Abstract
Background
Hand osteoarthritis is a common and disabling chronic joint disease with a lack of effective therapies. Emerging evidence suggests the role of local inflammation in causing pain in hand osteoarthritis. Corticosteroids are potent anti-inflammatory drugs used in many rheumatic diseases. The aim of this randomised, double-blind, placebo-controlled trial is to determine whether topical corticosteroid reduces pain over 6 weeks in patients with hand osteoarthritis.
Methods
One hundred participants with hand osteoarthritis will be recruited from the community in Melbourne, Australia, and randomly allocated in a 1:1 ratio to receive either topical Diprosone OV or placebo ointment administered 3 times daily on the painful hand joints for 6 weeks. The primary outcome is pain reduction (assessed by 100 mm visual analogue scale) at 6 weeks. The secondary outcomes include changes in pain and function assessed using Functional Index for Hand Osteoarthritis, Australian Canadian Osteoarthritis Hand Index, Michigan Hand Outcomes Questionnaire, and tender and swollen joint count at 6 weeks. Adverse events will be recorded. The primary analysis will be by intention to treat, including all participants in their randomised groups.
Discussion
This study will provide high-quality evidence to determine whether topical corticosteroid reduces pain over 6 weeks in patients with hand osteoarthritis, with major clinical and public health importance by informing clinical practice guidelines for the management of hand osteoarthritis and reducing the burden of the disabling disease.
Trial registration
Australian New Zealand Clinical Trials Registry (ANZCTR), ACTRN12620000599976. Registered 22 May 2020.
Similar content being viewed by others
Background
Hand osteoarthritis (OA) is a common chronic joint disease causing pain, functional disability, and decreased quality of life which result in substantial burden of disease [1, 2]. The lifetime risk of symptomatic hand OA is 39.8% in the general population, 47.2% for females and 24.6% for males [3]. In people aged 55 years and over, 67% of the women and 54.8% of the men have radiographic OA in at least one hand joint, with 20% having disabling pain [4]. Hand OA impairs the activities of daily living such as dressing and eating [5], with significant clinical importance in relation to health-related quality of life [2]. The healthcare costs for treating this condition are expected to escalate due to the ageing population and increasing obesity [6]. Disease-modifying drugs for hand OA are currently not available. In terms of clinical guidelines for the management of hand OA, the European League Against Rheumatism suggests topical therapy (non-steroidal anti-inflammatory drugs (NSAIDs) being first-line choice), oral analgesics (e.g. paracetamol and NSAIDs), and non-pharmacological therapy (education, assistive devices, exercises and orthoses) [7]. The American College of Rheumatology conditionally recommends management with topical capsaicin, topical NSAIDs, oral NSAIDs, as well as non-pharmacological therapy [8]. Although there is some evidence for at best, a short-term moderate effect of topical and oral NSAIDs on pain alleviation, evidence is conflicting for the efficacy of anti-inflammatory medications, such as corticosteroids and biologic agents [9]. There is an unmet need for effective therapies for hand OA.
Increasing evidence suggests that hand OA is a disease of the whole joint with local inflammation playing an important role in causing pain and disease progression [1]. Imaging studies have shown that most patients with hand OA have synovitis (~ 50%) [10, 11], and that painful joints are more likely to have synovitis than non-painful joints [10, 12, 13]. Moreover, synovitis has been shown to be the strongest predictor for progression of structural damage in hand OA [14,15,16,17]. Therefore, inflammation is a potential treatment target in hand OA, and therapies targeting synovitis may provide a novel approach for the management of hand OA. Glucocorticoids are potent multitargeted anti-inflammatory drugs which have been used in many rheumatic diseases because of their anti-inflammatory and immunosuppressive actions [18]. Randomised controlled trials have shown the effect of oral corticosteroids (prednisolone) on reducing pain and improving function in patients with hand OA over 6 weeks [19, 20], with little effect over 3 months [19, 21]. However, systemic corticosteroids are associated with significant adverse events including diabetes and osteoporosis [22, 23]. Intra-articular injections of corticosteroids are effective but the effects often last less than 1 month [24]. It is also technically difficult to perform in small hand joints so often performed under ultrasound guidance which adds significantly to the cost, inconvenience and timeliness of treatment. Topical delivery of corticosteroids provides a potential alternative approach to improving outcomes in hand OA. Topical corticosteroids are safe, inexpensive and commonly used for skin conditions. However, there have been no clinical trials examining the efficacy of topical corticosteroids in reducing pain in hand OA.
Objectives and hypothesis
We propose a randomised, double-blind, placebo-controlled trial to determine the effect of topical Diprosone OV ointment administered 3 times daily on painful hand joints compared to placebo in reducing pain and improving function in participants with hand OA over 6 weeks. It was hypothesised that topical Diprosone OV ointment would be more effective than placebo in reducing pain and improving function in participants with hand OA over 6 weeks.
Study design
This is a single center, parallel-group, randomised, double-blind, placebo-controlled trial over 6 weeks.
Trial registration
The trial was registered at the Australian New Zealand Clinical Trials Registry prior to recruitment commencing (ACTRN12620000599976, registered 22 May 2020). The trial reporting will be guided by the Consolidated Standards of Reporting Trials (CONSORT) Statement [25]. Protocol reporting is in accordance with the SPIRIT Statement (Standard Protocol Items: Recommendations for Interventional Trials) [26].
