Abstract
Background
With the aging population of society, the incidence rate of osteoporosis is increasing year by year. Early diagnosis of osteoporosis plays a significant role in the progress of disease prevention. As newly developed technology, computed tomography (CT) radiomics could discover radiomic features difficult to recognize visually, providing convenient, comprehensive and accurate osteoporosis diagnosis. This study aimed to develop and validate a clinical-radiomics model based on the monochromatic imaging of single source dual-energy CT for osteoporosis prediction.
Methods
One hundred sixty-four participants who underwent both single source dual-energy CT and quantitative computed tomography (QCT) lumbar-spine examination were enrolled in a study cohort including training datasets (n = 114 [30 osteoporosis and 84 non-osteoporosis]) and validation datasets (n = 50 [12 osteoporosis and 38 non-osteoporosis]). One hundred seven radiomics features were extracted from 70-keV monochromatic CT images. With QCT as the reference standard, a radiomics signature was built by using least absolute shrinkage and selection operator (LASSO) regression on the basis of reproducible features. A clinical-radiomics model was constructed by incorporating the radiomics signature and a significant clinical predictor (age) using multivariate logistic regression analysis. Model performance was assessed by its calibration, discrimination and clinical usefulness.
Results
The radiomics signature comprised 14 selected features and showed good calibration and discrimination in both training and validation cohorts. The clinical-radiomics model, which incorporated the radiomics signature and a significant clinical predictor (age), also showed good discrimination, with an area under the receiver operating characteristic curve (AUC) of 0.938 (95% confidence interval, 0.903–0.952) in the training cohort and an AUC of 0.988 (95% confidence interval, 0.967–0.998) in the validation cohort, and good calibration. The clinical-radiomics model stratified participants into groups with osteoporosis and non-osteoporosis with an accuracy of 94.0% in the validation cohort. Decision curve analysis (DCA) demonstrated that the radiomics signature and the clinical-radiomics model were clinically useful.
Conclusions
The clinical-radiomics model incorporating the radiomics signature and a clinical parameter had a good ability to predict osteoporosis based on dual-energy CT monoenergetic imaging.
Similar content being viewed by others
Introduction
Osteoporosis is a commonly encountered disease, and is characterized by decreased bone mineral density (BMD) and a damaged bone microstructure, resulting in an increased bone fragility and fracture susceptibility [1]. With the aging population of society, osteoporosis and osteoporosis-related fractures are leading causes of morbidity and mortality in the elderly [2, 3]. Hence, the early diagnosis of osteoporosis plays a significant role in the progress of disease prevention.
Currently, BMD measurement is considered clinically as an important means in the early diagnosis, prevention, and treatment of osteoporosis [4]. The reference standards for the BMD measurement include double-energy X-ray absorptiometry (DXA) and quantitative computed tomography (QCT) [5, 6]. DXA is used to measure the mean values of areal BMD (in g/cm2) for cortical bone and trabecular bone [7], whereas the accuracy of estimated bone mass value is limited by the inherent interference derived from hyperosteogeny, sclerosis, et al. [8, 9]. As a recognized approach for volumetric BMD (in mg/cm3) assessment of specifically trabecular bone [10], QCT holds a higher sensitivity on osteoporosis prediction than DXA [11,12,13,14], however, the application of QCT remains limited by the complex post-processing (extra calibration and standardized software) as well as higher radiation dose. In recent years, radiomics introduces a new quantitative approach for disease assessment. Based on the acquired radiography, computed tomography (CT) or magnetic resonance (MR) images, it applies advanced data model algorithms to extract high-throughput imaging features, and find radiomics features that are difficult to identify visually, so as to provide accurate disease diagnosis, malignant tumor classification, prognosis prediction and efficacy evaluation. Moreover, radiomics is more comprehensive and convenient, without adding additional radiation dose [15,16,17,18].
