Abstract
Background
Low back pain and sleep disturbance are common health problems worldwide which are also commonly observed among people after natural disasters. These symptoms are well known to coexist, and recent reports have indicated that sleep disturbance is a risk factor for low back pain. However, the influence of low back pain on sleep disturbance has rarely been assessed; therefore, this study aimed to clarify the association of low back pain with sleep disturbance, especially focusing on the frequency of low back pain, using 3-year cohort data after the Great East Japan Earthquake.
Methods
This study used the data obtained from people living in the disaster-affected areas after the Great East Japan Earthquake (n = 2,097). Low back pain and sleep disturbance were assessed at 4, 5, 6, and 7 years after the disaster. The frequency of low back pain was defined as the number of low back pain episodes at and before the evaluation time point and categorized into five groups such as absence, 1, 2, 3, and 4 at the fourth time point and four groups such as absence, 1, 2, and 3 at the third time point. Multivariate logistic regression analyses were conducted to assess the association of low back pain with sleep disturbance.
Results
Low back pain was significantly associated with sleep disturbance, and the association was stronger in participants with more frequent low back pain (adjusted odds ratios [95% confidence intervals],1.46 [1.10–1.95] in “1”; 2.02 [1.49–2.74] in “2”; 2.38 [1.67–3.40] in “3”; and 4.08 [2.74–6.06] in “4” in the frequency of low back pain) (P for trend < 0.001). Furthermore, antecedent low back pain was significantly associated with new-onset sleep disturbance, and the association was robust in more frequent low back pain (adjusted odds ratios [95% confidence intervals],1.60 [1.05–2.44] in “1”; 1.96 [1.20–3.21] in “2”; and 2.17 [1.14–4.14] in “3” in the frequency of low back pain) (P for trend = 0.007).
Conclusion
Our study showed that low back pain is strongly associated with sleep disturbance. Attention should be paid to low back pain to prevent and treat sleep disturbance, especially focusing on chronicity of low back pain.
Similar content being viewed by others
Background
Sleep disturbance is a common health problem worldwide. [1, 2] Sleep disturbance has been reported to often coexist with pain, [3, 4] and the association between sleep disturbance and pain has garnered attention. Low back pain (LBP) is one of the most common musculoskeletal pains, and its association with sleep disturbance has also been reported. Previous cross-sectional studies have shown that patients with LBP commonly complain of sleep disturbance, and patients with sleep disturbance have severe LBP. [5, 6] When considering the causal relationship between sleep disturbance and LBP, some longitudinal studies have further shown their association. [7,8,9,10,11,12,13,14] Most of these studies have assessed the influence of sleep disturbance on LBP and have shown that sleep disturbance is a risk factor for LBP and a predictor of poor recovery from LBP. [7,8,9, 11,12,13,14] In contrast, a previous study showed that antecedent LBP caused sleep disturbance. [10] Therefore, association between sleep disturbance and LBP is considered to be bidirectional; however, the influence of LBP on sleep disturbance has been rarely assessed and is unclear.
Moreover, sleep disturbance and LBP are common health problems after natural disasters. [15, 16] The Great East Japan Earthquake (GEJE) hit the northeastern coastal areas of Japan on March 11, 2011. [17] After the GEJE, a high prevalence of LBP and sleep disturbance was reported, [18, 19] and sleep disturbance was associated with the continuation and new onset of LBP in a dose-dependent manner. [20, 21] However, the influence of LBP on sleep disturbance has not been investigated after natural disasters. Clarifying the association between LBP and sleep disturbance is crucial in developing prevention or treatment strategies for them. This study aimed to elucidate the association between LBP and sleep disturbance, especially focusing on the frequency of LBP and association of antecedent LBP with new-onset sleep disturbance using 3-year cohort data after the GEJE.
