone volume fraction (BV/TV), trabecular thickness (Tb.Th), bone surface-to-volume ratio (BS/BV), and trabecular separation (Tb.Sp). ELISA was used for determining the level of bone alkaline phosphatase (BALP), amino-terminal propeptide of type I procollagen (PINP), osteocalcin (OCN), and C-terminal telopeptide of type I collagen (CTX-1) in serum; and Western blot for the protein level of Runt-related transcription factor 2 (Runx2), osteopontin (OPN), and collagen type I alpha 1 (COL1A1) in femoral tissues.
Results
MiR-210 expression was significantly decreased in femoral tissues of OVX rats. Overexpression of miR-210 could obviously increase BMD, BMC, BV/TV and Tb.Th, whereas significantly decrease BS/BV and Tb.Sp in femurs of OVX rats. Moreover, miR-210 also downregulated BALP and CTX-1 level, upregulated PINP and OCN level in the serum of OVX rats promoted the expression of osteogenesis-related markers (Runx2, OPN and COL1A1) in the femur of OVX rats. Additionally, further pathway analysis revealed that high expression of miR-210 activated the vascular endothelial growth factor (VEGF)/Notch1 signaling pathway in the femur of OVX rats.
Conclusion
High expression of miR-210 may improve the micromorphology of bone tissue and modulate bone formation and resorption in OVX rats by activating the VEGF/Notch1 signaling pathway, thereby alleviating osteoporosis. Consequently, miR-210 can serve as a biomarker for the diagnosis and treatment of osteoporosis in postmenopausal rats.
Similar content being viewed by others
Background
In recent years, the incidence of age-related diseases has been increasing annually with the serious aging population. Osteoporosis, a systemic bone disease, is characterized by low bone mass, deteriorative bone microarchitecture, elevated bone fragility and increased risk of bone fracture [1]. Clinically, osteoporosis is classified into primary, idiopathic and secondary osteoporosis [2]. According to the Chinese Center for Disease Control and Prevention, the total number of patients with osteoporosis in China in 2015 exceeded 160 million, with a prevalence of 12.4%, with the most affected individuals being postmenopausal women [3]. Postmenopausal osteoporosis (PMPO) is the most common type of primary osteoporosis in clinical practice. Postmenopausal women are more likely to suffer from osteoporosis (nearly 80%) due to many factors, such as aging and decreased estrogen [4,5,6]. In addition, the incidence of PMPO reaches around 50%, and it is difficult to cure; Worse, patients with PMPO show a younger tendency. [7, 8]. PMPO, as a global public health problem, is not only a serious mental and economic burden on society, families and patients, but also a challenge in the treatment of clinical departments such as orthopedics and endocrinology. Therefore, it is of great social significance and economic value to explore the pathologic mechanisms and molecular biomarkers for the diagnosis and treatment of menopausal osteoporosis.
MicroRNAs (miRNAs), a class of endogenous non-coding RNAs with 18–25 nucleotides in length, regulate gene expression by specifically binding to the 3’-UTR of target genes [9]. Moreover, miRNAs are involved in regulating a range of biological processes including cell differentiation, proliferation, invasion, metastasis, and apoptosis [10, 11]. Several studies have shown that abnormal expression of miRNAs can cause abnormal bone metabolism and then lead to osteoporosis. Yin et al. pointed out that miR-151a-3p was upregulated in osteoporosis, and it reduced femoral BMD and biomechanical parameters in OVX rats by targeting SOCS5 and activating JAK2/ STAT3 signaling, and then promoted PMPO [12]. Li et al. discovered that miR-23b-3p was upregulated in bone tissue of PMOP rats and promoted PMPO by targeting MRC2 and regulating the Wnt/β-catenin signaling pathway [13]. Additionally, several studies have revealed the role of some other miRNAs in alleviating osteoporosis. For example, Zhang et al. claimed that miR-187 accelerated osteogenic differentiation of human multipotent stromal cells (hMSCs) by directly regulating BARX2, played a significant therapeutic effect [14]. Wang et al. found that miR-655-3p inhibited the progression of osteoporosis by targeting LSD1 and activating the BMP-2/Smad signaling pathway [15]. Jiang et al. revealed that miR-218-5p promotes osteoblast differentiation and inhibits osteoclast formation through the ROBO1/DKK-1 pathway [16]. Therefore, it is convinced that miRNAs may be therapeutic targets for osteoporosis.