However, for conventional CT radiomics-based osteoporosis prediction, there are challenges in providing sufficient accuracy owing to the polychromatic images and beam-hardening artifacts. With the advances in technologies, dual-energy CT might be the solution. Derived from single source dual-energy CT, virtual monochromatic spectral (VMS) images are reconstructed from a pair of accurate material density images and mass attenuation coefficients. VMS imaging can yield improved image quality by reducing beam-hardening artifacts [19]. Single source dual-energy CT provides 101 sets of 40–140 keV VMS images for different clinical needs, resulting in more precise measurement of CT value which constitute radiomic features. Previous studies have shown that VMS images at approximately 70 keV (range, 67–72 keV) had lower image noise and a higher contrast-to-noise ratio (CNR) than 120-kVp CT images [20, 21]. Moreover, the radiation exposure dose of single source dual-energy CT with the volume-based adaptive statistical iterative reconstruction (ASiR-V) technique is equal to or lower than that of conventional CT [22,23,24]. But, reports on the use of radiomics analysis derived from single source dual-energy CT for osteoporosis prediction is still lacking.
Therefore, the purpose of our study was to explore the value of radiomics analysis based on the monochromatic imaging of single source dual-energy CT for osteoporosis prediction, with QCT referenced.
Methods
Study participants
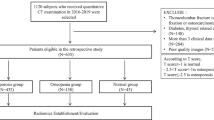
The institutional ethics committee of The First Affiliated Hospital of Hunan University of Chinese Medicine approved this retrospective study and the requirement to obtain informed consent from all participants was waived (no. HN-LL-KY-2021–019-01), the data were desensitized before using in order to protect the participant’s privacy. Data from participants who underwent both single source dual-energy CT and QCT lumbar-spine examination between September 2019 and August 2020 were collected. Clinical data including age, sex, and body mass index (BMI) were recorded before scanning. Participants were excluded if they were (a) with a presence of image artifacts; (b) with spinal tumor, spinal tumor-like infection or lesions, severe degenerative changes, hematologic disorder, and contrast enhanced scan; (c) with lumbar vertebral fracture, compression, and deformation; (d) with postoperative bone cement or metal implant [25]. Finally, a total of 164 participants [71 men (mean age: 49.8 ± 18.0 years, range: 22—87 years) and 93 women (mean age: 46.5 ± 17.4 years, range: 23—90 years)] were enrolled in the study. Participants were randomly allocated to training and validation cohorts in a 7: 3 ratio. The flowchart of participants selection is showed in Fig. 1. With QCT as the reference standard, diagnosis was performed according to the guidelines introduced by the International Society for Clinical Densitometry (ISCD) and American College of Radiology (ACR), osteoporosis of the spine was defined as a BMD value < 80 mg/cm3, and non-osteoporosis (osteopenia and normal) was defined as a BMD value ≥ 80 mg/cm3 [26].
Single source dual-energy CT and phantom-calibrated QCT imaging acquisition
In order to avoid additional radiation exposure and substantial costs in our study, all lumbar-spine examinations of the enrolled participants were simultaneously performed with a bone density calibration (BDC) phantom placed beneath the spine. CT examinations were performed on a single source dual-energy CT scanner (Revolution, GE Medical Systems, Milwaukee, WI, USA). The scan parameters were as follows: GSI helical mode with fast kilovoltage (KV)-switching between 80 and 140 kVp; tube current, 230 mA; beam collimation, 128 × 0.625 mm; rotation speed, 0.8 s/rot; helical pitch, 0.984; bone kernel; and 50% ASiR-V. Then, a series of contiguous 1.25-mm-thick VMS images at 70 keV were generated for subsequent BMD measurement and radiomics analysis [21]. The details of BMD measurement by phantom-calibrated QCT refer to our previous article [25].
Image segmentation
The volume-of-interest (VOI) was manually drawn on the axial 70-keV monochromatic images from single source dual-energy CT by reader 1 (L Yan, with 4 years of experience in medial image segmentation) using the 3D-Slicer software (version 4.11.20200930; http://www.slicer.org). The VOI with a diameter of 20 mm and a height of 12.5 mm was acquired in the middle of the L1-L5 vertebral bodies on eleven consecutive images. Typically, the boundary was set along the inner edge of vertebral cortex, and the areas of the cortical bone and the vertebral venous plexuses posteriorly were carefully avoided (Fig. 2). To assess interobserver variability in the segmentation, 33 participants were randomly selected and were manually segmented by reader 2 (Y He, with 2 years of experience in medial image segmentation) in the same way. The image segmentation was supervised by a radiologist (K Zhang, with 13 years of experience in musculoskeletal radiology).
