Abstract
Background
Children with idiopathic scoliosis (IS) have a high risk of osteoporosis and IS with low bone mineral density (BMD) are susceptible to curve progression. This review aims to explore the risk factors of low BMD in children with IS.
Methods
Studies were retrieved from 5 databases that were published up to January 2022. Search terms are keywords in titles or abstracts, including subject headings related to “Scoliosis”, “Bone Mineral Density”, and “Risk Factors”. Observational studies on risk factors of low BMD in children with IS were enrolled in this review. The number of studies, sample size, outcome measures, research type, endocrine, and lifestyle-related factors, gene/signal pathway, and other contents were extracted for qualitative analysis.
Results
A total of 56 studies were included in this scoping review. Thirty studies involved genetic factors that may affect BMD, including the Vitamin-D receptor gene, RANK/RANKL signal pathway, the function of mesenchymal stem cells, Runx2, Interleukin-6 (IL-6), and miR-145/β-catenin pathway. Eight studies mentioned the influence of endocrine factors on BMD, and the results showed that serum levels of IL-6, leptin and its metabolites, and ghrelin in children with IS were different from the age-matched controls. In addition, there were 18 articles on lifestyle-related factors related to low BMD in children with IS, consisting of physical activity, calcium intake, Vitamin D level, and body composition.
Conclusions
Genetic, endocrine, and lifestyle-related factors might relate to low BMD and even osteoporosis in IS. To prevent osteoporosis, the effectiveness of regular screening for low BMD risk factors in children with IS needs to be investigated. Additionally, clear risk factors suggest strategies for bone intervention. Future studies should consider the effectiveness of calcium and vitamin D supplements and physical activity in BMD improvement.
Similar content being viewed by others
Background
Idiopathic scoliosis (IS) is the most common type of scoliosis, defined as a three-dimensional structural deformity of the spine, and can be diagnosed when the Cobb angle is ≥ 10 [1]. The prevalence of IS varies widely worldwide, ranging from 0.59% to 12% [2, 3]. A meta-analysis has demonstrated that the peak age of IS in China is 14 to 15 years old, and the prevalence of IS in females is higher than in males [4]. Children with IS show a generalized low bone mineral density (BMD), which brings complications such as pain, fracture tendency, skeletal deformity, and abnormal bone development [5,6,7]. These problems greatly threaten the appearance and school activities of children with IS, but low BMD may be ignored in clinical practice because people focus on correcting deformity [8].
The risk of curve progression in IS children with low BMD was twice as high as those with normal BMD, suggesting that BMD may be one of the important prognostic factors for IS [9, 10]. Low BMD results in changes in bone mechanical properties, such as increased fragility and microfractures of vertebrae, and may lead to spinal asymmetry then [11]. IS children with low BMD also showed impaired ossification and bone growth, which may be associated with inconsistent degrees of curvature at different spinal sites, affecting the spinal structure and leading to the progression of IS [12].
There are many clinical interventions for children with IS to correct spinal deformity, including conservative and surgical protocols [13, 14]. However, therapeutic exercise, bracing, or surgical treatment effectiveness is strongly associated with BMD [15, 16]. For example, the mechanism of the brace is based on the external force that prevents the spine from collapsing, but the correction effect may be altered due to low BMD in children with IS [15, 17]. Some studies also suggested that surgical intervention was not recommended for IS children with severe osteoporosis, as low BMD is related to unstable spinal fusion [18]. As a result, ignorance of low BMD may influence the effectiveness of spinal curve correction and may even lead to intervention failure [19].
Osteoporosis is diagnosed when BMD is 2 or 2.5 standard deviations (SD) below the age and gender-matched standardized mean value. The prevalence of osteoporosis is reported to be higher in children with IS than in healthy controls [20]. Low BMD or osteoporosis in children with IS may be related to multiple factors, including abnormalities of bone metabolism regulated by congenital genetic defects [21] or endocrine factors [22]. As an issue closely related to living habits, low BMD may be mediated by lifestyle-related factors [23]. Low BMD in children with IS may be related to a variety of factors, while there is no sound theory. Although genetics, endocrines, and lifestyle-related factors are currently hotspots in the research area of bone metabolism in children with IS, no study summarizes the potential risk factors related to low BMD in children with IS. The summary of risk factors related to low BMD may contribute to preventing osteoporosis in children with IS and provide implications for future research regarding the intervention on osteoporosis in children with IS. Therefore, this scoping review aims to summarize the existing literature on the genetics, endocrines, and lifestyle factors related to low BMD in children with IS.
Methods
This scoping review was conducted under the guidance of Preferred Reporting Items for Systematic reviews and Meta-Analyses extension for Scoping Reviews (PRISMA-ScR) [24] and followed steps including study retrieval, evidence screening, and evaluation, data extraction, and synthesis of findings.
Stage 1 Study retrieval
Studies were limited to English and Chinese language and published before January 2022. One reviewer searched PubMed, Web of Science Core Collection, Embase, Cochrane Library, and Cumulative Index to Nursing and Allied Health Literature (CINAHL). Completed initial search for articles by January 20th, 2022. We retrieved risk factors for osteoporosis in children with IS using keywords or subject headings, including “idiopathic scoliosis”, “IS”, “bone mineral density”, “BMD”, “risk factor”, and their synonyms.
Stage 2 Evidence screening and evaluating
A comprehensive screening was conducted for the retrieved articles. Three reviewers (Y.Y., Z.C., Z.H.) screened the titles and abstracts of the retrieved articles independently to determine their relevance to the topic and assess their eligibility. They would check the full text of an article if necessary. Any disagreement would be solved by a discussion with a fourth reviewer (X.Z.). Eligibility criteria are shown in Table 1.
Stage 3 Quality assessment
Methodological quality was assessed in terms of different study types. To evaluate the risk of bias in randomized controlled trials (RCTs) and clinical controlled trials, we utilized version 2 of the Cochrane risk-of-bias tool for randomized trials (RoB 2) [25]. Rating case–control studies using the Newcastle–Ottawa Scale (NOS) [26] and the assessment of multiple systematic reviews (AMSTAR 2) for systematic reviews [27]. The NOS uses a semi-quantitative assessment of the star system to measure the quality of the study. It is divided into three sections: Selection, Comparability, and Exposure, with a total score of nine. Y.Y. and Z.C. evaluated the risk of bias in the retrieved articles independently, and disputes were discussed and weighed by Q.D.
Stage 4 Data extraction and synthesis
The data extraction was conducted by three reviewers (Y.Y., Z.C., Z.H.) and the results of the preliminary extracted data would be submitted to a fourth reviewer (Q.D.) for re-examining. Any differences would be further discussed to ensure consistency among reviewers. We extracted data from the enrolled articles, including author, publication year, number of publications, sample size, and risk factors related to low BMD (such as genetic, endocrine, or lifestyle-related factors). A qualitative approach was employed to describe and synthesize the risk factors because of the methodological heterogeneity of the included studies.