MiR-210 is a crucial target gene of hypoxia-inducible factor, which is closely related to angiogenesis.[17]. Specifically, miR-210 expression gradually increases under hypoxia, normoxia and the induction of vascular endothelial growth factor and its overexpression in endothelial cells can stimulate the formation of capillary tube-like structures and cell migration under [18, 19]. Studies have reported that miR-210 may promote osteogenic differentiation of bone marrow mesenchymal stem cells (BMSCs) by regulating the expression of VEGF, and can promote the formation of blood vessels and regulate bone regeneration [20]. In addition, it was reported that the Notch1-mediated signaling pathway is associated with the lower proliferation and differentiation capacity of BMSCs in postmenopausal patients with osteoporosis, which may be one of the reasons for the reduced bone mass in postmenopausal patients with osteoporosis [21]. However, the function of miR-210 in osteoporosis has not been proved. Therefore, a typical ovariectomized (OVX) rat model was constructed in this study to discuss the role of miR-210 in OVX rats. All in all, the objective of this paper was to provide scientific evidence and basis for the use of miR-210 as a therapeutic target for osteoporosis in OVX rats.
Materials and methods
Model rat construction and grouping treatment
Thirty female SD rats (body weight: 180–220 g; age: 6 weeks) were included in this study, and then a postmenopausal osteoporotic rat model was established by ovariectomy [22]. Specifically, thirty rats were randomly divided into 5 groups with 6 rats in each group after adaptive feeding, including Sham group, OVX group, OVX + NC group, OVX + miR-210 mimic group and OVX + miR-210 inhibitor group. The rats in each group were fasted for 12 h and then anesthetized intraperitoneally with a concentration of 2% pentobarbital sodium injection. After the rats lost their corneal reflexes and consciousness, all four limbs of the rats in four OVX groups were fixed in a supine position. The linea alba, 3.5 cm from the vaginal orifice, was then used as an incision and a longitudinal incision of 1.5-2 cm was made using a scalpel. Continually, the subcutaneous tissue was separated with blunt dissection, followed by an incision of muscularis and peritoneum. Finally, the abdominal cavity was opened, the uterus was found, and the ovary was removed. Then the OVX rats were treated four weeks after the establishment of the OVX model as follows: OVX group, OVX rats were injected intravenously into the tail vein with an equal volume of normal saline (5 µL). OVX + NC group, OVX rats were injected intravenously into the tail vein with a negative vector (5 µL). OVX + miR-210 mimic group, OVX rats were injected intravenously into the tail vein with miR-210 overexpression vector (5 µL). OVX + miR-210 inhibitor group, OVX rats were injected intravenously into the tail vein with miR-210 inhibitor (5 µL). All injections were given weekly for a total of 8 weeks. Whereas in Sham group, the adipose tissue near ovaries was excised at an equal ovarian volume before suturing. To avoid infection, all rats received intramuscular injection of penicillin G for 3 consecutive days after surgery. Incidentally, rats were allowed to eat and drink freely and were routinely housed throughout the experiment. All rats were sacrificed at the end of the processing cycle, and the femur and serum from each group were collected for further index testing.
Micro computed tomography (Micro CT)
Micro computed tomography (Micro CT) was utilized for scanning the microstructure of femoral trabeculae in rats of each group, and the system SkyScan1276 for imaging whole femurs at 10 μm isotropic voxel size. Specifically, samples fixed in 4% paraformaldehyde were tested, and the scanning conditions included an X-ray tube potential (85 kv), an X-ray intensity (200 ja), and an exposure time (400 ms) for image reconstruction. A 3-D image was obtained from a 2-D image by distance transformation of the grayscale image. Then 3D and 2D analyses were performed using a software analyzer. Bone microstructure analysis was conducted in the region of interest, and finally, bone mineral density (BMD), bone mineral content (BMC), trabecular bone volume fraction (BV/TV), trabecular thickness (Tb.Th), bone surface-to-volume ratio (BS/BV), and trabecular separation (Tb.Sp) were determined [23].
