Abstract
Background
In this study, we try to investigate the effect of antibiotic bone cement in patients with infected diabetic foot ulcer (DFU).
Methods
This is a retrospective study, including fifty-two patients with infected DFU who had undergone treated between June 2019 and May 2021. Patients were divided into Polymethylmethacrylate (PMMA) group and control group. 22 patients in PMMA group received antibiotic bone cement and regular wound debridement, and 30 patients in control group received regular wound debridement. Clinical outcomes include the rate of wound healing, duration of healing, duration of wound preparation, rate of amputation, and frequency of debridement procedures.
Results
In PMMA group, twenty-two patients (100%) had complete wound healing. In control group, twenty-eight patients (93.3%) had wound healing. Compared with control group, PMMA group had fewer frequencies of debridement procedures and shorter duration of wound healing (35.32 ± 3.77 days vs 44.37 ± 7.44 days, P < 0.001). PMMA group had five minor amputation, while control group had eight minor amputation and two major amputation. Regarding the rate of limb salvage, there was no limb lose in PMMA group and two limb losses in control group.
Conclusion
The application of antibiotic bone cement is an effective solution for infected DFU treatment. It can effectively decreased the frequency of debridement procedures and shorten the healing duration in patients with infected DFU.
Similar content being viewed by others
Key messages
In this study, we try to investigate the effect of antibiotic bone cement in patients with infected diabetic foot ulcer. Patients treated with antibiotic bone cement were included in this retrospective study. Antibiotic bone cement treatment can effectively decreased the frequency of debridement procedures and shorten the healing duration in patients with infected DFU.
Diabetic foot ulcer (DFU) is a common complication of diabetes mellitus [1]. Impaired wound healing in diabetic patients can lead to infections, chronic ulcers with a recurrence rate of 66% and even lower extremity amputation, which significantly affects the patients’ quality of life [2].
Current treatment guidelines for DFU recommend foot wound debridement, glycemic control, infection management, revascularization, and decompression to promote healing [3]. Wound infection is a predictor of poor wound healing and amputation [4]. It can develop and spread rapidly and cause significant and irreversible tissue damage. A correct understanding of diabetic foot infection and the application of antibiotic treatment are the key to improve the efficacy. With the main advantage of high drug concentration on the target site and low risk of systemic toxicity, local antibiotic therapy acts as an effective treatment [5]. Antibiotic bone cement has been widely used in the treatment of infected arthroplasty and osteomyelitis [6, 7]. It has the dual function of preventing soft tissue contracture and delivering antibiotics locally to bone and soft tissue by elution [8]. Polymethylmethacrylate (PMMA) is the major bone cement material in the orthopedic procedures. There are few similar studies on the application of antibiotic bone cement as an effective method for infected diabetic foot ulcer. In this present study, we retrospectively reviewed our experience on the use of antibiotic bone cement for infected DFU treatment.
Materials and methods
Study design
This is a single-center, retrospective study including patients with infected DFU treated from June 2019 to May 2021. Patients who meet the following inclusion and exclusion criteria were recruited. This retrospective study was approved by our institutional review board.
Inclusion criteria
Type 2 diabetes.
An ankle-brachial index (ABI) > 0.7, and at least one of the anterior tibial artery, posterior tibial artery, and peroneal artery can reach the level of the ankle joint.
Infected diabetic foot was defined as Grade IIIB or Grade IVB according to the Texas University Classification [9] and Grade 2, 3 or 4 according to the Wagner classification [10]. Diagnosis is based on patient’s history, clinical sign, radiographic examination, laboratory evaluation and positive bacterial culture. We apply preoperative X-ray, bone biopsies and surgeon’s experience to judge whether there is osteomyelitis.
Exclusion Criteria
Patients with chronic wound due to vasculitis, pyoderma gangrenosum, pressure ulcer, or wound infections not related to DM; known or suspect malignancy of current ulcer; currently undergoing radiation or chemotherapy [11].
Patients were split into two groups based on the surgical procedure. Patients treated with regular wound debridement were defined as control group. The PMMA group included patients who received antibiotic bone cement and regular wound debridement.