Results
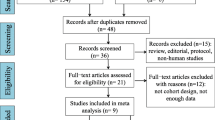
A total of 1498 records, including four from the reference lists, were first retrieved from the five databases. After removing 419 duplicated and 1019 unrelated studies, we finally included 56. There were 30 studies [6, 28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56] on genetic risk factors related to low BMD in children with IS, eight studies on endocrine risk factors [57,58,59,60,61,62,63,64], and 18 studies [5, 65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81] on lifestyle-related risk factors. A total of 10,768 participants with IS were included in the 56 included studies, consisting of 295 male patients with IS in 14 studies [30, 31, 33, 38, 40, 43, 44, 48, 51, 56, 63,64,65,66].
Tables S1, S2, and S3 show our assessment of the risk of bias. The overall bias of RCTs and clinical controlled trials was between some concerns and high. According to the methodological quality evaluation results of AMSTAR 2, three systematic reviews have critically low overall confidence. The risk of bias score of 50 case–control studies ranged from five to nine points, with 12 studies scoring nine points, indicating the quality of included studies was generally good.
Part I: Bone mineral density
We found five studies [82,83,84,85,86] investigating the prevalence of osteoporosis in children with idiopathic scoliosis (IS). The results showed that the prevalence of osteoporosis in children with IS was 4.1% to 28.1%, with a median prevalence of 12.2%. In children without IS, the prevalence of osteoporosis ranged from 0% to 3.7% with a median prevalence of 2.1%.
Part II: Genetic factors
Table 2 shows the possible genetic mechanisms underlying low BMD in children with IS. BsmI is an important factor in BMD, which is made up of two nucleotide variants A-G in the genotype of the vitamin D receptor (VDR) gene's eight introns [50]. Our results showed that low BMD might be correlated to VDR BsmI rs1544410 polymorphism in Asian children with IS [6, 28, 31, 50]. Osteoprotegerin (OPG) is a receptor activator of nuclear factor kappa-B ligand (RANKL) decoy receptor that prevents RANKL from binding to its receptor receptor activator of nuclear factor kappa-B (RANK), preventing osteoclast development and activation [40]. Ten included studies [30, 32, 36, 40, 42, 44, 48, 51, 52, 54] showed that G → C mutation in OPG gene site 1181 and T single nucleotide polymorphism in adiponectin gene site rs7639352 might be associated with low BMD. In addition, BMD in children with IS may be decreased by the knockdown or overexpression of miR-145/β-catenin via inhibiting osteocyte function. Low BMD in AIS may be related to the dropped osteogenic differentiation ability in the mesenchymal stem cells (MSCs) mechanism [39, 55, 56]. Three studies showed that low Runx2 expression might be related to low BMD [43,44,45]. G-c polymorphism at the -174 and -572 sites of the Interleukin-6 (IL-6) gene promoter was associated with low BMD, and adiponectin may be a mediator in this pathway of bone development [33, 34, 51].
The genes related to low BMD in children with IS may consist of WNT16 [29], LBX1 in muscle and bone [37], or the insulin-like growth factor-I gene [49]. Our results also showed a potential relationship between low BMD and genetic variants of CHD7 [47], estrogen receptor gene polymorphism [46], or single nucleotide polymorphism [38].
Part III: Endocrine factors
Table 3 shows the influence of endocrine factors on BMD in children with IS. Many endocrine factors mediate bone development and our results showed that the endocrine factors associated with low BMD in children with IS consisted of serum leptin and soluble leptin receptor (SLR) [57, 59,60,61,62], sclerostin [58], ghrelin [62], and IL-6 [63]. Nontargeted plasma metabolite analysis also showed that there might be an association between oxoglutarate, L-arginine arginine, N-acetylaspartate, citrate, and bone mass in children with IS [64].
Part IV: Lifestyle-related factors
Lifestyle-related factors related to BMD include serum vitamin D/25-hydroxyvitamin D (vitamin D/25(OH)D) [65,66,67,68, 72, 73], calcium intake [5, 69, 72, 73, 75,76,77,78,79, 81], physical activity [69, 72,73,74, 76, 77, 79, 80], grip strength [70], whole-body vibration [71]. Although no significant differences in diary calcium intake between the IS group and the control group [5, 69, 72, 73, 75,76,77,78,79, 81], calcium intake and serum vitamin D/25(OH)D levels may be correlated with low BMD [5, 65,66,67,68,69, 72, 73, 75,76,77,78,79, 81]. Improving grip strength may bring extra benefits to BMD in children with IS [70]. But the effect of whole-body vibration on BMD in children with IS needs further exploration [71]. The influence of lifestyle-related factors on bone health in children with IS is shown in Table 4.
Discussion
In this study, we investigated the possible risk factors, including genetic, endocrine, and lifestyle-related factors of low BMD in children with IS. A total of 6 genetic factors are mentioned, including the VDR gene, RANK/RANKL signal pathway, the function of MSCs, Runx2, IL-6 related gene, and miR-145/β-catenin pathway. Endocrine factors included serum leptin and SLR, sclerostin, ghrelin, IL-6, and some nontargeted plasma metabolites, which may be associated with low BMD in children with IS. In addition, lifestyle-related factors such as vitamin D level, calcium intake, physical activity, and body composition also affect bone health in children with IS.
Bone mineral density
There might be a trend that the BMD of children with IS is significantly lower than that of healthy individuals [85, 87]. In a general way, BMD lagging the same age and gender population by 1 to 2 SD was defined as osteopenia, while a lag beyond 2 or 2.5 SD was defined as osteoporosis [88]. Notably, the prevalence of osteoporosis in children with IS was much higher than in healthy controls, suggesting that osteoporosis may be a comorbidity in IS patients involved in the onset or progress of IS. From this perspective, this review extensively retrieves multiple factors that provide evidence for osteoporosis in children with IS. However, one study showed no significant differences in femoral neck BMD Z-score between children with IS and the control group [89]. The possible reason for the difference is that in this study, the control group was children who needed orthopedic surgery and might also have low BMD.
Genetic factors
VDR is a ligand-dependent nuclear transcription factor [90]. The VDR gene is located on chromosome 12q13 and can rapidly mediate the function of vitamin D [31]. It has been reported that VDR gene polymorphism was related to serum vitamin D concentration, and BsmI site polymorphism had an important impact on VDR activity [91]. Existing studies suggest that the frequency of the Bb phenotype at the BsmI site of the VDR gene is significantly increased in children with IS, but whether this is the cause of low BMD is still controversial.