qRT-PCR
Femurs from each group were collected. And total RNA from femurs was extracted using Trizol (Sigma, USA), followed by the detection of the concentration and purity of total RNA with Nanodrop software. Next, cDNA was synthesized on the basis of the instructions of the reverse transcription PCR kit (Takara, Japan). Then the treated cDNA was collected to synthesize miR-210 according to the real-time PCR kit (Takara, Japan). The primer sequences used were shown in Table 1, and the data was analyzed by the 2−ΔΔCt method.
Enzyme-linked immunosorbent assay (ELISA)
Blood from all rats was collected into coagulation-promoting tubes, then centrifugation at 1000 g was performed at 4 ℃ for 15 min. The serum was separated by a pipette for subsequent measurement. The level of bone alkaline phosphatase (BALP) kit, amino-terminal propeptide of type I procollagen (PINP), osteocalcin (OCN) and C-terminal telopeptide of type I collagen (CTX-1) in rat serum were assessed according to the instructions of Elisa kit (Nanjing Jiancheng, China).
Western blot
The tissue proteins from each group of rat femurs were extracted on ice according to the instructions of RIPA lysis Solution (Solarbio, China). The protein concentration was determined by a BCA kit (Solarbio, China). The 30 µg protein was separated by Sodium Dodecyl Sulfate - Polyacrylamide Gel Electrophoresis (SDS-PAGE). After electrophoresis, the target protein was transferred to a polyvinylidene fluoride (PVDF) membrane, and the membrane was sealed in 5% skimmed milk powder for 2 h. Then diluted primary antibody (CST, USA) was added for incubation overnight in a shaker at 4 ℃. After washing three times with TBST, diluted secondary antibodies (ZSGB-BIO, China) were added for another 1-h incubation at room temperature. Finally, the protein bands were developed using enhanced chemiluminescence (ECL) chemiluminescence and then photographed and archived. Image-pro plus software was employed to calculate the grayscale of the bands, and the expression level of the target protein was analyzed with β-actin as an internal control.
Statistics analysis
The data were expressed as mean ± standard deviation (SD). SPSS 24. 0 software was used for statistical analysis. The student t-test was used for comparing the differences between the two groups, and one-way ANOVA for comparing the differences between multiple groups. P < 0.05 was considered as the criterion for significant differences.
Results
Downregulation of miR-210 expression in femoral tissue of ovariectomized rats
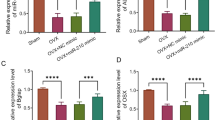
In order to clarify the function of miR-210 in menopausal rats, we established a PMPO rat model (OVX group) by ovariectomy and overexpressed and knocked down miR-210 respectively by tail vein injection. The expression level of miR-210 in the femoral tissue of the OVX group was much lower than that of the Sham group (P < 0.01); in addition, tail vein injection of miR-210 mimic successfully elevated miR-210 expression level while tail vein injection of miR-210 inhibitor markedly inhibited miR-210 expression level in the femoral tissue of OVX rats (P < 0.01); and there was no significant difference in the expression of miR-210 between the OVX group and OVX + NC group (P > 0.05) (Fig. 1).
Effect of miR-210 on the microstructure of femoral trabeculae in ovariectomized rats
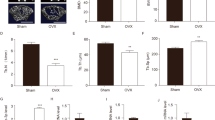
Micro-CT was used to detect the effects of treatment with miR-210 mimic or miR-210 inhibitor on bone mass loss in the OVX rats. Representative 2D and 3D Micro-CT images were shown in Fig. 2A. The results showed that the miR-210 improved the extensive bone mass loss in the OVX rat model. In addition, the effect of miR-210 on the femoral structure of OVX rats was assessed by measuring the indicators such as BMD, BMC, BV/TV, Tb.Th, BS/BV and Tb.Sp. The results revealed that, compared with the Sham group, BMD, BMC, BV/TV, and Tb.Th in the OVX group was significantly lower (P < 0.01), but BS/BV and Tb.Sp were obviously higher (P < 0.01). It indicated that a PMPO rat model was successfully constructed by ovariectomy. Besides, overexpression of miR-210 could significantly increase the BMD, BMC, BV/TV, and Tb.Th of the femur in OVX rats and notably decrease BS/BV and Tb.Sp; while knocking down of miR-210 obviously decreased BMD, BMC, BV/TV, Tb.Th and increased BS/BV and Tb.Sp (Fig. 2B-G). The above suggested that miR-210 could alleviate PMPO in OVX rats.