Materials and methods
Study design and participants
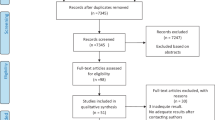
The present study used one part of the data of a cohort study conducted among people living in disaster-affected areas after the GEJE, such as Ogatsu, Oshika, and Ajishima areas in Ishinomaki City and Wakabayashi ward in Sendai City, Japan. [15, 18, 22] This cohort aimed to assess the mental and physical health conditions of the people living in these areas and to support them. It has been started 3 months after the GEJE and has been continued annually. The initial population of the cohort included all people living in the three areas in Ishinomaki City and people living in the prefabricated houses in Wakabayashi ward in Sendai City. To assess the association between LBP and sleep disturbance, the present study used the 3-year longitudinal study data of people (18 years or over) at 4 (defined as the first time point), 5 (second time point), 6 (third time point), and 7 (fourth time point) years after the GEJE because follow-up rates in these periods were comparatively high. Individuals who had participated in the previous survey were recruited. Self-reported questionnaires and informed consent forms were mailed to the participants (n = 4,324). The number of questionnaire responders at the first time point was 3,032 (70.1%), and the response rates at the second, third, and fourth time points were 86.9% (2,635/3,032), 89.6% (2,361/2,635), and 89.8% (2,119/2,361), respectively. Individuals with missing data on sleep conditions at the third or fourth time point were excluded (n = 22) because sleep disturbance at these time points were used for the analyses, and 2,097 were included in the present study (Fig. 1).
Outcome variable (sleep disturbance)
Participants’ sleep conditions were assessed using the Athens Insomnia Scale (AIS). The AIS consists of eight questions, with each question rated from 0 to 3 (0, no problem at all; 3, very serious problem). Sleep disturbance was defined as a score of ≥ 6/24 on the AIS. [23] We used the information on sleep conditions at the fourth time point to assess the association between LBP and sleep disturbance and sleep conditions at the third and fourth time points to assess the influence of antecedent LBP on the onset of sleep disturbance.
Main predictor (LBP)
LBP was assessed using a self-report questionnaire at four time points. Participants were asked if they had LBP in the last few days; they were classified into “absence” or “presence” of LBP groups at each time point. The frequency of LBP at the third time point was defined as the number of “presence” of LBP at the first, second, and third time points and categorized into four groups: absence, 1, 2, and 3. Furthermore, the frequency of LBP at the fourth time point was defined as the number of “presence” of LBP at the first, second, third, and fourth time points and was categorized into five groups: absence, 1, 2, 3, and 4.
Covariates
The following variables at the third or fourth time points were included in the analysis as covariates because they were considered potential confounding factors: sex, age, body mass index, living area, smoking and drinking habits, comorbid conditions, working status, walking time per day, living status, economic conditions, psychological conditions, and social network. Psychological conditions were assessed using the Kessler Psychological Distress Scale-6 (K-6), which consists of six mental health questions rated from 0 to 4. Psychological distress was defined as a score of ≥ 10/24 on the K-6. [24] Social network was assessed using the Lubben Social Network Scale-6 (LSNS-6), which consists of six social network questions rated from 0 to 5. Social isolation was defined as a score of < 12/30 on the LSNS-6. [25] These variables were categorized as shown in Table 1.
Statistical analysis
The chi-square test was used to compare covariates due to the absence or presence of LBP at the fourth time point. The association between LBP and sleep disturbance was assessed using crude and multivariate logistic regression analyses, and odds ratios (ORs) and 95% confidence intervals (95% CIs) were calculated. The outcome of interest was sleep disturbance at the fourth time point. First, the main predictor was set as LBP and its frequency at the fourth time point to assess the association between LBP and sleep disturbance. The variables at the fourth time point were used as covariates in the analysis. Second, participants without sleep disturbance at the third time point were selected, and the main predictor was set as LBP and its frequency at the third time point to assess the influence of antecedent LBP on the onset of sleep disturbance. The variables at the third time point were used as covariates in the analysis. SPSS (version 24.0: IBM Corp., Armonk, NY) was used for the analyses, and a P-value of < 0.05 was considered significant.