RANKL activates osteoclasts by binding to RANK molecules on the cell membrane of osteoclasts, while OPG competes with RANK to bind RANKL to inhibit the function of osteoclasts and their precursors [92, 93]. The balance of RANKL/RANK and OPG is vital in chronic arthritis, hormone-induced osteoporosis, etc. The serum RANKL concentration in children with scoliosis is abnormally increased, while the OPG level is similar to that of the average population. When the ratio of RANKL/RANK to OPG is unbalanced, osteoclasts are excessively activated, and bone resorption is enhanced, ultimately leading to a decrease in BMD. It was also found that the expression of Mir-145 and adiponectin was up-regulated in IS, the former inhibiting the production of OPG while the latter stimulating the production of RANKL [94].
MSCs play a key role in bone growth, they can differentiate into osteoprogenitor cells and cartilage progenitor cells and can affect osteoclast function by regulating RANKL and OPG [95]. At present, it is believed that the osteogenic differentiation ability of MSCs in children with IS is decreased may be due to various reasons. For example, the expression of pyruvate kinase isoenzyme M2 (PKM2) in stem cells is up-regulated, which inhibits cell differentiation [55]. ERK1/2 signaling is activated by the downregulation of heat shock protein HSP70, which promotes osteogenic differentiation and proliferation of MSCs [56]. Additionally, Runx2 is a key transcription regulator of osteoblast differentiation and maturation, and its downregulation also inhibits the osteogenic differentiation of MSCs [96].
In bone tissue, IL-6 is produced by osteoblasts, monocytes, and T cells and plays a prominent role in bone metabolism by promoting osteoclastogenesis and osteoclast activity. In the IL-6 gene promoter region, the -174G/C polymorphism and the -572G/C polymorphism alters levels of plasma IL-6. Because the promoter activity with the C allele is 40% lower than that with the G allele, osteoporosis is common in the GG phenotypic population [97]. The -572G allele was shown to be much more common in the Asian IS population, while the -174 locus polymorphism was found to be more prevalent in Europeans [97].
Endocrine factors
Leptin is involved in bone remodeling through direct or indirect means, acting on the development of bone diseases such as osteoporosis [98]. Studies proposed that low serum leptin and low SLR levels might related to low BMD [59, 62]. Leptin is produced by white adipocytes, and binds to leptin receptors on the membranes of osteoblasts and chondrocytes, stimulating signaling pathways such as JAK2, STAT3, and MAPK to affect bone cell development and differentiation [99]. Leptin regulates the hypothalamic-pituitary function and affects the levels of thyroid hormone, growth hormone, cortisol, and steroids, and further regulates bone metabolism [99]. The possible mechanism of leptin in regulating BMD may be through the OPG/RANKL signaling pathway that enhances osteoblasts and inhibits osteoclastogenesis [100]. In contrast, the SLR binds to circulating leptin and suppresses signaling pathways [101]. At present, it is believed that the mechanism of leptin impacting BMD in children with IS may be multi-faceted, including insufficient leptin secretion, abnormal expression of SLR, and low sensitivity of bone tissue to leptin signaling [102].
IL-6 affects the differentiation and function of osteoclasts, and studies have shown that IL-6 is significantly associated with hip BMD in women with insufficient calcium intake [103]. IL-6 can be produced directly in cells by lipopolysaccharide, which stimulates IL-6 by inducing a series of intermediate cytokines IL-1 and tumor necrosis factor (TNF) [104]. Excessive secretion of IL-6 may be one of the essential factors causing low BMD in children with IS [63].
Lifestyle-related factors
Identifying the underlying risk factor of osteoporosis in children or teenagers and eliminating risk factors that lead to low BMD should be the initial steps in treatment. Bisphosphonates have been demonstrated to have therapeutic effects on osteoporosis in children by inhibiting the mevalonate pathway, but their safety is unknown. Potential side effects include inhibiting cartilage production [105], raising the chance of atypical femoral bone [106], and interfering with tooth eruption [107].
Nutrition is the most effective means of optimizing peak BMD [108]. The putative mechanism by which calcium intake affects bone health is through increased BMD, which also increases muscle and increases activity [109]. While the median calcium intake of children with IS was 517.7 mg/d, which was only 50% of the reference intake [69]. Vitamin D and calcium supplements are interventions for low BMD patients [110].
Exercise reduces the production of inflammatory factors such as IL-6 and TNF-a and increases the secretion of cytokines that inhibit bone resorption, such as IL-2 and IL-10 [111]. Studies showed that girls with IS may be more reluctant to exercise than other children [69]. Long-term weight-bearing physical activity programs have been proven to improve BMD and structural characteristics without causing harm, and the benefits last over time [112]. A healthy diet and regular exercise are preventive factors against osteoporosis in children with IS. Children with IS, on the other hand, have low body fat and lean body weight. Although the link between this inclination and low BMD has yet to be established, we believe that a balanced protein and fat consumption will benefit the skeletal development of children with IS [113].
Factors connection
The association between genetics, endocrines, and lifestyle factors may help to understand the pathogenesis of osteoporosis in children with IS.
Approximately 60–80% of the variation in bone structure was caused by genetic factors and developed before adolescence [114]. Genetic factors may regulate BMD by influencing hormone secretion and hormone receptors related to bone synthesis and absorption [33]. For example, the variation of IL-6 related genes may affect the BMD via the endocrine pathway in children with IS because IL-6 is a critical cytokine that promotes bone resorption. A study showed that G-c polymorphism at the -174 sites of the IL-6 gene promoter in children with IS might be associated with serum IL-6 levels [33]. When IL-6 is excessively secreted, it will disrupt the balance of bone metabolism and cause low BMD in children with IS [63]. In addition, VDR BsmI polymorphism plays a key role in bone metabolism regulation [50]. The VDR gene codifies instructions for making VDR protein respond to 25(OH)D [115]. After combining with 25(OH)D, VDR regulates the proliferation and differentiation of osteoblasts, osteoclasts, and chondrocytes [90]. Mutations in the VDR gene may lead to abnormal expression or non-expression of VDR [28]. For those children with IS who have deficits in the VDR gene, low BMD may be detected even if serum 25(OH)D is in the normal range [6].
Some lifestyles will disrupt the endocrine balance, leading to bone synthesis or absorption disorders. Leptin levels are positively correlated with BMD in children with IS [59]. Studies have shown that physical activity, especially moderate-vigorous physical activity, may promotes the body's responsiveness to leptin [116, 117]. Long-term pro-inflammatory dietary patterns like trans-hydrogenated fats and fried food can lead to inflammation [118]. One study showed that people who have taken pro-inflammatory food for a long time had a lower level of the lumbar spine and total hip BMD than those who have a healthy diet [119]. One of the possible explanations may be that long-term pro-inflammatory dietary patterns may stimulate excessive secretion of IL-6, which reduces the BMD of children with IS.