Medical care
Preoperative and postoperative medical care was the same for both groups of patients, except for the different surgical procedures. Appropriate medical treatment included blood glucose regulation, perfusion improvement by prostaglandins or antiplatelet drugs, appropriate antibiotics administration, and routine sterile dressing change. Tissue samples were taken for microbiological analysis. Sensitive antibiotics were selected for intravenous application according to the results of drug susceptibility test. It was switched to oral therapy when the patients was clinically improving. During the treatment period, blood glucose was monitored daily, and oral hypoglycemic drugs, such as metformin, acarbose, etc., were used. To control blood glucose, subcutaneous injection of short-acting and long-acting insulin were applied, and the dose was dynamically adjusted until the wound healed.
Surgical procedures
For PMMA group, treatment was divided into two stages: the first to treat the diabetic wound infection and the second to reconstruct the wound defect. In debridement, we removed and debrided nonviable infected soft tissues and bones. The edges of debridement were achieved until the soft tissues and bones presented generally healthy. After thorough soft tissue and bone debridement, the wound was covered with antibiotic cement. We used PMMA (Smith & Nephew, TN, USA) premixed with gentamicin and added vancomycin to the powder (2 g vancomycin per 40 g mix) before mixing the powder and liquid. The wound was covered with gauze dressings and changed every two days.
Two weeks after PMMA implantation, we removed the antibiotic cement. The second stage surgery that reconstructing the soft tissue defect was conducted when there were no clinical signs and symptoms of infection. Otherwise, further debridement and PMMA implantation were performed. It depended on their clinical signs, laboratory evaluation and clinical experience of surgeons. The standard to decide when to perform the reconstructive procedures included that the wound was fresh enough, the bacterial culture was negative, the blood glucose was well controlled, and there was no or mild anemia, etc. Soft tissue defect was reconstructed with skin grafting, skin flap coverage or closed primarily. After wound healing, we continued to follow the patients monthly for three consecutive visits.
For control group, after primary debridement, the wound was covered with the negative pressure wound therapy system (VSD Medical Science and Technology Co. Ltd., Wuhan, China). This device promoted wound healing by removing fluid from open wounds, preparing the wound bed for closure, reducing edema, and promoting formation and perfusion of granulation tissue [12]. Patients received continuous debridement weekly until clinical signs and infectious symptoms were free. Soft tissue defect was reconstructed with skin grafting, skin flap coverage or closed primarily.
Outcomes
Clinical outcomes include the rate of wound healing, healing duration, rate of amputation, and frequency of debridement procedures. We defined the healing rate as the percentage of patients whose wounds healed at a given time point (wound size of 0 cm and Wagner score of 0 for each wound). The amputation rate is defined as the percentage of patients who lost a limb or a part of it at a given time point [13]. The indication of amputation included (1) all efforts to treat progressive diabetic foot infection remain insufficient, (2) progressive necrosis or gangrene, (3) intractable pain, and (4) acute arterial occlusion. Minor amputation is defined as below level of the ankle, which require the preservation of a functional foot to stand and walk without a prosthesis [14]. Major amputation refers to above, through or below knee loss of a limb, and represents failed limb salvage [15].The duration of healing is defined as the time from the initial surgery to complete wound healing. The duration of wound preparation is defined as the time from initial surgery to reconstructive procedures. Clinical evaluation of infection symptoms include swelling, exudate, odor, surrounding cellulitis, tissue necrosis, etc. [16].
Statistical analysis is performed with SPSS 18.0 software in this study. Data are expressed as means and standard deviations. Differences between groups are assessed with Student’s t-test or Mann–Whitney U test. The frequencies of the data are evaluated with Fisher’s exact test. The value is assumed to be significant at p-value < 0.05.
Results
After the assessment of 72 patients, 20 patients did not meet the study selection criteria and were excluded and 52 patients were included to the study (Fig. 1). The demographics data of the included study population were presented in Table 1. In this study, twenty-two patients with infected DFU (9 males and 13 females, aged 52.81 ± 9.78 years) were enrolled in PMMA group. Thirty patients with infected DFU (11 males and 19 females, aged 54.83 ± 8.64 years) were enrolled in control group. We also assessed the severity of any diabetic foot infection using the Infectious Diseases Society of America/International Working Group on the Diabetic Foot classification scheme and all included patients were moderate infection (grade 3 or grade 3(O)). There was no significant difference between the two groups in age, gender, BMI, Fasting blood glucose, HbA1c, SCr, BUN, and ABI (P > 0.05). Patients in both groups were type 2 diabetes mellitus. Table 2 presented the clinical data for each patient.