Results
The prevalence of LBP at each time point was 26.3% (551/2,097), 25.1% (526/2,097), 26.6% (558/1,097), and 26.8% (561/2,097) at the first, second, third, and fourth time points, respectively. The prevalence of sleep disturbance at the third and fourth time points was 34.0% (712/2,097) and 33.2% (697/2,097), respectively. The baseline characteristics of the participants at the fourth time point are listed in Table 1. Participants with LBP were more likely to be smokers and drinkers and have comorbid conditions such as hypertension and myocardial infarction, poor economic conditions, psychological distress, and social isolation. LBP was significantly associated with sleep disturbance at the fourth time point, and the adjusted OR (95% CI) was 2.21 (1.76–2.77), using the absence of LBP as a reference (P < 0.001) (Table 2). Furthermore, LBP frequency was significantly associated with sleep disturbance, and the adjusted ORs (95% CIs) were 1.46 (1.10–1.95) in “1,” 2.02 (1.49–2.74) in “2,” 2.38 (1.67–3.40) in “3,” and 4.08 (2.74–6.06) in “4” in the frequency of LBP at the fourth time point, using the absence of LBP as a reference (P for trend < 0.001) (Table 3).
Among the participants without sleep disturbance at the third time point, the prevalence of the onset of sleep disturbance at the fourth time point was 12.3% (171/1,385). LBP at the third time point was significantly associated with the onset of sleep disturbance at the fourth time point, and the adjusted OR (95% CI) was 1.83 (1.24–2.69), using the absence of LBP as a reference (P = 0.002) (Table 4). Additionally, the frequency of LBP at the third time point was significantly associated with the onset of sleep disturbance at the fourth time point, and the adjusted ORs (95% CIs) were 1.60 (1.05–2.44) in “1,” 1.96 (1.20–3.21) in “2,” and 2.17 (1.14–4.14) in “3” in the frequency of LBP at the third time point, using the absence of LBP as a reference (P for trend = 0.007) (Table 5).
Discussion
The present study revealed that LBP was associated with sleep disturbance, and this association was robust in participants with more frequent LBP episodes. Furthermore, antecedent LBP was associated with the onset of sleep disturbance, and the influence was stronger in participants with more frequent LBP episodes.
Sleep disturbance has been reported to coexist with LBP. [5, 6, 26, 27] Most of these studies have assessed sleep disturbance in patients with LBP. People with chronic LBP tend to have difficulty initiating sleep, reduced sleeping time, and lower sleep efficiency. [28] The rate of sleep disturbance among patients with chronic LBP was reported to be 50–60%. [6, 26] In the present study, 48.5% of the participants with LBP had sleep disturbance, even though LBP was not limited to chronic LBP; however, the rate was higher compared to 27.7% among the participants without LBP. Moreover, the association between LBP and sleep disturbances was significant after adjusting for potential confounding factors. Although several factors such as sex, age, and psychological and socioeconomic factors are associated with both LBP and sleep disturbance, [29,30,31,32] LBP is considered to be independently associated with sleep disturbance. In addition, previous studies have shown that LBP intensity correlates with the severity of sleep disturbance. [27, 33] These studies indicated that LBP and sleep disturbance are associated in a dose-dependent manner. Therefore, it is speculated that the frequency of LBP further affects the association between LBP and sleep disturbance. However, to the best of our knowledge, no study has assessed the association between LBP and sleep disturbance due to the frequency of LBP. The present study clearly showed that the association between LBP and sleep disturbance is stronger in patients with more frequent LBP. LBP is associated with sleep disturbance, and this association is considered robust among people with chronic LBP.