Effect of miR-210 on the microarchitecture of femoral trabeculae in ovariectomized rats. A: Representative Micro-CT images of 2D and 3D demonstrating that OVX-induced bone loss was improved by miR-210. B-G: Micro computed tomography (Micro CT) to measure bone mineral density (BMD, B), bone mineral content (BMC, C), trabecular bone volume fraction (BV/TV, D), bone surface-to-volume ratio (BS/BV, E), trabecular thickness (Tb.Th, F) and trabecular separation (Tb.Sp, G), **P < 0.01 vs. Sham group, #P < 0.05 and ##P < 0.01 vs. OVX + NC group
Effect of miR-210 on the level of bone markers in the serum of ovariectomized rats
Studies have indicated that bone turnover markers (BTM) such as BALP, PINP, OCN and CTX-1 are commonly applied for monitoring osteoporosis [24, 25]. Therefore, the levels of BALP, PINP, OCN, and CTX-1 in the serum of OVX rats were detected. And the outcomes showed that, in comparison with the Sham group, the level of BALP and CTX-1 was significantly higher (P < 0.01) while the level of PINP and OCN was obviously lower (P < 0.01) in the OVX group; the OVX + miR-210 mimic group exhibited significantly lower levels of BALP and CTX-1, while higher levels of PINP and OCN in serum compared with the OVX + NC group; and the level of BALP and CTX-1 in serum of OVX + miR-210 inhibitor group was significantly increased while the level of PINP and OCN was obviously decreased (Fig. 3A-D).
Effect of miR-210 on the level of osteogenesis-related biomarkers in the femur of ovariectomized rats
The effect of miR-210 on the expression of osteogenesis-related biomarkers such as Runt-related transcription factor 2 (Runx2), osteopontin (OPN), and collagen type I alpha 1 (COL1A1) was further assessed by Western blot. The protein level of Runx2, OPN and COL1A1 in the femur of OVX rats was obviously lower than that in the Sham group (P < 0.01); overexpression of miR-210 significantly increased the protein expression level of Runx2, OPN and COL1A1 in the femur of OVX rats, while miR-210 inhibitor markedly decreased the protein expression level of Runx2, OPN and COL1A1 (P < 0.01) (Fig. 4A/B). All of these indicated that miR-210 could significantly increase the expression of osteogenesis-related biomarkers in the femur of OVX rats.
Effect of miR-210 on VEGF/Notch1 signaling pathway in the femur of OVX rats
Wang et al. stated that miR-210 induced protein expression of VEGF and Notch1 in HUVECs cells [26], and Lou et al. also proved that miR-210 overexpression could promote angiogenesis after cerebral ischemia by upregulating Notch1 signaling molecules [27]. To explore whether miR-210 also played a protective role in OVX rats by regulating the VEGF/Notch1 pathway, the expression of proteins associated with the VEGF/Notch1 pathway was examined. The results presented that the protein level of VEGF, Notch1 and Jagged1 in the femur of rats in the OVX group was much lower compared with that in the Sham group (P < 0.01). And the protein level of VEGF, Notch1 and Jagged1 in the femur of rats in the OVX + miR-210 mimic group was significant higher than that in OVX + NC group (P < 0.01), while that in the OVX + miR-210 inhibitor group was significantly lower (P < 0.01) (Fig. 5A/B). The above findings suggested that miR-210 could activate the VEGF/Notch1 signaling pathway in the femur of OVX rats.