Radiomics feature extraction
PyRadiomics [27], an open-source python package recommended for standardized radiomics analysis workflow [28], was used to extract 107 radiomics features from each VOI. These 107 extracted features were designed to characterize the VOIs in seven parts: 18 first-order statistics features, 14 shape features, 24 Gy level co-occurrence matrix (GLCM) features, 16 Gy level size zone matrix (GLSZM) features, 16 Gy level run length matrix (GLRLM) features, 5 neighboring gray tone difference matrix (NGTDM) features, and 14 Gy level dependence matrix (GLDM) features. All images were resampled to a voxel size of 1 × 1 × 1 mm to standardize the voxel spacing, and were quantized with a quantization range of mean ± 3 × SD and 25 bins without filtering.
Details of the radiomics features are described (Supplementary Data S1).
Selection of radiomics features
The following three steps were undertaken to select robust radiomics features.
First, the intraclass correlation coefficients between the VOI-based radiomics features extracted from multiple cases (the cases of 33 randomly selected and segmented by reader 1 and reader 2) were obtained to determine reproducibility. Stable features with an intraclass correlation coefficient (ICC) > 0.90 were accepted and were included for subsequent analysis.
Second, to further reduce dimensionality, the univariate statistical tests (two-sample t test) between osteoporosis and non-osteoporosis groups were examined, and features were retained with p < 0.05.
Finally, the least absolute shrinkage and selection operator (LASSO) regression [29] was performed with penalty parameter tuning using five fold cross-validation, and features with non-zero coefficients were selected.
Models construction and evaluation
Radiomics model
A radiomics signature was constructed from linear combinations of the selected features weighted by their respective LASSO coefficients.
Clinical-radiomics model
For each of the four variables (radiomics signature and three clinical parameters [age, sex, BMI]), the univariate logistic regression analysis was performed. Significant parameters from the univariate analysis were selected and were included in the multivariate logistic regression analysis to construct a clinical-radiomics model.
The performance of the two models was assessed in terms of calibration and discrimination. Calibration was quantified with the Hosmer Lemeshow goodness-of-fit test. Discrimination was assessed with the area under the receiver operating characteristics (ROC) curve. The optimal cutoff values for classifying participants according to osteoporosis or non-osteoporosis were determined on the highest Youden index (sensitivity + specificity—1) value in the ROC curves of the training cohort. Accuracy, specificity, sensitivity, negative predictive value (NPV), and positive predictive value (PPV) were obtained.
The performance of the two models was then tested in the validation cohort.
Clinical use
Decision curve analysis (DCA) was conducted to estimate the clinical usefulness of the radiomics signature and the clinical-radiomics model by calculating the net benefits for a range of threshold probabilities in the validation cohort.
Statistical analysis
All statistical analyses were performed with IBM SPSS Statistics 25 and R software (version 3.6.1, https://www.r-project.org). Student or the Mann–Whitney test was utilized to analyze continuous variables, and the chi-square or Fisher’s exact test was used to analyze the differences between the groups in categorical variables. P values < 0.05 were considered statistically significant.
Results
Demographic and clinical characteristics
The demographic and clinical characteristics of osteoporosis and non-osteoporosis participants are summarized in Table 1. There were 114 participants in the training cohort (30 osteoporosis and 84 non-osteoporosis) and 50 participants in the validation cohort (12 osteoporosis and 38 non-osteoporosis). There was no significant difference in participants’ demographic and clinical characteristics between the training and validation datasets (Table 2).
Radiomics feature selection
Among the 107 radiomics features, 71 stable features were identified with an ICC > 0.90 and were included for subsequent analysis (Supplementary Data S2). Then, these 71 features were further reduced to 23 features by applying the two-sample t test. Finally, the LASSO regression model led to the selection of 14 features with non-zero coefficients, which consisted of seven first-order statistics features and seven texture features (Table 3; Fig. 3).