Regarding the causal relationship between LBP and sleep disturbance, some longitudinal studies have shown that antecedent sleep disturbance is associated with the onset of LBP among healthcare workers, [14] firefighters, [8] people after a natural disaster, [21] and the general population. [9] Additionally, other reports have shown that sleep disturbance is associated with poor recovery from pain in patients with LBP. [7, 12, 13] These reports indicate that sleep disturbance is a risk factor for LBP. However, only a few longitudinal studies have assessed the influence of LBP on sleep disturbances. Morelhão et al. reported that high LBP intensity in older patients was associated with poor sleep quality 6 months later. [10] The present study also showed that antecedent LBP was significantly associated with the onset of sleep disturbance 1 year later, even after adjustment for potential confounding factors. Although there have been only a few reports assessing the influence of pain on sleep disturbance, previous studies have shown that prior pain severity predicts subsequent sleep disturbance among patients with rheumatoid arthritis or orofacial pain. [34, 35] Furthermore, people with musculoskeletal pain, including LBP, would have a higher rate of sleep disturbance compared with those without the pain 1 year later. [19, 36] Regarding the influence of pain on sleep disturbance, it is hypothesized that pain prevents the initiation or continuation of sleep. [37] In addition, brain structure controlling nociception modulates sleep states, [38] and pain and sleep disturbance can occur due to a common neurobiological dysfunction. [37] A previous longitudinal study showed that preceding sleep disturbance was associated with onset of LBP, and the effect was stronger along with longer duration and increased frequency of sleep disturbance. [21] Conversely, the present study is the first to show that the influence of antecedent LBP on the onset of sleep disturbance is robust in participants with more frequent LBP. LBP leads to the onset of sleep disturbance in a dose-dependent manner, such as intensity [10] and frequency of LBP, as shown in this study. Sleep disturbance is considered a prospective symptom among people with LBP, and the onset of sleep disturbance can lead to poor recovery from LBP, [7, 13] and this interaction is assumed to be stronger dose-dependently. Understanding the mutual relationship between LBP and sleep disturbance is critical to prevent and treat these symptoms, especially focusing on chronicity.
This study has some limitations. First, LBP was assessed using a self-reported questionnaire, and pain was not quantified. The intensity of LBP is considered to affect sleep disturbance, which should be examined in future studies. Second, LBP and sleep disturbance were assessed at four time points over 3 years, and those for periods other than these time points were not clear, which may have affected the results. Finally, participants were people living in areas affected by the GEJE. Although 4 years have passed since the GEJE, the effects of the disaster may remain, and the generalizability of the results of the present study is not clarified.
In conclusion, LBP was associated with sleep disturbance among people living in places affected by the GEJE, and the association was robust in those with more frequent LBP. Furthermore, antecedent LBP was associated with the onset of sleep disturbance, and the effect was stronger in patients with more frequent LBP.
Availability of data and materials
All data generated or analyzed during this study are not publicly available due to ethical concerns, but are available from the corresponding author on reasonable request.
Change history
10 January 2023
A Correction to this paper has been published: https://doi.org/10.1186/s12891-023-06141-2
Abbreviations
- LBP:
-
Low back pain
- GEJE:
-
Great East Japan Earthquake
- OR:
-
Odds ratio
- 95% CI:
-
95% confidence interval
References
Liu X, Uchiyama M, Kim K, et al. Sleep loss and daytime sleepiness in the general adult population of Japan. Psychiatry Res. 2000;93:1–11.
Morphy H, Dunn KM, Lewis M, et al. Epidemiology of insomnia: a longitudinal study in a UK population. Sleep. 2007;30:274–80.
Finan PH, Goodin BR, Smith MT. The association of sleep and pain: an update and a path forward. J Pain. 2013;14:1539–52.
Lautenbacher S, Kundermann B, Krieg JC. Sleep deprivation and pain perception. Sleep Med Rev. 2006;10:357–69.
van de Water AT, Eadie J, Hurley DA. Investigation of sleep disturbance in chronic low back pain: an age- and gender-matched case-control study over a 7-night period. Man Ther. 2011;16:550–6.
Tang NK, Wright KJ, Salkovskis PM. Prevalence and correlates of clinical insomnia co-occurring with chronic back pain. J Sleep Res. 2007;16:85–95.