Clinical implications
A comprehensive screening protocol should be designed based on the risk factors related to low BMD found in this review because a wide-ranged screening may be an effective step to improve bone strength and prognosis. The screening protocol may include risk gene analysis (e.g., VDR gene and IL-6 gene), endocrine test (e.g., serum leptin and SLR, sclerostin, ghrelin, and IL-6), and review of daily living (e.g., nutrition and physical activities). Lifestyle-related factors might be appropriate interventional targets for low BMD in children with IS, as there are many rehabilitation programs for lifestyle modification. Future studies may be needed to determine which type and frequency of physical activity are best for improving BMD in children with IS. Calcium and vitamin D are necessary for treating low BMD, and daily calcium and vitamin D intakes for children with IS needs to be investigated.
Osteoporosis refers to people with low BMD, altered bone microarchitecture, increased fragility, and fracture risk [120]. This definition is consistent with the effects of the three factors summarized in this review. However, according to the latest criteria on diagnosing osteoporosis in children and adolescents, a history of clinically meaningful fractures is mandatory, in addition to a low BMD score of less than -2.0. Otherwise, the children should present a nontraumatic vertebral compression fracture [121]. Our included articles have insufficient information to draw relationships between children with IS and osteoporosis. Although it is clear that these risk factors are associated with low BMD and changes in bone quality in children with IS. More research on this population and osteoporosis is needed in the future.
Conclusions
This scoping review showed that BMD was generally lower in children with IS than in asymptomatic controls. Genetic, endocrine, and lifestyle-related factors might be associated with low BMD in children with IS. Bone synthesis or absorption may be directly regulated by endocrine factors, while genetic and lifestyle-related factors may influence BMD via the endocrine pathway. Comprehensive screening for low BMD risk factors may be reasonable to prevent osteoporosis and progression in children with IS. Future studies may consider targeted intervention protocols, including lifestyle modifications, to improve the low BMD in children with IS.
Availability of data and materials
The datasets used and/or analyzed during the current scoping review are available from the corresponding authors on reasonable request.
Abbreviations
- IS:
-
Idiopathic scoliosis
- BMD:
-
Bone mineral density
- IL-6:
-
Interleukin-6
- SD:
-
Standard deviation
- PRISMA-ScR:
-
Preferred Reporting Items for Systematic reviews and Meta-Analyses extension for Scoping Reviews
- CINAHL:
-
Cumulative Index to Nursing and Allied Health Literature
- RCTs:
-
Randomized controlled trials
- RoB 2:
-
Version 2 of the Cochrane risk-of-bias tool for randomized trials
- NOS:
-
Newcastle–Ottawa Scale
- AMSTAR 2:
-
Assessment of multiple systematic reviews
- VDR:
-
Vitamin D receptor
- OPG:
-
Osteoprotegerin
- RANKL:
-
Receptor activator of nuclear factor kappa-B ligand
- RANK:
-
Receptor activator of nuclear factor kappa-B
- MSCs:
-
mesenchymal stem cells
- SLR:
-
soluble leptin receptor
- vitamin D/25(OH)D:
-
vitamin D/25-hydroxyvitamin D
- PKM2:
-
pyruvate kinase isoenzyme M2
- TNF:
-
tumor necrosis factor
References
Cheng JC, Castelein RM, Chu WC, Danielsson AJ, Dobbs MB, Grivas TB, et al. Adolescent idiopathic scoliosis. Nature Rev Dis Primers. 2015;1:15030.
Negrini S, Donzelli S, Aulisa AG, Czaprowski D, Schreiber S, de Mauroy JC, et al. 2016 SOSORT guidelines: orthopaedic and rehabilitation treatment of idiopathic scoliosis during growth. Scoliosis Spinal Disord. 2018;13(1):1–48.
Wong HK, Hui JH, Rajan U, Chia HP. Idiopathic scoliosis in Singapore school children: a prevalence study 15 years into the screening program. Spine (Phila Pa 1976). 2005;30(10):1188–96.
Zhang H, Guo C, Tang M, Liu S, Li J, Guo Q, et al. Prevalence of scoliosis among primary and middle school students in Mainland China: a systematic review and meta-analysis. Spine (Phila Pa 1976). 2015;40(1):41–9.
Cheung CSK, Lee WTK, Tse YK, Lee KM, Guo X, Qin L, et al. Generalized osteopenia in adolescent idiopathic scoliosis- association with abnormal pubertal growth, bone turnover, and calcium intake? Spine (Phila Pa 1976). 2006;31(3):330–8.
Suh KT, Eun IS, Lee JS. Polymorphism in vitamin D receptor is associated with bone mineral density in patients with adolescent idiopathic scoliosis. Eur Spine J. 2010;19(9):1545–50.
Cheuk KY, Zhu TY, Yu FW, Hung VW, Lee KM, Qin L, et al. Abnormal bone mechanical and structural properties in adolescent idiopathic scoliosis: a study with finite element analysis and structural model index. Calcif Tissue Int. 2015;97(4):343–52.
Lam TP, Ng BK, Cheung LW, Lee KM, Qin L, Cheng JC. Effect of whole body vibration (WBV) therapy on bone density and bone quality in osteopenic girls with adolescent idiopathic scoliosis: a randomized, controlled trial. Osteoporos Int. 2013;24(5):1623–36.
Yip BH, Yu FW, Wang Z, Hung VW, Lam TP, Ng BK, et al. Prognostic value of bone mineral density on curve progression: a longitudinal cohort study of 513 girls with adolescent idiopathic scoliosis. Sci Rep. 2016;6:39220.
Hung VWY, Qin L, Cheung CSK, Lam TP, Ng BKW, Tse YK, et al. Osteopenia: a new prognostic factor of curve progression in adolescent idiopathic scoliosis. J Bone Joint Surg Am. 2005;87(12):2709–16.
Zhao F-D, Pollintine P, Hole B, Adams M, Dolan P. Vertebral fractures usually affect the cranial endplate because it is thinner and supported by less-dense trabecular bone. Bone. 2009;44(2):372–9.
Wang WJ, Hung VWY, Lam TP, Ng BKW, Qin L, Lee KM, et al. The association of disproportionate skeletal growth and abnormal radius dimension ratio with curve severity in adolescent idiopathic scoliosis. Eur Spine J. 2010;19(5):726–31.
Monticone M, Ambrosini E, Cazzaniga D, Rocca B, Ferrante S. Active self-correction and task-oriented exercises reduce spinal deformity and improve quality of life in subjects with mild adolescent idiopathic scoliosis. Results of a randomised controlled trial. Eur Spine J. 2014;23(6):1204–14.
Lotan S, Kalichman L. Manual therapy treatment for adolescent idiopathic scoliosis. J Bodyw Mov Ther. 2019;23(1):189–93.