The clinical outcomes were presented in Table 3. In PMMA group, twenty-two patients (100%) had complete wound healing (Figs. 2 and 3). The mean of wound healing time was 35.32 ± 3.77 days with the average number of debridement procedures of 1.50 ± 0.51. The mean time of wound preparation in PMMA groups was 19.82 ± 5.29 days. Minor amputation was reported in five patients (22.7%). In control group, twenty-eight patients (93.3%) had wound healing. The mean duration of wound healing was 44.37 ± 7.44 days with the average number of debridement procedures of 2.13 ± 0.86. The mean time of wound preparation in control groups was 28.20 ± 7.53 days. There were eight minor amputation (26.7%) and two major amputation (6.7%) in control group. With regard to the rate of limb salvage, there was no limb lose in PMMA group and two limb loss in control group. None of patients reported ulcer recurrence in 3 months’ follow-up.
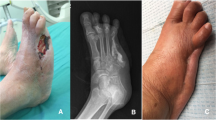
Clinical case: female, 52 years old, Wagner grade 4. A Initial wound before surgical debridement; B The nonviable, infected soft tissues and necrotic toes were debrided; C The defect was filled with antibiotic bone cement; D Antibiotic bone cement was removed after 2 weeks; E The wound was covered with skin grafting; D The wound was completely healed at follow-up
Clinical case: female, 45 years old, Wagner grade 2. A Initial wound before surgical debridement; B The nonviable and infected soft tissues were debrided; C The defect was filled with antibiotic bone cement; D Antibiotic bone cement was removed after 2 weeks; E The wound was covered with skin grafting; D The wound was completely healed at follow-up
Wound microflora pathogens isolated were presented in Table 4. Staphylococcus aureus is the most prevalent genera that isolated pathogens in both groups, followed by Escherichia coli, Enterococcus faecalis, and Enterobacter cloacae. There was no significant difference in cultivation results between both groups.
Discussion
In this retrospective study, we found that antibiotic bone cement treatment can effectively decreased frequency of debridement procedures and shorten the healing duration in patients with infected DFU. It is effective as an adjunct to extensive debridement for salvage of infected DFU and reduces the probability of amputation in patients.
Debridement involves removing all devitalised, contaminated or foreign material in or near the wound until surrounding healthy tissue is shown and it is widely used in diabetic foot care [17]. It plays a key role in infection control, and speeds the healing process in most patients with diabetic foot wounds. If progressive tissue necrosis or further deep infection occurs, surgical debridement should be repeated. Piaggesi et al. evaluated the efficacy of DFU surgical debridement compared to conventional non-surgical management. Compared with conventional treatment, surgical debridement has proved to be an effective methods for DFU patients in terms of healing time, complications, and recurrence [18].
Even with the well-established principles to managing DFU, there is still room for improvement in DFU treatment. Since the first report in 1970, PMMA-based antibiotic bone cement system has been extensively studied in the treatment of osteomyelitis and in the prevention of artificial hip/knee replacement-associated bone infection [19]. As we all known, the release of antibiotic in the antibiotic bone cement to control local infection is achieved by direct dissolution at the surface and diffusion from the bulk. In a large prospective study of DFU patients, the presence of infection was associated with a 50% increased risk of minor amputation compared to ulcer patients without infection [20]. The application of antibiotic bone cement to deliver antibiotic in patients with diabetic foot infection might be an effective adjuvant therapy.
In PMMA group, the mean of wound healing time was 35.10 ± 3.61 with the average number of debridement procedures of 1.55 ± 0.51 while the mean duration of wound healing was 44.37 ± 7.44 with the average number of debridement procedures of 2.06 ± 0.88 in control group. The mean time of wound preparation in PMMA groups was shorter when compared with it in control groups (19.82 ± 5.29 days vs 28.20 ± 7.53 days). The antibiotic bone cement treatment can effectively decreased frequency of debridement procedure and shorten healing duration in diabetic patients with foot infection. Regard with the rate of limb salvage, there was no limb lose in PMMA group and two limb losses in control group. Limb salvage according to “Recommended standards for reports dealing with lower extremity ischemia” is applicable to the treatment results of interventions aimed at avoiding major amputation [21]. It might be an effective adjunct to extensive debridement for limb salvage.