Alsaadi SM, McAuley JH, Hush JM, et al. Poor sleep quality is strongly associated with subsequent pain intensity in patients with acute low back pain. Arthritis Rheumatol. 2014;66:1388–94.
Lusa S, Miranda H, Luukkonen R, et al. Sleep disturbances predict long-term changes in low back pain among finnish firefighters: 13-year follow-up study. Int Arch Occup Environ Health. 2015;88:369–79.
Mork PJ, Vik KL, Moe B, et al. Sleep problems, exercise and obesity and risk of chronic musculoskeletal pain: the norwegian HUNT study. Eur J Public Health. 2014;24:924–9.
Morelhão PK, Gobbi C, Christofaro DGD, et al. Bidirectional association between sleep quality and low back pain in older adults: a longitudinal observational study. Arch Phys Med Rehabil. 2022;103:1558–64.
Pinheiro MB, Ho KK, Ferreira ML, et al. Efficacy of a sleep quality intervention in people with low back pain: protocol for a feasibility randomized co-twin controlled trial. Twin Res Hum Genet. 2016;19:492–501.
Rhon DI, O’Hagan E, Mysliwiec V, et al. Does disordered sleep moderate the relationship between pain, disability and downstream health care utilization in patients with low back pain?: a longitudinal cohort from the US Military Health System. Spine (Phila Pa 1976). 2019;44:1481–91.
Skarpsno ES, Mork PJ, Nilsen TIL, et al. Influence of sleep problems and co-occurring musculoskeletal pain on long-term prognosis of chronic low back pain: the HUNT study. J Epidemiol Community Health. 2020;74:283–9.
Vinstrup J, Jakobsen MD, Andersen LL. Poor sleep is a risk factor for low-back pain among healthcare workers: prospective cohort study. Int J Environ Res Public Health. 2020;17:996.
Sugawara Y, Tomata Y, Sekiguchi T, et al. Social trust predicts sleep disorder at 6 years after the Great East Japan earthquake: data from a prospective cohort study. BMC Psychol. 2020;8:69.
Angeletti C, Guetti C, Ursini ML, et al. Low back pain in a natural disaster. Pain Pract. 2014;14:E8–16.
Ishigaki A, Higashi H, Sakamoto T, et al. The Great East-Japan Earthquake and devastating tsunami: an update and lessons from the past great earthquakes in Japan since 1923. Tohoku J Exp Med. 2013;229:287–99.
Yabe Y, Hagiwara Y, Sekiguchi T, et al. A 5-year longitudinal study of low back pain in survivors of the Great East Japan Earthquake. Spine (Phila Pa 1976). 2021;46:695–701.
Yabe Y, Hagiwara Y, Sekiguchi T, et al. Higher incidence of sleep disturbance among survivors with musculoskeletal pain after the Great East Japan Earthquake: a prospective study. Tohoku J Exp Med. 2018;244:25–32.
Yabe Y, Hagiwara Y, Sekiguchi T, et al. Sleep disturbance is associated with new onset and continuation of lower back pain: a longitudinal study among survivors of the Great East Japan Earthquake. Tohoku J Exp Med. 2018;246:9–14.
Yabe Y, Hagiwara Y, Sekiguchi T, et al. Association between sleep disturbance and low back pain: a 3-year longitudinal study after the Great East Japan Earthquake. Spine (Phila Pa 1976). 2022;47:361–8.
Hagiwara Y, Yabe Y, Sugawara Y, et al. Influence of living environments and working status on low back pain for survivors of the Great East Japan Earthquake. J Orthop Sci. 2016;21:138–42.
Soldatos CR, Dikeos DG, Paparrigopoulos TJ. Athens Insomnia Scale: validation of an instrument based on ICD-10 criteria. J Psychosom Res. 2000;48:555–60.
Kessler RC, Andrews G, Colpe LJ, et al. Short screening scales to monitor population prevalences and trends in non-specific psychological distress. Psychol Med. 2002;32:959–76.