Discussion
Due to the increasing aging of the global population, the incidence of osteoporosis has increased substantially in recent years, and the mortality rate caused by osteoporotic fractures also reaches 15-30% [28]. Osteoporotic fractures significantly increase disability, morbidity and mortality [29]. In recent years, evidence has revealed the regulatory role of miRNAs in osteoblast growth, differentiation and function [30, 31]. BMD and BMC can effectively evaluate the degree of bone loss. Additionally, BV/TV and BS/BV can well reflect the bone mass of cancellous bone, bone cortex and the bone mass, respectively. As for Tb.Sp and Tb.Th, they are mainly adopted to assess the spatial morphological structure of bone trabeculae [32]. BALP, OCN and P1NP are markers of bone formation; CTXI is an indicator of bone resorption [24]. Runx2, OPN and COL1a1 serve as markers of osteogenesis [33]. Experimental animal models of osteoporosis are suitable tools for the study of osteoporosis, and among these models, the OVX rat model is the most commonly used [34]. In this study, a rat model of osteoporosis (OVX rat model) was also constructed by ovariectomy. OVX rats exhibited significant decreases in BMD, BMC, BV/TV, and Tb.Th, a notable increase in BS/BV and Tb.Sp in the femur, an obvious upregulation in the level of BALP and CTX-1, and a marked downregulation in the level of PINP, OCN, and the femur of Runx2, OPN, and COL1A1 protein level in the serum. These results are consistent with previous osteoporosis research [35]. The above outcomes of this study suggested that a PMPO rat model was successfully established by ovariectomy.
In this study, the expression of miR-210 was significantly downregulated in the femoral tissues of OVX rats, indicating that miR-210 may be involved in regulating osteoporosis in OVX rats. Overexpression of miR-210 significantly increased BMD, BMC, BV/TV and Tb.Th and decreased BS/BV and Tb.Sp in femurs of OVX rats; while after inhibition of miR-210, BMD, BMC, BV/TV and Tb.Th were decreased, and BS/BV and Tb.Sp were increased. Further, overexpression of miR-210 significantly decreased the level of BALP and CTX-1 in the serum of OVX rats, and significantly increased the level of PINP, OCN and the protein level of Runx2, OPN and COL1A1 in the femur, while after inhibition of miR-210, BALP and CTX-1 were increased, and PINP, OCN, Runx2, OPN and COL1A1 were decreased. These findings are consistent with previous results, such as Garmilla-Ezquerra et al. also found that the miR-210 expression in the discovery stage was close to statistical significance (p = 0.06) [35]. Meanwhile, Mizuno et al. revealed that miR-210 level was upregulated during osteoblastic differentiation, and it positively regulated osteoblastic differentiation of mouse mesenchymal ST2 cells [16]. The results above showed that miR-210 was associated with osteoblast activity in osteoporotic.
As is known to all, VEGF not only is one of the most vital molecules regulating vascular development and angiogenesis but also plays a key role in bone development [36]. Research has demonstrated that VEGF protein expression in tibial metaphysis is significantly reduced in patients with ovariectomy-induced osteoporosis [37]. The increased expression of VEGF in the periosteum and angiogenesis of osteoporotic rats may be closely related to the increased bone resorption activity in the periosteal regions [17]. Notch, as a pivotal regulator of vascular density, not only inhibits the proliferation of endothelial cells but also regulates the expression of VEGF receptors. The absence or ectopic activation of Notch signaling leads to an increase or decrease in vascular density, respectively [38]. Sun found that Notch could regulate the mineralization of osteoblasts by regulating the expression of Runx2, OPN and COL1A1 [39]. In addition, previous studies have shown that miR-210 plays a key role through VEGF/Notch signaling pathways in neuronal apoptosis in cerebral infarction [40] and angiogenesis in cerebral ischemia-reperfusion [41]. In this study, protein levels of VEGF, Notch1 and Jagged1 were significantly elevated in OVX rats with overexpression of miR-210, while after inhibition of miR-210, the above protein level was significantly decreased. It is suggested that overexpression of miR-210 may ameliorate osteoporosis in menopausal rats by activating the VEGF/ Notch1 signaling pathway.