El Hawary R, Zaaroor-Regev D, Floman Y, Lonner BS, Alkhalife YI, Betz RR. Brace treatment in adolescent idiopathic scoliosis: risk factors for failure—a literature review. Spine J. 2019;19(12):1917–25.
Ammann P, Rizzoli R. Bone strength and its determinants. Osteoporos Int. 2003;14(3):13–8.
Smit TH. Adolescent idiopathic scoliosis: the mechanobiology of differential growth. JOR spine. 2020;3(4):e1115.
FitzPatrick SK, Casemyr NE, Zurakowski D, Day CS, Rozental TD. The effect of osteoporosis on outcomes of operatively treated distal radius fractures. J Hand Surg. 2012;37(10):2027–34.
Sun X, Wu T, Liu Z, Zhu Z, Qian B, Zhu F, et al. Osteopenia predicts curve progression of adolescent idiopathic scoliosis in girls treated with brace treatment. J Pediatr Orthop. 2013;33(4):366–71.
Cheng JC, Hung VW, Lee WT, Yeung HY, Lam TP, Ng BK, et al. Persistent osteopenia in adolescent idiopathic scoliosis–longitudinal monitoring of bone mineral density until skeletal maturity. Stud Health Technol Inform. 2006;123:47–51.
Xu F, Li W, Yang X, Na L, Chen L, Liu G. The roles of epigenetics regulation in bone metabolism and osteoporosis. Front Cell Develop Biol. 2021;8:619301.
Zhang W, Yang G-J, Wu S-X, Li D-Q, Xu Y-B, Ma C-H, et al. The guiding role of bone metabolism test in osteoporosis treatment. Am J Clin Exp Immunol. 2018;7(2):40.
Wang Y, Wang L, Sun Y, Wu M, Ma Y, Yang L, et al. Prediction model for the risk of osteoporosis incorporating factors of disease history and living habits in physical examination of population in Chongqing, Southwest China: based on artificial neural network. BMC Public Health. 2021;21(1):1–10.
Tricco AC, Lillie E, Zarin W, O’Brien KK, Colquhoun H, Levac D, et al. PRISMA extension for scoping reviews (PRISMA-ScR): checklist and explanation. Ann Intern Med. 2018;169(7):467–73.
Sterne JA, Savović J, Page MJ, Elbers RG, Blencowe NS, Boutron I, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:14898.
Peterson J, Welch V, Losos M, Tugwell P. The Newcastle Ottawa scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. Ottawa: Ottawa Hosp Res Inst. 2011;2(1):1–12.
Shea BJ, Reeves BC, Wells G, Thuku M, Hamel C, Moran J, et al. AMSTAR 2: a critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. BMJ. 2017;358:j4008.
Chen WJ, Qiu Y, Zhu F, Zhu ZZ, Sun X, Liu Z, et al. Vitamin D receptor gene polymorphisms: no association with low bone mineral density in adolescent idiopathic scoliosis girls. Chin J Surg. 2008;46(15):1183–6.
Cheng KL, Li QQ, Wang Y, Zhang J, Lam TP, Hung A, et al. Lower WNT16 expression in patients with adolescent idiopathic scoliosis - potential link to lower bone mass in AIS? Stud Health Technol Inform. 2021;280:23–8.
Chiru M. Adolescent idiopathic scoliosis and osteopenia. Maedica. 2011;6(1):17–22.
Dai J, Lv ZT, Huang JM, Cheng P, Fang H, Chen AM. Association between polymorphisms in vitamin D receptor gene and adolescent idiopathic scoliosis: a meta-analysis. Eur Spine J. 2018;27(9):2175–83.
Eun IS, Park WW, Suh KT, Kim JI, Lee JS. Association between osteoprotegerin gene polymorphism and bone mineral density in patients with adolescent idiopathic scoliosis. Eur Spine J. 2009;18(12):1936–40.
Gao J, Zhang L, Liu Z, Yao S, Gao S. Correlation analysis between interleukin 6 polymorphism and adolescent idiopathic scoliosis susceptibility and bracing effectiveness. Chin J Rep Reconstr Surg. 2018;32(6):678–84.
Lee JS, Suh KT, Eun IS. Polymorphism in interleukin-6 gene is associated with bone mineral density in patients with adolescent idiopathic scoliosis. J Bone Joint Surg Br. 2010;92(8):1118–22.
Lee WYW, Zhang J, Wang Y, Leung RKK, Lam TP, Qiu Y, et al. miR-145 overexpression impairs osteocytes structure and function in Adolescent Idiopathic Scoliosis. J Bone Miner Res. 2017;32:S352.
Liu Z, Qiu Y, Wang B, Ma W, Zhu F, Zhu Z, et al. The relationship between RANKL/OPG and the decreased bone mass in adolescent idiopathic scoliosis patients. Stud Health Technol Inform. 2008;140:345.
Man GCW, Tang NLS, Lee WYW, Zhang J, Ng BKW, Xu L, et al. Expression of LBX1 in muscle and bone of adolescent idiopathic scoliosis in Chinese. J Orthop Res. 2017;35(S1):S1.
Moon ES, Kim HS, Sharma V, Park JO, Lee HM, Moon SH, et al. Analysis of single nucleotide polymorphism in adolescent idiopathic scoliosis in Korea: for personalized treatment. Yonsei Med J. 2013;54(2):500–9.
Park WW, Suh KT, Kim JI, Kim SJ, Lee JS. Decreased osteogenic differentiation of mesenchymal stem cells and reduced bone mineral density in patients with adolescent idiopathic scoliosis. Eur Spine J. 2009;18(12):1920–6.
Popa O, Anton MC, Vladoiu S, Manda D, Ianas O. Osteocalcin, OPG and RANKL circulating levels in adolescent idiopathic scoliosis. Endocr Abstr. 2010;22:P106.
Qiu XS, Tang NL, Yeung HY, Qiu Y, Cheng JC. Genetic association study of growth hormone receptor and idiopathic scoliosis. Clin Orthop Relat Res. 2007;462:53–8.
Suh KT, Lee SS, Hwang SH, Kim SJ, Lee JS. Elevated soluble receptor activator of nuclear factor-κB ligand and reduced bone mineral density in patients with adolescent idiopathic scoliosis. Eur Spine J. 2007;16(10):1563–9.
Sun C, Qiu Y, Yin G, Shu H, Liu Z, Wang XH, et al. Abnormal expression and significance of Runx2 in osteoblasts of adolescent idiopathic scoliosis patients. Chin J Surg. 2009;47(19):1495–8.
Sun C, Yin G, Yeung H, Tang NLS, Cheng JCY, Qiu Y. Abnormal expression of Runx2, RANKL and osteoprotegerin in osteoblasts from adolescent idiopathic scoliosis. Stud Health Technol Inform. 2010;158:196.