There is no reliable evidence on the priority selection of effective antibiotic type according to the existing clinical practice guidelines. Based on bacterial culture results and drug sensitivity of wound secretion, moderate and severe diabetic foot infection are typically treated from 2 to 4 weeks of intravenous antibiotic therapy with 4 to 6 weeks of bone infection treatment [22]. Once the clinical symptoms and signs of infections resolved, antibiotics can often be discontinued [23]. Usually, emergency surgery is needed to control infection. However, there is a time interval between specimen culture and pathogen identification. Therefore, it’s hard to get a cultured antibacterial spectrum before surgery. Gram-negative bacteria were more abundant in diabetic foot infection in warm-countries and the Staphylococcus is among the most prevalent genera that isolated pathogens in nearly every series in the literature [24, 25], as well as in our study. The most common mixed antibiotics are vancomycin and gentamicin. Vancomycin is a glycopeptide antibiotic that is primarily effective against gram-positive such as Staphylococcus aureus. Gentamicin is an aminoglycoside antibiotic and has broad-spectrum antimicrobial activity. The synergistic action of two antibiotics in bone cement has longer bactericidal activity than single antibiotic-loaded bone cement [26]. The coupling of a glycopeptide with an aminoglycoside covers both Gram-negative and Gram-positive bacteria [27].
Generally, it is worth noting that few studies have demonstrated the advantages of antibiotic bone cement in clinical treatment of infected DFU. Three similar studies such as Liu et al. [5], Ehya et al. [28] and Melamed et al. [6] have found the adjunctive antibiotic bone cement to improve the outcomes in surgically treated diabetic foot osteomyelitis or infected diabetic foot ulcer. Liu et al. reported that the healing duration was 13.1 ± 3.7 weeks in the PMMA group and 26.4 ± 7.8 weeks in the control group. The mean of healing time was 79.4 days (95% CI, 71–90) in the PMMA group and 101.7 days (95% CI, 93–110) in the control group in the study by Mendame. These findings are similar to our results that antibiotic bone cement can effectively shorten healing duration in patients with infected DFU. However, the average healing time in these studies are longer than ours. The prolonged wound preparation time may have contributed to the poor outcome. One of the key issues for the treatment of DFU is the prolonged wound healing time, which may have resulted from the prolonged wound preparation time. The wound preparation period is a very subjective process, and it is depended very much on the doctor’s will. In our study, it is still quite long from the beginning of the treatment to the healing. The standard to decide when to perform the reconstructive procedures included that the wound was fresh enough, the bacterial culture was negative, the blood glucose was well controlled, and there was no or mild anemia, etc. However, clinical signs such as granulation growth or freshness, wound exudation, etc. depend on the experience of the surgeon and may therefore influence the treatment options of the attending doctor. Standardization are needed to decide when to perform the reconstructive procedures.
In this study, we reviewed our experience on the management of diabetic foot infection by inclusion of patients with Texas classification IIIB and IVB or Wagner grades 2, 3 and 4 in the final analysis. The application of antibiotic bone cement on the defect from the surgical debridement of nonviable and infected soft tissue to treat infected DFU could achieve a satisfying medical outcome. The current outcomes should be assessed in light of some limitations, which mainly given that the analysis was retrospective. Larger and more prospective studies are still required to further evaluate these treatment option. In addition, all patients in the present study received vancomycin in the antibiotic bone cement. With continuous exploration and dialogue with infectious disease experts, it is more appropriate to decide which antibiotics to add according to the antibiotic sensitivity data.
During the application of antibiotic bone cement, some disadvantages should be concerned. It is worthy of note the surgeon waited for the antibiotic bone cement-mixed body temperature to drop significantly, in order to avoid exothermic heating of the surrounding tissues. In addition, the antibiotic bone cement may lead to poor wound drainage after it filled the residual dead space. We could make holes on the cement-mixed body during the last period of polymerization to promote drainage. Finally, the surgeon’s experience influences the final outcomes.