Construction, performance, and validation of the models
For radiomics model, the radiomics signature was constructed from the 14 robust features as follows:
Radiomics signature = 1.453 + (0.090 ∙ 90th percentile) + (0.121 ∙ maximum) + (0.523 ∙ mean) + (0.147 ∙ median) + (0.081 ∙ minimum) + (-0.044 ∙ skewness) + (-0.493 ∙ total energy) + (0.023 ∙ idmn) + (-0.026 ∙ large dependence high gray level emphasis) + (-0.047 ∙ small dependence low gray level emphasis) + (-0.021 ∙ large area low gray level emphasis) + (0.018 ∙ low gray level zone emphasis) + (0.026 ∙ small area low gray level emphasis) + (0.005 ∙ busyness).
For clinical-radiomics model, the results of the univariate and multivariate analyses are summarized in Table 4. Among the clinical parameters, age was found to be a significant predictor. A clinical-radiomics model was constructed based on the radiomics signature and a significant clinical predictor as follows:
\(\mathrm{ln}\left[\mathrm{P}/\left(1-\mathrm{P}\right)\right]\) = 4.989 + (1.143 ∙ radiomics signature) + (-0.094 ∙ age), where \(\mathrm{P}\) is the predicted probability of osteoporosis.
The diagnostic performance of the above two models is summarized in Table 5, and the receiver operating characteristic curves and calibration curves were plotted (Fig. 4). The clinical-radiomics model showed good discrimination (AUC, 0.938 in training cohort and 0.988 in validation cohort), and calibration according to the Hosmer–Lemeshow test in both training (p = 0.880) and validation (p = 0.905) cohorts. The radiomics signature also showed good discrimination and calibration performance in both training (AUC, 0.914; Hosmer–Lemeshow test, p = 0.810) and validation (AUC, 0.902; Hosmer–Lemeshow test, p = 0.758) cohorts.
Clinical use
The DCA result for the two models is shown in Fig. 5. The decision curve showed that if the threshold probability of a doctor or patient is > 10%, using the radiomics signature and the clinical-radiomics model to predict osteoporosis leads to a higher overall net benefit than the treat-none and treat-all-patients schemes.
Discussion
In our study, we developed and validated two models (radiomics signature and clinical-radiomics model) based on the monochromatic imaging of single source dual-energy CT for osteoporosis prediction. The proposed clinical-radiomics model, incorporating the radiomics signature and a significant clinical predictor (age), stratified participants into osteoporosis and non-osteoporosis groups with higher accuracies (88.6% in training and 94.0% in validation cohorts) than the radiomics signature alone.
A recent study has demonstrated that a deep learning-based method could achieve fully automatic identification of vertebral osteoporosis, osteopenia, and normal bone mineral density in 120 kVp polychromatic CT images [30]. In fact, the true single-energy CT value is defined as the attenuation value after the monochromatic X-rays passing through the detected tissue. But, in conventional single-source CT, the polychromaticity of the X-rays commonly causes averaging attenuation effect and beam-hardening artifacts in the image, and leads to inaccurate measurement of CT values [31], which may have a potential effect on the accuracy of the deep learning-based study. The recently developed dual-energy CT imaging uses single source and fast kVp-switching between 80 and 140 kVp to simulate the imaging of materials under monochromatic X-ray source by analyzing the information during dual-energy data acquisition to produce VMS images. By doing so, single source dual-energy CT could greatly reduce beam-hardening artifacts and generate images with more accurate CT attenuation values at every energy level. Moreover, in previous studies [20], the approximately 70 keV monochromatic image has characterized by lower image noise and a higher CNR, and has potentially replaced the 120 kVp polychromatic image used in conventional image processing. It also reported that the 70 keV monochromatic image had the lowest artifact index among all energy levels [31]. So, in our study, the 70 keV monochrome image with improved image quality was selected thus looked forward to better diagnostic efficiency.