In summary, we constructed an OVX rat model and found that miR-210 was decreased in OVX rats and its high expression could alleviate osteoporosis in postmenopausal rats. We have explored the role and mechanism of miR-210 in postmenopausal osteoporosis in vivo for the first time, but there are still the following limitations: the specific mechanism of miR-210 was obscure because only the VEGF/Notch1 signaling pathway activity was determined after knockdown or overexpression of miR-210, and it is not clear whether miR-210 also plays a role in improving osteoporosis through other signal pathways in this study. Meanwhile, we have not further verified this mechanism through pathway inhibitors. In addition, miRNAs mainly act on target genes. It’s unclear whether miR-210 can activate the VEGF/Notch signal pathway by targeting downstream target genes, thus alleviating osteoporosis. Further experiments are needed to explore and verify the above findings in order to provide a more detailed theoretical basis for miR-210 as a marker of osteoporosis.
Conclusion
In conclusion, miR-210 expression is significantly downregulated in femoral tissues of OVX rats, and its high expression improves the microstructure of bone tissue, regulates bone formation and resorption in OVX rats, and then alleviates osteoporosis. Besides, mechanistic studies suggest that it may play these roles by activating the VEGF/ Notch1 signaling pathway. MiR-210 can serve as a diagnostic and therapeutic biomarker for the treatment of osteoporosis in postmenopausal rats.
Data availability
The dataset supporting the conclusions of this article is available at our institution contacting the corresponding author.
Abbreviations
- PMPO:
-
postmenopausal osteoporosis
- OVX:
-
ovariectomized
- Micro CT:
-
Micro computed tomography
- BMD:
-
bone mineral density
- BMC:
-
bone mineral content
- BV/TV:
-
trabecular bone volume fraction
- Tb.Th:
-
trabecular thickness
- BS/BV:
-
bone surface-to-volume ratio
- Tb.Sp:
-
trabecular separation
- BALP:
-
bone alkaline phosphatase
- OCN:
-
osteocalcin
- OPN:
-
osteopontin
- COL1A1:
-
collagen type I alpha 1
- VEGF:
-
vascular endothelial growth factor
References
Rachner TD, Khosla S, Hofbauer LC. Osteoporosis: now and the future. Lancet. 2011;377(9773):1276–87.
Marcucci G, Brandi ML. Rare causes of osteoporosis. Clin Cases Miner Bone Metab. 2015;12(2):151–6.
Price CT, Koval KJ, Langford JR. Silicon: a review of its potential role in the prevention and treatment of postmenopausal osteoporosis. Int J Endocrinol. 2013;2013:316783.
Zhang C, Wang Y, Zhang CL, Wu HR. Prioritization of candidate metabolites for postmenopausal osteoporosis using multi-omics composite network. Exp Ther Med. 2019;17(4):3155–61.
Kerschan-Schindl K, Mikosch P, Obermayer-Pietsch B, Gasser RW, Dimai HP, Fahrleitner-Pammer A, et al. Current controversies in clinical management of postmenopausal osteoporosis. Exp Clin Endocrinol Diabetes. 2014;122(8):437–44.
Gambacciani M, Levancini M. Management of postmenopausal osteoporosis and the prevention of fractures. Panminerva Med. 2014;56(2):115–31.
Black DM, Rosen CJ, Clinical Practice. Postmenopausal osteoporosis. N Engl J Med. 2016;374(3):254–62.
Paik J, Scott LJ, Romosozumab. A review in postmenopausal osteoporosis. Drugs Aging. 2020;37(11):845–55.
Kanekura K, Nishi H, Isaka K, Kuroda M. MicroRNA and gynecologic cancers. J Obstet Gynaecol Res. 2016;42(6):612–7.
Kwan JY, Psarianos P, Bruce JP, Yip KW, Liu FF. The complexity of microRNAs in human cancer. J Radiat Res. 2016;57(Suppl 1):i106–11.
Li Z, Yu X, Shen J, Law PT, Chan MT, Wu WK. MicroRNA expression and its implications for diagnosis and therapy of gallbladder cancer. Oncotarget. 2015;6(16):13914–21.
Fu Y, Xu Y, Chen S, Ouyang Y, Sun G. MiR-151a-3p promotes postmenopausal osteoporosis by targeting SOCS5 and activating JAK2/STAT3 signaling. Rejuvenation Res. 2020;23(4):313–23.