Wang WJ, Sun C, Liu Z, Sun X, Zhu F, Zhu ZZ, et al. Transcription factor runx2 in the low bone mineral density of girls with adolescent idiopathic scoliosis. Orthop Surg. 2014;6(1):8–14.
Wu J, Qiu Y, Zhang L, Sun Y, Chen X. Association of estrogen receptor gene polymorphisms with bone mineral density in adolescent idiopathic scoliosis. Osteoporos Int. 2007;18:S69–70.
Wu Z, Dai Z, Yuwen W, Liu Z, Qiu Y, Cheng JC, et al. Genetic variants of CHD7 are associated with adolescent idiopathic scoliosis. Spine (Phila Pa 1976). 2021;46(11):E618-e24.
Xiao L, Zhang H, Wang Y, Li J, Yang G, Wang L, et al. Dysregulation of the ghrelin/RANKL/OPG pathway in bone mass is related to AIS osteopenia. Bone. 2020;134:115291.
Yeung HY, Tang NL, Lee KM, Ng BK, Hung VW, Kwok R, et al. Genetic association study of insulin-like growth factor-I (IGF-I) gene with curve severity and osteopenia in adolescent idiopathic scoliosis. Stud Health Technol Inform. 2006;123:18–24.
Yin X, Wang HD, Guo JD, Zhang L, Zhang YP, Li L, et al. Association of vitamin D receptor BsmI rs1544410 and ApaI rs7975232 polymorphisms with susceptibility to adolescent idiopathic scoliosis a systematic review and meta-analysis. Med. 2018;97(2):e9627.
Zhang HQ, Wang LJ, Liu SH, Li J, Xiao LG, Yang GT. Adiponectin regulates bone mass in AIS osteopenia via RANKL/OPG and IL6 pathway. J Trans Med. 2019;17:64.
Zhang J, Lee WYW, Chen H, Tam EMS, Man GC, Lam TP, et al. Dysfunctional osteogenic and osteocytic activity in adolescent idiopathic scoliosis (AIS). Scol Spinal Disord. 2017;12(Suppl 1):17.
Zhang JJ, Chen HX, Leung RKK, Choy KW, Lam TP, Ng BKW, et al. Aberrant miR-145-5p/-catenin signal impairs osteocyte function in adolescent idiopathic scoliosis. FASEB J. 2018;32(12):6537–49.
Zhou S, Wang WJ, Zhu ZZ, Sun X, Zhu F, Yu Y, et al. Increased expression of receptor activator of nuclear factor-kappa B ligand in osteoblasts from adolescent idiopathic scoliosis patients with low bone mineral density. J Huazhong Univ Sci Technolog Med Sci. 2012;32(5):686–90.
Zhuang Q, Li J, Wu Z, Zhang J, Sun W, Li T, et al. Differential proteome analysis of bone marrow mesenchymal stem cells from adolescent idiopathic scoliosis patients. PLoS One. 2011;6(4):18834.
Zhuang Q, Mao W, Xu P, Li H, Sun Z, Li S, et al. Identification of differential genes expression profiles and pathways of bone marrow mesenchymal stem cells of adolescent idiopathic scoliosis patients by microarray and integrated gene network analysis. Spine (Phila Pa 1976). 2016;41(10):840–55.
Lam EMS, Lee W, Cheuk KY, Lam TP, Ng BKW, Lee SKM, et al. Morphological and bone strength indices in girls with adolescent idiopathic scoliosis and their correlations with leptin and soluble leptin receptor. Scoliosis. 2015;10:1.
Lee WYW, Chen H, Cheuk KY, Tam EMS, Lam TP, Ng BKW, et al. How does serum sclerostin level correlate with bone mass and bone quality in adolescent idiopathic scoliosis? J Orthop Res. 2017;35:1755.
Qiu Y, Sun X, Qiu X, Li W, Zhu Z, Zhu F, et al. Decreased circulating leptin level and its association with body and bone mass in girls with adolescent idiopathic scoliosis. Spine(Phila Pa 1976). 2007;32(24):2703–10.
Tam EM, Yu FW, Hung VW, Liu Z, Liu KL, Ng BK, et al. Are volumetric bone mineral density and bone micro-architecture associated with leptin and soluble leptin receptor levels in adolescent idiopathic scoliosis?–A case-control study. PLoS ONE. 2014;9(2):e87939.
Tam EMS, Yu FWP, Hung VWY, Yu WS, Liu Z, Lam TP, et al. Correlation of morphological and bone strength indices with serum leptin and soluble leptin receptor in girls with adolescent idiopathic scoliosis: a pilot study. Osteoporos Int. 2013;24:S631–2.
Wang Q, Wang C, Hu W, Hu F, Liu W, Zhang X. Disordered leptin and ghrelin bioactivity in adolescent idiopathic scoliosis (AIS): a systematic review and meta-analysis. J Orthop Surg Res. 2020;15(1):1–9.
Wu J, Qiu Y, Zhang L, Sun YF, Chen X. Changes of bone mineral density in association with serum interleukin-6 in adolescent idiopathic scoliosis. Chin J Clin Rehab. 2005;9(10):223–5.
Xiao L, Yang G, Zhang H, Liu J, Guo C, Sun Y. Nontargeted metabolomic analysis of plasma metabolite changes in patients with adolescent idiopathic scoliosis. Mediators Inflamm. 2021;2021:5537811.
Alsiddiky A, Alfadhil R, Al-aqel M, Ababtain N, Almajed N, Bakarman K, et al. Assessment of serum vitamin D levels in surgical adolescent idiopathic scoliosis patients. BMC Pediatr. 2020;20(1):1–5.
Balioglu MB, Aydin C, Kargin D, Albayrak A, Atici Y, Tas SK, et al. Vitamin-D measurement in patients with adolescent idiopathic scoliosis. J Pediatr Orthop-Part B. 2017;26(1):48–52.
Batista R, Martins DE, Hayashi LF, Lazaretti-Castro M, Puertas EB, Wajchenberg M. Association between vitamin D serum levels and adolescent idiopathic scoliosis. Scoliosis. 2014;9:1.
Catan L, Cerbu S, Amaricai E, Suciu O, Horhat DI, Popoiu CM, et al. Assessment of static plantar pressure, stabilometry, vitamin d and bone mineral density in female adolescents with moderate idiopathic scoliosis. Int J Environ Res Public Health. 2020;17(6):2167.
Cheng JCY, Lau JTF, Ho S, Guo X. Nutrition and physical activity as possible factors affecting bone mineral status in adolescent idiopathic scoliosis: cross-sectional and case-control studies. Hong Kong Med J. 2007;13(3):33–5.