Several studies have applied radiomics to predict osteoporosis. He et al. proposed the classification method of normal vs. osteopenia, normal vs. osteoporosis, and osteopenia vs. osteoporosis using radiomics based on T1-wighted and T2-wighted sagittal lumbar spine magnetic resonance (MR) images, and the AUC was 0.810, 0.797, and 0.769, respectively [32]. Compared with the results of their study, our models applied to CT images showed superior discrimination performance. In one recent study by Lim et al., abdomen-pelvic CT (APCT)-based radiomics analysis with a machine learning algorithm was used to predict femoral osteoporosis [33]. In their study, a machine learning classifier could classify participants into osteoporosis and non-osteoporosis with accuracy of 92.9% and 92.7% in the training and validation cohorts, respectively, indicating radiomics approach based on CT images has good potential for osteoporosis prediction. In our study, to enhance robustness, we employed similar method to select reproducible features from multiple cases (ICC > 0.90), and our models achieved a comparable accuracy for vertebral osteoporosis prediction. Further, in our study, to develop a more holistic model, we synchronously incorporated the radiomics signature and a significant clinical predictor (age). In line with previous studies, the ages of the participants with osteoporosis were significantly older than those with non-osteoporosis [34, 35].
Most previous studies [32, 33, 36] had a common problem that the areal BMD (in g/cm2) measured by DXA was considered as the reference standard, which may have a potential effect on the accuracy of these studies. It is well known that QCT can measure true volumetric BMD (in mg/cm3) and provide more accurate BMD values than DXA. Moreover, there may have better agreement between volumetric BMD and the radiomic features of VOIs. To date, few studies on radiomics of osteoporosis have used QCT as the reference standard. In our study, with QCT as reference, the proposed models had a good ability to predict osteoporosis, showing that QCT and VOIs are more corresponding. Radiomics analysis may be a new approach for the diagnosis of osteoporosis.
Radiomics features are quantitatively extracted agnostic features that are considered to reflect intra-region heterogeneity [37]. Our radiomics signature included seven first order statistics features and seven texture features, respectively describing the distribution and spatial arrangement of voxel intensities within the VOI. Among the fourteen features, original_firstorder_Mean was the most predictive feature with the largest LASSO coefficient, representing the average gray level intensity within the VOI. Several studies [38] have shown a significant association between BMD-decrease and the first order statistics change, including mean CT Hounsfield unit (HU). Moreover, some complex texture features have been reported to be linked to known pathological changes in the osteoporotic bone, such as GLCM features [39,40,41,42]. In our study, we found that osteoporosis had a lower mean gray level intensity than non-osteoporosis, which should be highly related to the processes that occur in osteoporosis. Osteoporosis causes bone mineral loss and destruction of the delicate bony microstructure especially trabecular bone which is more active metabolically [43]. Therefore, we speculated that osteoporosis would show lower mean intensity of gray levels within a VOI due to decreased bone calcium and increased fatty marrow than non-osteoporosis. These subtle differences in statistics and pathology reflect the difference in heterogeneity of these two groups, which are difficult to identify by visual assessment of CT images.
This study has several limitations that should be noted. First, as a retrospective study with a relatively small sample size, there is a possibility of participant selection bias in our cohorts. Second, the proposed models were established on the basis of data obtained from a single center and single race. Therefore, it is our future work to prove the validity through prospective multi-center and multi-ethnic studies. Third, our study did not directly show the advantage of using dual-energy CT monochromatic images in constructing radiomics signature over the conventional kVp images. Finally, our study cohort was divided into two groups: osteoporosis and non-osteoporosis groups, which consist of normal and osteopenia participants. And this was due to the purpose of our study was to develop and validate models based on the monochromatic imaging of single source dual-energy CT for osteoporosis prediction. Thus, this study was focused on osteoporosis prediction. In the future, we will aim to evaluate whether radiomics analysis is possible to predict osteoporosis, osteopenia, and normal status accurately.
In conclusion, our proposed models, with QCT regarded as the reference standard and the VOI-based radiomics features extracted from the 70 keV monochromatic images, had a good ability to predict osteoporosis. The predictive models could serve as potential decision support tools for both radiologists and clinicians.
Availability of data and materials
The datasets in this study are available on request from the corresponding author. The datasets are not publicly available due to privacy or ethical restrictions.
References
Compston JE, McClung MR, Leslie WD. Osteoporosis. Lancet. 2019;393:364–76.
Hofbauer LC, Rachner TD. More DATA to guide sequential osteoporosis therapy. Lancet. 2015;386:1116–8.