Sone T, Nakaya N, Sugawara Y, et al. Longitudinal association between time-varying social isolation and psychological distress after the Great East Japan Earthquake. Soc Sci Med. 2016;152:96–101.
Alsaadi SM, McAuley JH, Hush JM, et al. Prevalence of sleep disturbance in patients with low back pain. Eur Spine J. 2011;20:737–43.
Bahouq H, Allali F, Rkain H, et al. Prevalence and severity of insomnia in chronic low back pain patients. Rheumatol Int. 2013;33:1277–81.
Kelly GA, Blake C, Power CK, et al. The association between chronic low back pain and sleep: a systematic review. Clin J Pain. 2011;27:169–81.
Morin CM, Belanger L, LeBlanc M, et al. The natural history of insomnia: a population-based 3-year longitudinal study. Arch Intern Med. 2009;169:447–53.
Matsumoto S, Yamaoka K, Inoue M, et al. Social ties may play a critical role in mitigating sleep difficulties in disaster-affected communities: a cross-sectional study in the Ishinomaki area. Japan Sleep. 2014;37:137–45.
Fujii T, Matsudaira K. Prevalence of low back pain and factors associated with chronic disabling back pain in Japan. Eur Spine J. 2013;22:432–8.
Pincus T, Burton AK, Vogel S, et al. A systematic review of psychological factors as predictors of chronicity/disability in prospective cohorts of low back pain. Spine (Phila Pa 1976). 2002;27:E109-20.
Burgess HJ, Burns JW, Buvanendran A, et al. Associations between sleep disturbance and chronic pain intensity and function: a test of direct and indirect pathways. Clin J Pain. 2019;35:569–76.
Nicassio PM, Wallston KA. Longitudinal relationships among pain, sleep problems, and depression in rheumatoid arthritis. J Abnorm Psychol. 1992;101:514–20.
Riley JL 3rd, Benson MB, Gremillion HA, et al. Sleep disturbance in orofacial pain patients: pain-related or emotional distress? Cranio. 2001;19:106–13.
Jansson-Fröjmark M, Boersma K. Bidirectionality between pain and insomnia symptoms: a prospective study. Br J Health Psychol. 2012;17:420–31.
Roehrs TA. Workshop Participants. Does effective management of sleep disorders improve pain symptoms? Drugs 2009;69 Suppl 2:5–11.
Smith MT, Haythornthwaite JA. How do sleep disturbance and chronic pain inter-relate? Insights from the longitudinal and cognitive-behavioral clinical trials literature. Sleep Med Rev. 2004;8:119–32.
Acknowledgements
None.
Funding
This study was supported by a Health Sciences Research Grant for Health Services (H23-Tokubetsu-Shitei-002, H24-Kenki-Shitei-002, H25-Kenki-Shitei-002 [Fukko]); Ministry of Health, Labour and Welfare, Japan; and a Grant-in-Aid for Scientific Research (A; 21H04845) from the Japan Society for the Promotion of Science.
Author information
Authors and Affiliations
Contributions
YY, YH, and IT contributed to the design of the study. YY, YH, and YS were responsible for data collection and supervision of the study. YY performed statistical analysis. YY and YH wrote the manuscript. YS and IT helped to analyze the data and draft the manuscript. The author(s) read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study protocol was reviewed and approved by the Ethics Committee of Tohoku University Graduate school of Medicine (approval number, 201192). Written informed consent was obtained from all the participants. All the protocols were followed in accordance with the 1964 Helsinki declaration and its later amendments.
Consent for publication
Not applicable.
Competing interests
The authors declare no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original version of this article was revised: the duplicate affiliations were removed.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Yabe, Y., Hagiwara, Y., Sugawara, Y. et al. Low back pain is associated with sleep disturbance: a 3-year longitudinal study after the Great East Japan Earthquake. BMC Musculoskelet Disord 23, 1132 (2022). https://doi.org/10.1186/s12891-022-06106-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12891-022-06106-x