Cheuk KY, Hung VWY, Yu FWP, Wong LLN, Lee WYW, Cheng JCY, et al. Unique correlation pattern between bone qualities and handgrip strength in adolescent idiopathic scoliosis (AIS) girls. Scol Spinal Disord. 2018;13(Suppl 1):O12.
Christen P, Marano G, Wayne Lee YW, Lam TP, Müller R. Whole body vibration therapy triggers load-driven bone formation in adolescents with idiopathic scoliosis. J Bone Miner Res. 2017;32:S187.
Lam TP, Ng BKW, Mak QWY, Tam EMS, Lee KM, Qin L, et al. Vitamin D level and its correlation with bone mineral density in girls with Adolescent Idiopathic Scoliosis (AIS). Osteoporos Int. 2013;24(Suppl 4):S640.
Lam TP, Wah Ng BK, Lee KM, Hung ALH, Tam EMS, Cheung FTF, et al. Serum 25 (OH) vitamin D level and its correlation with bone mineral density in girls with adolescent idiopathic scoliosis (AIS). Scoliosis. 2015;10:1.
Lau RW, Cheuk KY, Ng BK, Tam EM, Hung AL, Cheng JC, et al. Effects of a home-based exercise intervention (E-Fit) on bone density, muscle function, and quality of life in girls with Adolescent Idiopathic Scoliosis (AIS): a pilot randomized controlled trial. Int J Environ Res Public Health. 2021;18(20):10899.
Lee WT, Cheng JC, Cheung CS, Guo X. Inadequate calcium intake is a significant determinant on generalised osteopenia in Hong Kong Chinese adolescents with idiopathic scoliosis. J Hyg Res. 2003;32(6):568–72.
Lee WT, Cheung CS, Tse YK, Guo X, Qin L, Ho SC, et al. Generalized low bone mass of girls with adolescent idiopathic scoliosis is related to inadequate calcium intake and weight bearing physical activity in peripubertal period. Osteoporos Int. 2005;16(9):1024–35.
Lee WTK, Cheung CSK, Chau WW, Tse YK, Qin L, Cheng JCY. Poor calcium intake and inadequate physical activity are associated with generalized osteopenia throughout puberty in adolescents with idiopathic scoliosis (AIS). Osteoporos Int. 2006;17:403–4.
Lee WTK, Cheung CSK, Chau WW, Tse YK, Qin L, Cheng JCY. Systemic osteopenia in adolescent idiopathic scoliosis (AIS) is associated with increased bone turnover, growth disturbance and inadequate calcium intake - a study of 900 adolescent girls. Osteo Int. 2006;17:432.
Normand E, Franco A, Parent S, Moreau A, Marcil V. Metabolic, anthropometric and nutritional profile of girls with adolescent idiopathic scoliosis: a pilot study. J Bone Mineral Res. 2018;33:143.
Tobias JH, Fairbank J, Harding I, Taylor HJ, Clark EM. Association between physical activity and scoliosis: a prospective cohort study. Int J Epidemiol. 2019;48(4):1152–60.
Yang G, Lam TP, Pang H, Yip B-K, Lee WY, Hung A-H, et al. A six years longitudinal cohort study on the changes in bone density and bone quality up to peak bone mass in adolescent idiopathic scoliosis with and without 2 years of calcium and vitamin d supplementation. J Bone Miner Res. 2020;35(SUPPL 1):78–9.
Du Q, Zhou X, Li JA, He XH, Liang JP, Zhao L, et al. Quantitative ultrasound measurements of bone quality in female adolescents with idiopathic scoliosis compared to normal controls. J Manipulative Physiol Ther. 2015;38(6):434–41.
Sadat-Ali M, Al-Othman A, Bubshait D, Al-Dakheel D. Does scoliosis causes low bone mass? A comparative study between siblings. Eur Spine J. 2008;17(7):944–7.
Szalay EA, Bosch P, Schwend RM, Buggie B, Tandberg D, Sherman F. Adolescents with idiopathic scoliosis are not osteoporotic. Spine (Phila Pa 1976). 2008;33(7):802–6.
Tahvildari BP, Erfani MA, Nouraei H, Sadeghian M. Evaluation of bone mineral status in adolescent idiopathic scoliosis. Clin Orthop Surg. 2014;6(2):180–4.
Cheng JC, Qin L, Cheung CS, Sher AH, Lee KM, Ng SW, et al. Generalized low areal and volumetric bone mineral density in adolescent idiopathic scoliosis. J Bone Miner Res. 2000;15(8):1587–95.
Li XF, Li H, Liu ZD, Dai LY. Low bone mineral status in adolescent idiopathic scoliosis. Eur Spine J. 2008;17(11):1431–40.
Karaguzel G, Holick MF. Diagnosis and treatment of osteopenia. Rev Endocr Metab Disord. 2010;11(4):237–51.
Wang Z, Chen H, Yu YE, Zhang J, Cheuk KY, Ng BK, et al. Unique local bone tissue characteristics in iliac crest bone biopsy from adolescent idiopathic scoliosis with severe spinal deformity. Sci Rep. 2017;7:40265.
MacDonald PN, Baudino TA, Tokumaru H, Dowd DR, Zhang C. Vitamin D receptor and nuclear receptor coactivators: crucial interactions in vitamin D-mediated transcription. Steroids. 2001;66(3–5):171–6.
Wang TJ, Zhang F, Richards JB, Kestenbaum B, van Meurs JB, Berry D, et al. Common genetic determinants of vitamin D insufficiency: a genome-wide association study. Lancet. 2010;376(9736):180–8.
Baek KH, Oh KW, Lee WY, Tae HJ, Rhee EJ, Han JH, et al. Changes in the serum sex steroids, IL-7 and RANKL-OPG system after bone marrow transplantation: influences on bone and mineral metabolism. Bone. 2006;39(6):1352–60.
García Palacios V, Robinson LJ, Borysenko CW, Lehmann T, Kalla SE, Blair HC. Negative regulation of RANKL-induced osteoclastic differentiation in RAW264.7 Cells by estrogen and phytoestrogens. J Biol Chem. 2005;280(14):13720–7.
Fu Y, Hu X, Gao Y, Li K, Fu Q, Liu Q, et al. LncRNA ROR/miR-145-5p axis modulates the osteoblasts proliferation and apoptosis in osteoporosis. Bioengineered. 2021;12(1):7714–23.
Bielby R, Jones E, McGonagle D. The role of mesenchymal stem cells in maintenance and repair of bone. Injury. 2007;38(Suppl 1):S26-32.
Komori T. Runx2, an inducer of osteoblast and chondrocyte differentiation. Histochem Cell Biol. 2018;149(4):313–23.
Wang Z, Yang Y, He M, Wang R, Ma J, Zhang Y, et al. Association between interleukin-6 gene polymorphisms and bone mineral density: a meta-analysis. Genet Test Mol Biomarkers. 2013;17(12):898–909.