In conclusion, the application of antibiotic bone cement is an effective solution for infected DFU treatment. It can effectively decreased frequency of debridement and shorten healing duration in patients with infected DFU. However, more evidence studies are required to strengthen these conclusions.
Availability of data and materials
All data generated or analysed during this study are included in this published article.
References
Dai J, Yu M, Chen H, Chai Y. Association Between Serum 25-OH-Vitamin D and Diabetic Foot Ulcer in Patients With Type 2 Diabetes. Front Nutr. 2020;7:109.
Everett E, Mathioudakis N. Update on management of diabetic foot ulcers. Ann N Y Acad Sci. 2018;1411(1):153–65.
Alexiadou K, Doupis J. Management of diabetic foot ulcers. Diabetes Ther. 2012;3(1):4.
Acar E, Kacıra BK. Predictors of Lower Extremity Amputation and Reamputation Associated With the Diabetic Foot. J Foot Ankle Surg. 2017;56(6):1218–22.
Liu C, You JX, Chen YX, Zhu WF, Wang Y, Lv PP, Zhao F, Li HY, Li L. Effect of Induced Membrane Formation Followed by Polymethylmethacrylate Implantation on Diabetic Foot Ulcer Healing When Revascularization Is Not Feasible. J Diabetes Res. 2019;2019:2429136.
Melamed EA, Peled E. Antibiotic impregnated cement spacer for salvage of diabetic osteomyelitis. Foot Ankle Int. 2012;33(3):213–9.
Lachiewicz PF, Wellman SS, Peterson JR. Antibiotic Cement Spacers for Infected Total Knee Arthroplasties. J Am Acad Orthop Surg. 2020;28(5):180–8.
Ferrao P, Myerson MS, Schuberth JM, McCourt MJ. Cement spacer as definitive management for postoperative ankle infection. Foot Ankle Int. 2012;33(3):173–8.
Armstrong DG, Lavery LA, Harkless LB. Treatment-based classification system for assessment and care of diabetic feet. J Am Podiatr Med Assoc. 1996;86(7):311–6.
Wagner FW Jr. The dysvascular foot: a system for diagnosis and treatment. Foot Ankle. 1981;2(2):64–122.
Zhang X, Dai J, Li L, Chen H, Chai Y. NLRP3 Inflammasome Expression and Signaling in Human Diabetic Wounds and in High Glucose Induced Macrophages. J Diabetes Res. 2017;2017:5281358.
Liu S, He CZ, Cai YT, Xing QP, Guo YZ, Chen ZL, Su JL, Yang LP. Evaluation of negative-pressure wound therapy for patients with diabetic foot ulcers: systematic review and meta-analysis. Ther Clin Risk Manag. 2017;13:533–44.
Dai J, Jiang C, Sun Y, Chen H. Autologous platelet-rich plasma treatment for patients with diabetic foot ulcers: a meta-analysis of randomized studies. J Diabetes Complications. 2020;34(8):3.
Rutherford RB, Baker JD, Ernst C, Johnston KW, Porter JM, Ahn S, Jones DN. Recommended standards for reports dealing with lower extremity ischemia: revised version. J Vasc Surg. 1997;26(3):517–38.
Moxey PW, Gogalniceanu P, Hinchliffe RJ, Loftus IM, Jones KJ, Thompson MM, Holt PJ. Lower extremity amputations–a review of global variability in incidence. Diabet Med. 2011;28(10):1144–53.
Gadepalli R, Dhawan B, Sreenivas V, Kapil A, Ammini AC, Chaudhry R. A clinico-microbiological study of diabetic foot ulcers in an Indian tertiary care hospital. Diabetes Care. 2006;29(8):1727–32.
Edwards J, Stapley S. Debridement of diabetic foot ulcers. Cochrane Database Syst Rev. 2010;2010(1):CD003556.
Piaggesi A, Schipani E, Campi F, Romanelli M, Baccetti F, Arvia C, Navalesi R. Conservative surgical approach versus non-surgical management for diabetic neuropathic foot ulcers: a randomized trial. Diabet Med. 1998;15(5):412–7.