Pisani P, Renna MD, Conversano F, Casciaro E, Di Paola M, Quarta E, Muratore M, Casciaro S. Major osteoporotic fragility fractures: risk factor updates and societal impact. World J Orthop. 2016;7:171–81.
Lorentzon M, Cummings SR. Osteoporosis: the evolution of a diagnosis. J Intern Med. 2015;277:650–61.
Berry SD, Samelson EJ, Pencina MJ, McLean RR, Cupples LA, Broe KE, Kiel DP. Repeat bone mineral density screening and prediction of hip and major osteoporotic fracture. JAMA. 2013;310:1256–62.
Wu Y, Guo Z, Fu X, Wu J, Gao J, Zeng Q, Fu H, Cheng X. The study protocol for the China Health Big Data (China Biobank) project. Quant Imaging Med Surg. 2019;9:1095–102.
Ott SM. Methods of determining bone mass. J Bone Miner Res. 1991;6 Suppl 2:S71-6.
Ito M, Hayashi K, Yamada M, Uetani M, Nakamura T. Relationship of osteophytes to bone mineral density and spinal fracture in men. Radiology. 1993;189:497–502.
Rand T, Seidl G, Kainberger F, Resch A, Hittmair K, Schneider B, Glüer CC, Imhof H. Impact of spinal degenerative changes on the evaluation of bone mineral density with dual energy X-ray absorptiometry (DXA). Calcif Tissue Int. 1997;60:430–3.
Engelke K. Quantitative computed tomography-current status and new developments. J Clin Densitom. 2017;20:309–21.
Engelke K, Libanati C, Liu Y, Wang H, Austin M, Fuerst T, Stampa B, Timm W, Genant HK. Quantitative computed tomography (QCT) of the forearm using general purpose spiral whole-body CT scanners: accuracy, precision and comparison with dual-energy X-ray absorptiometry (DXA). Bone. 2009;45:110–8.
Li N, Li XM, Xu L, Sun WJ, Cheng XG, Tian W. Comparison of QCT and DXA: osteoporosis detection rates in postmenopausal women. Int J Endocrinol. 2013;2013:895474.
Löffler MT, Jacob A, Valentinitsch A, Rienmüller A, Zimmer C, Ryang YM, Baum T, Kirschke JS. Improved prediction of incident vertebral fractures using opportunistic QCT compared to DXA. Eur Radiol. 2019;29:4980–9.
Xu XM, Li N, Li K, Li XY, Zhang P, Xuan YJ, Cheng XG. Discordance in diagnosis of osteoporosis by quantitative computed tomography and dual-energy X-ray absorptiometry in Chinese elderly men. J Orthop Translat. 2019;18:59–64.
Liu Z, Zhang XY, Shi YJ, Wang L, Zhu HT, Tang Z, Wang S, Li XT, Tian J, Sun YS. Radiomics analysis for evaluation of pathological complete response to neoadjuvant chemoradiotherapy in locally advanced rectal cancer. Clin Cancer Res. 2017;23:7253–62.
Wang Y, Liu W, Yu Y, Liu JJ, Xue HD, Qi YF, Lei J, Yu JC, Jin ZY. CT radiomics nomogram for the preoperative prediction of lymph node metastasis in gastric cancer. Eur Radiol. 2020;30:976–86.
Rastegar S, Vaziri M, Qasempour Y, Akhash MR, Abdalvand N, Shiri I, Abdollahi H, Zaidi H. Radiomics for classification of bone mineral loss: a machine learning study. Diagn Interv Imaging. 2020;101:599–610.
Zhang B, Tian J, Dong D, Gu D, Dong Y, Zhang L, Lian Z, Liu J, Luo X, Pei S, Mo X, Huang W, Ouyang F, Guo B, Liang L, Chen W, Liang C, Zhang S. Radiomics features of multiparametric MRI as novel prognostic factors in advanced nasopharyngeal carcinoma. Clin Cancer Res. 2017;23:4259–69.
Pessis E, Campagna R, Sverzut JM, Bach F, Rodallec M, Guerini H, Feydy A, Drapé JL. Virtual monochromatic spectral imaging with fast kilovoltage switching: reduction of metal artifacts at CT. Radiographics. 2013;33:573–83.