Li R, Ruan Q, Yin F, Zhao K. MiR-23b-3p promotes postmenopausal osteoporosis by targeting MRC2 and regulating the Wnt/beta-catenin signaling pathway. J Pharmacol Sci. 2021;145(1):69–78.
Zhang J, Zhang T, Tang B, Li J, Zha Z. The miR-187 induced bone reconstruction and healing in a mouse model of osteoporosis, and accelerated osteoblastic differentiation of human multipotent stromal cells by targeting BARX2. Pathol Res Pract. 2021;219:153340.
Wang XJ, Liu JW, Liu J. MiR-655-3p inhibits the progression of osteoporosis by targeting LSD1 and activating BMP-2/Smad signaling pathway. Hum Exp Toxicol. 2020;39(10):1390–404.
Jiang HT, Liu L, Liu SY, Xu TT, Zhang ZP, MIzoguchi I. Mir-218-5p promotes osteoblast differentiation and inhibits osteoclast formation through the ROBO1/DKK-1 pathway during bone remodeling. J Biol Reg Homeos Ag. 2022;36(4):937–45.
Mizuno Y, Tokuzawa Y, Ninomiya Y, Yagi K, Yatsuka-Kanesaki Y, Suda T, et al. miR-210 promotes osteoblastic differentiation through inhibition of AcvR1b. FEBS Lett. 2009;583(13):2263–8.
Ivan M, Huang X. miR-210: fine-tuning the hypoxic response. Adv Exp Med Biol. 2014;772:205–27.
Fasanaro P, D’Alessandra Y, Di Stefano V, Melchionna R, Romani S, Pompilio G, et al. MicroRNA-210 modulates endothelial cell response to hypoxia and inhibits the receptor tyrosine kinase ligand Ephrin-A3. J Biol Chem. 2008;283(23):15878–83.
Liu XD, Cai F, Liu L, Zhang Y, Yang AL. MicroRNA-210 is involved in the regulation of postmenopausal osteoporosis through promotion of VEGF expression and osteoblast differentiation. Biol Chem. 2015;396(4):339–47.
Tao J, Chen S, Yang T, Dawson B, Munivez E, Bertin T, et al. Osteosclerosis owing to Notch gain of function is solely rbpj-dependent. J Bone Miner Res. 2010;25(10):2175–83.
Khedgikar V, Gautam J, Kushwaha P, Kumar A, Nagar GK, Dixit P, et al. A standardized phytopreparation from an indian medicinal plant (Dalbergia sissoo) has antiresorptive and bone-forming effects on a postmenopausal osteoporosis model of rat. Menopause. 2012;19(12):1336–46.
Gomes CC, Freitas DQ, Medeiros Araujo AM, Ramirez-Sotelo LR, Yamamoto-Silva FP, de Freitas Silva BS, et al. Effect of Alendronate on Bone microarchitecture in irradiated rats with Osteoporosis: Micro-CT and histomorphometric analysis. J Oral Maxillofac Surg. 2018;76(5):972–81.
Migliorini F, Maffulli N, Spiezia F, Tingart M, Maria PG, Riccardo G. Biomarkers as therapy monitoring for postmenopausal osteoporosis: a systematic review. J Orthop Surg Res. 2021;16(1):318.
Zhang R, Yang M, Li Y, Liu H, Ren M, Tao ZS. Effect of alendronate on the femoral metaphyseal defect under carbamazepine in ovariectomized rats. J Orthop Surg Res. 2021;16(1):14.
Wang J, Zhang Y, Xu F. Function and mechanism of microRNA-210 in acute cerebral infarction. Exp Ther Med. 2018;15(2):1263–8.
Lou YL, Guo F, Liu F, Gao FL, Zhang PQ, Niu X, et al. miR-210 activates notch signaling pathway in angiogenesis induced by cerebral ischemia. Mol Cell Biochem. 2012;370(1–2):45–51.
Gierlotka M, Zdrojewski T, Wojtyniak B, Polonski L, Stokwiszewski J, Gasior M, et al. Incidence, treatment, in-hospital mortality and one-year outcomes of acute myocardial infarction in Poland in 2009–2012–nationwide AMI-PL database. Kardiol Pol. 2015;73(3):142–58.