Chen XX, Yang T. Roles of leptin in bone metabolism and bone diseases. J Bone Miner Metab. 2015;33(5):474–85.
Reid IR, Baldock PA, Cornish J. Effects of leptin on the skeleton. Endocr Rev. 2018;39(6):938–59.
Lateef M, Baig M, Azhar A. Serum Leptin and Bone Turnover Markers in Postmenopausal Osteoporosis. Topics in Osteoporosis [Internet]. 2013. Available from: http://dx.doi.org/10.5772/54527.
Rodrigo C, Tennekoon KH, Karunanayake EH, De Silva K, Amarasinghe I, Wijayasiri A. Circulating leptin, soluble leptin receptor, free leptin index, visfatin and selected leptin and leptin receptor gene polymorphisms in sporadic breast cancer. Endocr J. 2017;64(4):393–401.
Liang G, Gao W, Liang A, Ye W, Peng Y, Zhang L, et al. Normal leptin expression, lower adipogenic ability, decreased leptin receptor and hyposensitivity to Leptin in adolescent idiopathic scoliosis. PLoS ONE. 2012;7(5):e36648.
Czerny B, Kaminski A, Kurzawski M, Kotrych D, Safranow K, Dziedziejko V, et al. The association of IL-1β, IL-2, and IL-6 gene polymorphisms with bone mineral density and osteoporosis in postmenopausal women. Eur J Obstet Gynecol Reprod Biol. 2010;149(1):82–5.
Rachoń D, Myśliwska J, Suchecka-Rachoń K, Semetkowska-Jurkiewicz B, Zorena K, Łysiak-Szydłowska W. Serum interleukin-6 levels and bone mineral density at the femoral neck in post-menopausal women with type 1 diabetes. Diabet Med. 2003;20(6):475–80.
Battaglia S, Dumoucel S, Chesneau J, Heymann MF, Picarda G, Gouin F, et al. Impact of oncopediatric dosing regimen of zoledronic acid on bone growth: preclinical studies and case report of an osteosarcoma pediatric patient. J Bone Miner Res. 2011;26(10):2439–51.
Aspenberg P, Schilcher J. Atypical femoral fractures, bisphosphonates, and mechanical stress. Curr Osteoporos Rep. 2014;12(2):189–93.
Lézot F, Chesneau J, Navet B, Gobin B, Amiaud J, Choi Y, et al. Skeletal consequences of RANKL-blocking antibody (IK22-5) injections during growth: mouse strain disparities and synergic effect with zoledronic acid. Bone. 2015;73:51–9.
Cromer B, Harel Z. Adolescents: at increased risk for osteoporosis? Clin Pediatr. 2000;39(10):565–74.
Ondrak KS, Morgan DW. Physical activity, calcium intake and bone health in children and adolescents. Sports Med. 2007;37(7):587–600.
Ferrari S, Bianchi ML, Eisman JA, Foldes AJ, Adami S, Wahl DA, et al. Osteoporosis in young adults: pathophysiology, diagnosis, and management. Osteoporos Int. 2012;23(12):2735–48.
Yuan Y, Chen X, Zhang L, Wu J, Guo J, Zou D, et al. The roles of exercise in bone remodeling and in prevention and treatment of osteoporosis. Prog Biophys Mol Biol. 2016;122(2):122–30.
Gómez-Bruton A, Matute-Llorente Á, González-Agüero A, Casajús JA, Vicente-Rodríguez G. Plyometric exercise and bone health in children and adolescents: a systematic review. World J Pediatr. 2017;13(2):112–21.
Miyagi M, Saito W, Imura T, Nakazawa T, Shirasawa E, Kawakubo A, et al. Body composition in Japanese girls with adolescent idiopathic scoliosis. Spine Surg Relat Res. 2021;5(2):68–74.
Hartman C, Hochberg Z, Shamir R. Osteoporosis in pediatrics. IMAJ-RAMAT GAN-. 2003;5(7):509–15.
Bahrami A, Sadeghnia HR, Tabatabaeizadeh SA, Bahrami-Taghanaki H, Behboodi N, Esmaeili H, et al. Genetic and epigenetic factors influencing vitamin D status. J Cell Physiol. 2018;233(5):4033–43.
Alessa HB, Chomistek AK, Hankinson SE, Barnett JB, Rood J, Matthews CE, et al. objective measures of physical activity and cardiometabolic and endocrine biomarkers. Med Sci Sports Exerc. 2017;49(9):1817–25.
Blüher S, Panagiotou G, Petroff D, Markert J, Wagner A, Klemm T, et al. Effects of a 1-year exercise and lifestyle intervention on irisin, adipokines, and inflammatory markers in obese children. Obesity. 2014;22(7):1701–8.
Bartolozzi E. The natural approach to osteoporosis. Clin Cases Miner Bone Metab. 2015;12(2):111–5.
Fang Y, Zhu J, Fan J, Sun L, Cai S, Fan C, et al. Dietary Inflammatory Index in relation to bone mineral density, osteoporosis risk and fracture risk: a systematic review and meta-analysis. Osteoporos Int. 2021;32(4):633–43.
Bianchi ML. Osteoporosis in children and adolescents. Bone. 2007;41(4):486–95.
Bishop N, Arundel P, Clark E, Dimitri P, Farr J, Jones G, et al. Fracture prediction and the definition of osteoporosis in children and adolescents: the ISCD 2013 Pediatric Official Positions. J Clin Densitom. 2014;17(2):275–80.
Acknowledgements
Not applicable.
Funding
This work was supported by the General Program of National Natural Science Foundation of China (81972030), Three-year Action Plan of Shanghai Municipality to Further Accelerate the Inheritance, Innovation and Development of Traditional Chinese Medicine (2021–2023) [ZY(2021- 2023)- 0201–05] and Clinical Research Plan of SHDC (SHDC 2020CR3041B).
Author information
Authors and Affiliations
Contributions
Q.D. and X.Z. conceived and supervised the scoping review and led the drafting. Y.Y., Z.C., and Z.H. retrieved, screened the articles, and extracted the data. All the authors involved in the drafting and approved the manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1. Table S1.
Risk of bias Assessmentby Newcastle-Ottawa Scale for Case-Control Studies. Table S2. Risk of BiasAssessment by RoB 2 for Randomized Controlled Trials. Table S3. Risk of BiasAssessment by AMSTAR 2 for Systematic Reviews.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Yang, Y., Chen, Z., Huang, Z. et al. Risk factors associated with low bone mineral density in children with idiopathic scoliosis: a scoping review. BMC Musculoskelet Disord 24, 48 (2023). https://doi.org/10.1186/s12891-023-06157-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12891-023-06157-8