Matsumoto K, Jinzaki M, Tanami Y, Ueno A, Yamada M, Kuribayashi S. Virtual monochromatic spectral imaging with fast kilovoltage switching: improved image quality as compared with that obtained with conventional 120-kVp CT. Radiology. 2011;259:257–62.
Yamada Y, Jinzaki M, Hosokawa T, Tanami Y, Abe T, Kuribayashi S. Abdominal CT: an intra-individual comparison between virtual monochromatic spectral and polychromatic 120-kVp images obtained during the same examination. Eur J Radiol. 2014;83:1715–22.
Li W, Li A, Wang B, Niu X, Cao X, Wang X, Shi H. Automatic spectral imaging protocol and iterative reconstruction for radiation dose reduction in typical hepatic hemangioma computed tomography with reduced iodine load: a preliminary study. Br J Radiol. 2018;91:20170978.
Lv P, Zhou Z, Liu J, Chai Y, Zhao H, Guo H, Marin D, Gao J. Can virtual monochromatic images from dual-energy CT replace low-kVp images for abdominal contrast-enhanced CT in small- and medium-sized patients? Eur Radiol. 2019;29:2878–89.
Zheng S, Dong Y, Miao Y, Liu A, Zhang X, Wang B, Ge Y, Liu Y, Wang S. Differentiation of osteolytic metastases and Schmorl’s nodes in cancer patients using dual-energy CT: advantage of spectral CT imaging. Eur J Radiol. 2014;83:1216–21.
Zhou S, Zhu L, You T, Li P, Shen H, He Y, Gao H, Yan L, He Z, Guo Y, Zhang Y, Zhang K. In vivo quantification of bone mineral density of lumbar vertebrae using fast kVp switching dual-energy CT: correlation with quantitative computed tomography. Quant Imaging Med Surg. 2021;11:341–50.
American College of Radiology (2018) ACR–SPR–SSR practice parameter for the performance of musculoskeletal quantitative computed tomography (QCT). Available online: https://www.acr.org/-/media/ACR/Files/Practice-Parameters/QCT.pdf, Accessed 7 Nov 2018.
van Griethuysen JJM, Fedorov A, Parmar C, Hosny A, Aucoin N, Narayan V, Beets-Tan RGH, Fillion-Robin JC, Pieper S, Aerts H. Computational radiomics system to decode the radiographic phenotype. Cancer Res. 2017;77:e104–7.
Lambin P, Leijenaar RTH, Deist TM, Peerlings J, de Jong EEC, van Timmeren J, Sanduleanu S, Larue R, Even AJG, Jochems A, van Wijk Y, Woodruff H, van Soest J, Lustberg T, Roelofs E, van Elmpt W, Dekker A, Mottaghy FM, Wildberger JE, Walsh S. Radiomics: the bridge between medical imaging and personalized medicine. Nat Rev Clin Oncol. 2017;14:749–62.
Tibshirani R. Regression Shrinkage and Selection via the Lasso. J Roy Stat Soc: Ser B (Methodol). 1996;58:267–88.
Fang Y, Li W, Chen X, Chen K, Kang H, Yu P, Zhang R, Liao J, Hong G, Li S. Opportunistic osteoporosis screening in multi-detector CT images using deep convolutional neural networks. Eur Radiol. 2021;31:1831–42.
He T, Qian X, Zhai R, Yang Z. Computed tomography number measurement consistency under different beam hardening conditions: comparison between dual-energy spectral computed tomography and conventional computed tomography imaging in phantom experiment. J Comput Assist Tomogr. 2015;39:981–5.
He L, Liu Z, Liu C, Gao Z, Ren Q, Lei L, Ren J. Radiomics based on lumbar spine magnetic resonance imaging to detect osteoporosis. Acad Radiol. 2021;28:e165–71.
Lim HK, Ha HI, Park SY, Han J. Prediction of femoral osteoporosis using machine-learning analysis with radiomics features and abdomen-pelvic CT: a retrospective single center preliminary study. PLoS One. 2021;16:e0247330.