Oh EJ, Oh SH, Lee IS, Kwon OS, Lee JH. Antibiotic-eluting hydrophilized PMMA bone cement with prolonged bactericidal effect for the treatment of osteomyelitis. J Biomater Appl. 2016;30(10):1534–44.
van Battum P, Schaper N, Prompers L, Apelqvist J, Jude E, Piaggesi A, Bakker K, Edmonds M, Holstein P, Jirkovska A, et al. Differences in minor amputation rate in diabetic foot disease throughout Europe are in part explained by differences in disease severity at presentation. Diabet Med. 2011;28(2):199–205.
Kontopodis N, Tavlas E, Papadopoulos G, Pantidis D, Kafetzakis A, Chalkiadakis G, Ioannou C. Effectiveness of Platelet-Rich Plasma to Enhance Healing of Diabetic Foot Ulcers in Patients With Concomitant Peripheral Arterial Disease and Critical Limb Ischemia. Int J Low Extrem Wounds. 2016;15(1):45–51.
Lipsky BA, Senneville É, Abbas ZG, Aragón-Sánchez J, Diggle M, Embil JM, Kono S, Lavery LA, Malone M, van Asten SA, et al. Guidelines on the diagnosis and treatment of foot infection in persons with diabetes (IWGDF 2019 update). Diabetes Metab Res Rev. 2020;36(Suppl 1):e3280.
Frykberg RG, Wukich DK, Kavarthapu V, Zgonis T, Dalla Paola L. Surgery for the diabetic foot: A key component of care. Diabetes Metab Res Rev. 2020;36(Suppl 1):e3251.
Tiwari S, Pratyush DD, Dwivedi A, Gupta SK, Rai M, Singh SK. Microbiological and clinical characteristics of diabetic foot infections in northern India. J Infect Dev Ctries. 2012;6(4):329–32.
Jnana A, Muthuraman V, Varghese VK, Chakrabarty S, Murali TS, Ramachandra L, Shenoy KR, Rodrigues GS, Prasad SS, Dendukuri D, et al. Microbial Community Distribution and Core Microbiome in Successive Wound Grades of Individuals with Diabetic Foot Ulcers. Appl Environ Microbiol. 2020;86(6):e02608–19.
Warburg O. Versuche und uberledbeudem carcinomgewebe (methoden). Biochem Z. 1923;142:317–33.
Bistolfi A, Massazza G, Verné E, Massè A, Deledda D, Ferraris S, Miola M, Galetto F, Crova M. Antibiotic-loaded cement in orthopedic surgery: a review. ISRN Orthop. 2011;2011:290851.
Mendame Ehya RE, Zhang H, Qi B, Yu A. Application and Clinical Effectiveness of Antibiotic-Loaded Bone Cement to Promote Soft Tissue Granulation in the Treatment of Neuropathic Diabetic Foot Ulcers Complicated by Osteomyelitis: A Randomized Controlled Trial. J Diabetes Res. 2021;2021:9911072.
Acknowledgements
Not applicable.
Funding
Sponsorship for this study and article processing charges was supported by a grant from the Shanghai Municipal Health Commission (20204Y0430).
Author information
Authors and Affiliations
Contributions
Conceptualization, JZ Dai; Investigation, JZ Dai and Y Zhou; Methodology, Y Zhou and SS Mei; Writing – original draft, JZ Dai; Writing – review & editing, JZ Dai and H Chen. All authors have read and approved the manuscript, and ensure that this is the case.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
The studies involving human participants were reviewed and approved by the Ethic Review Board of Shanghai Six People’s Hospital affiliated to Shanghai Jiao Tong University. All methods were carried out in accordance with relevant guidelines and regulations. Consent was obtained from all subjects and/or their legal guardian(s). Informed consent was obtained from all participants involved in the study.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Dai, J., Zhou, Y., Mei, S. et al. Application of antibiotic bone cement in the treatment of infected diabetic foot ulcers in type 2 diabetes. BMC Musculoskelet Disord 24, 135 (2023). https://doi.org/10.1186/s12891-023-06244-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12891-023-06244-w