Johnell O, Kanis JA. An estimate of the worldwide prevalence and disability associated with osteoporotic fractures. Osteoporos Int. 2006;17(12):1726–33.
Fukao T, Fukuda Y, Kiga K, Sharif J, Hino K, Enomoto Y, et al. An evolutionarily conserved mechanism for microRNA-223 expression revealed by microRNA gene profiling. Cell. 2007;129(3):617–31.
Mizuno Y, Yagi K, Tokuzawa Y, Kanesaki-Yatsuka Y, Suda T, Katagiri T, et al. miR-125b inhibits osteoblastic differentiation by down-regulation of cell proliferation. Biochem Biophys Res Commun. 2008;368(2):267–72.
Kai C, Xiaodong H, Lingdi L, Weiming R, Dongdong W. Predictive value of Micro-CT parameters for postoperative recurrence of Osteoporotic Vertebral Compression Fractures. Imaging Sci Photochem. 2022;40(1):22–7.
Park JH, Son YJ, Lee CH, Nho CW, Yoo G. Circaea mollis Siebold & Zucc. Alleviates postmenopausal osteoporosis in a mouse model via the BMP-2/4/Runx2 pathway. BMC Complement Med Ther. 2020;20(1):123.
Yousefzadeh N, Kashfi K, Jeddi S, Ghasemi A. Ovariectomized rat model of osteoporosis: a practical guide. EXCLI J. 2020;19:89–107.
Tao ZS, Li TL, Xu HG, Yang M. Hydrogel contained valproic acid accelerates bone-defect repair via activating notch signaling pathway in ovariectomized rats. J Mater Sci Mater Med. 2021;33(1):4.
Haigh JJ. Role of VEGF in organogenesis. Organogenesis. 2008;4(4):247–56.
Maharaj AS, D’Amore PA. Roles for VEGF in the adult. Microvasc Res. 2007;74(2–3):100–13.
Suchting S, Freitas C, le Noble F, Benedito R, Breant C, Duarte A, et al. The notch ligand Delta-like 4 negatively regulates endothelial tip cell formation and vessel branching. Proc Natl Acad Sci U S A. 2007;104(9):3225–30.
Sun X. A comparative study on the regulatory mechanism of notch signaling pathway in postmenopausal osteoporosis rats by Zuo and Yougui Wan. Volume 5. Liaoning University of Traditional Chinese Medicine; 2016.
Jiang YL, Liu WW, Wang Y, Yang WY. MiR-210 suppresses neuronal apoptosis in rats with cerebral infarction through regulating VEGF-notch signaling pathway. Eur Rev Med Pharmacol Sci. 2021;25(1):2.
Xu SY, Zeng CL, Ni SM, Peng YJ. The Angiogenesis Effects of Electro-acupuncture treatment via Exosomal miR-210 in cerebral ischemia-reperfusion rats. Curr Neurovasc Res. 2022;19(1):61–72.
Acknowledgements
We thanks to the Guangdong Medical Laboratory Animal Center for providing the site for the relevant animal experiments.
Funding
This research was supported by Inner Mongolia University Science and Technology Research Project (NJZY22046) and Baotou Health Science and Technology Plan Project (wsjkwkj004).
Author information
Authors and Affiliations
Contributions
Li-Jue Ren and Xiao-Hui Zhu designed the study, conducted animal experiments, and drafted the paper. Jiu-Ting Tan, Xiang-Yu Lv, and Yan Liu collected and analyzed the data. All authors have read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This experiment was approved by Experimental animal ethics committee of Guangdong Medical Experimental Center (C202212-2). All methods were carried out in accordance with relevant guidelines and regulations. All methods are reported in accordance with ARRIVE guidelines.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no conflict of interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Ren, LJ., Zhu, XH., Tan, JT. et al. MiR-210 improves postmenopausal osteoporosis in ovariectomized rats through activating VEGF/Notch signaling pathway. BMC Musculoskelet Disord 24, 393 (2023). https://doi.org/10.1186/s12891-023-06473-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12891-023-06473-z