NIH Consensus Development Panel on Osteoporosis Prevention, Diagnosis, and Therapy. Osteoporosis prevention, diagnosis, and therapy. JAMA. 2001;285:785–95.
Kelsey JL. Risk factors for osteoporosis and associated fractures. Public Health Rep. 1989;104(Suppl):14–20.
Booz C, Noeske J, Albrecht MH, Lenga L, Martin SS, Yel I, Huizinga NA, Vogl TJ, Wichmann JL. Diagnostic accuracy of quantitative dual-energy CT-based bone mineral density assessment in comparison to Hounsfield unit measurements using dual x-ray absorptiometry as standard of reference. Eur J Radiol. 2020;132:109321.
Gillies RJ, Kinahan PE, Hricak H. Radiomics: images are more than pictures. They Are Data Radiology. 2016;278:563–77.
Zou D, Li W, Deng C, Du G, Xu N. The use of CT Hounsfield unit values to identify the undiagnosed spinal osteoporosis in patients with lumbar degenerative diseases. Eur Spine J. 2019;28:1758–66.
Kawashima Y, Fujita A, Buch K, Li B, Qureshi MM, Chapman MN, Sakai O. Using texture analysis of head CT images to differentiate osteoporosis from normal bone density. Eur J Radiol. 2019;116:212–8.
Muehlematter UJ, Mannil M, Becker AS, Vokinger KN, Finkenstaedt T, Osterhoff G, Fischer MA, Guggenberger R. Vertebral body insufficiency fractures: detection of vertebrae at risk on standard CT images using texture analysis and machine learning. Eur Radiol. 2019;29:2207–17.
S R, R M. An Automatic Bone Disorder Classification Using Hybrid Texture Feature Extraction with Bone Mineral Density. Asian Pac J Cancer Prev. 2018;19:3517–23.
Valentinitsch A, Trebeschi S, Kaesmacher J, Lorenz C, Löffler MT, Zimmer C, Baum T, Kirschke JS. Opportunistic osteoporosis screening in multi-detector CT images via local classification of textures. Osteoporos Int. 2019;30:1275–85.
Engelke K, Adams JE, Armbrecht G, Augat P, Bogado CE, Bouxsein ML, Felsenberg D, Ito M, Prevrhal S, Hans DB, Lewiecki EM. Clinical use of quantitative computed tomography and peripheral quantitative computed tomography in the management of osteoporosis in adults: the 2007 ISCD Official Positions. J Clin Densitom. 2008;11:123–62.
Acknowledgements
Not applicable.
Funding
This work was supported by the National Natural Science Foundation of China (grant number 81603482), China Postdoctoral Science Foundation (grant number 2017M622586), Key Discipline Construction Project of Hunan University of Chinese Medicine (grant number 4901–020000200806), Hunan Province “Domestic First-class Cultivation Discipline” Open Fund Project of Integrated Traditional Chinese and Western Medicine (grant number 2020ZXYJH07), Hunan Province Science and Technology Talent Support Project - Young Outstanding Science and Technology Worker Training Program (grant number 2022TJ-N05) and Scientific Research Project of Hunan Provincial Department of Education (grant number 22B0401).
Author information
Authors and Affiliations
Contributions
(I) Conception and design: K Zhang, X Hu, H Gao; (II) Administrative support: P Li, H Shen, K Zhang; (III) Provision of study materials or patients: S Chen, T You, J Li, Z Zhong; (IV) Collection and assembly of data: J Wang, S Zhou; (V) Data analysis and interpretation: J Wang, L Yan, Y He, M Luo; (VI) Manuscript writing: All authors; (VII) Final approval of manuscript: All authors.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the ethics committee/IRB of The First Affiliated Hospital of Hunan University of Chinese Medicine (no. HN-LL-KY-2021–019-01) and individual consent for this retrospective analysis was waived.
Consent for publication
Not applicable.
Competing interests
All authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Wang, J., Zhou, S., Chen, S. et al. Prediction of osteoporosis using radiomics analysis derived from single source dual energy CT. BMC Musculoskelet Disord 24, 100 (2023). https://doi.org/10.1186/s12891-022-06096-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12891-022-06096-w