Ethics approval
Ethics approval has been obtained from Alfred Hospital Ethics Committee (117/20) and registered at Monash University Human Research Ethics Committee (24219). Written informed consent will be obtained from all the participants.
Methods
Study setting and participants
Eligible participants with hand OA will be recruited from the community in Melbourne, Australia via advertisements and from medical practitioners.
Inclusion criteria
Participants aged ≥40 years with symptomatic radiological hand OA will be recruited. We will also invite those who contacted us to take part in the clinical trial of methotrexate for hand OA but could not take part due to concern about using methotrexate on the part of the participants or contraindications to its use. As recommended by the Osteoarthritis Research Society International (OARSI) clinical trials guidelines for hand OA [27], a pain score of at least 40 on a 100 mm visual analogue scale (VAS) and radiologic OA (Kellgren and Lawrence grade ≥ 2) in ≥1 hand joint will be required for study entry. Additional inclusion criteria include stable analgesic requirements (including NSAIDs) for at least 4 weeks, stable doses of chondroitin or glucosamine for 4 months, no corticosteroids via any route for at least 3 months.
Exclusion criteria
(i) concomitant rheumatic disease, inflammatory joint disease, psoriatic arthritis, ankylosing spondylitis, or gout; (ii) allergic reaction to other medicines containing betamethasone dipropionate, any other corticosteroid(s), or any of the ingredients of Diprosone OV ointment (i.e. betamethasone as dipropionate, propylene glycol, white beeswax, propylene glycol monostearate, and soft white paraffin); (iii) contraindication to topical corticosteroids (e.g. untreated bacterial, fungal, or viral skin lesions; widespread plaque psoriasis; skin conditions with ulcers); (iv) unable to complete informed consent; (v) pregnancy, breast feeding, or trying to become pregnant.
Study timeline
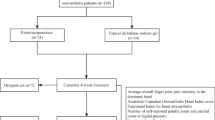
This trial began recruitment in October 2020. Recruitment is estimated to be completed in January 2022 with the study finalized by 6-week follow-up and data collection in March 2022. Figure 1 shows trial participation and study procedure.
Randomisation, allocation concealment, and blinding
Allocation of participants in a 1:1 ratio to either topical corticosteroid or placebo group will be based on computer generated random numbers prepared and securely held by the Alfred Health Clinical Trials Pharmacy. Block randomisation will be performed, stratified by gender given the higher prevalence of hand OA in females compared with males [4]. The use of a central automated allocation procedure with security in place will ensure the allocation cannot be accessed or influenced by any person. Participants, assessors and statisticians will be blinded to group allocation. Allocation concealment and blinding will be ensured by: (1) study medication being dispensed by the Alfred Health Clinical Trials Pharmacy; (2) use of an identical placebo ointment; (3) subjective measures being taken by assessors blinded to group allocation. Emergency unblinding will be allowed in limited situations that impact on the safety of study participants. Code-break for the full randomisation schedule will be maintained by the Alfred Health Clinical Trials Pharmacy, and be used in case of the need for emergency unblinding. Participants who are unblinded will be withdrawn from treatment and be requested to complete questionnaires at 6 weeks.
Intervention
Participants will be randomised to receive topical corticosteroid or placebo. Placebo will be of the same colour and appearance as the corticosteroid ointment. They are required to apply a thin layer of the study ointment to cover the painful hand joints 3 times per day for 6 weeks.
Corticosteroid
Diprosone OV (betamethasone dipropionate in Optimised Vehicle) ointment. Diprosone OV contains the active ingredient betamethasone dipropionate which is a potent corticosteroid used to decrease inflammation, redness, itchiness and discomfort of some skin conditions. Diprosone OV ointment is used to treat persistent or severe dermatitis, eczema, and acute or chronic psoriasis.
Placebo
Plain paraffin ointment.
Safety
Adverse events will be recorded throughout the study. Photos of the hands, back and front, will be taken at baseline and 6 weeks to be reviewed by a dermatologist for safety check, mainly looking for dilatation of vessels, skin wrinkling, and hypopigmentation. Photos at 3 weeks will be taken and reviewed if there is any concern.
Compliance
The remaining ointment will be weighed at the end of the study to document compliance. Weekly telephone contact over the 6 weeks will be conducted to address any concerns. This will help to mitigate non-compliance.
Concomitant medication
To maintain the pragmatic nature of the trial, there are no restrictions about the use of concomitant analgesic medications. Participants will be allowed to continue the treatments being taken at the screening visit for the duration of the trial. If a participant is experiencing severe pain and requires an increase in analgesic dose, the use of paracetamol, topical or oral NSAIDs or opioids, or a combination of these will be permitted, with the reason for the dose increase and the dose used to be documented.
Study procedure
Table 1 shows the study procedure. Volunteers will be telephone screened and undergo an x-ray of the symptomatic hand (standardized posteroanterior view) to confirm radiologic disease. In the case of bilateral symptomatic hands, the most symptomatic hand will undergo x-ray. In the case of both hands being symptomatic to an equal degree, the dominant hand will undergo x-ray. The study visits will be onsite or via telehealth. Consent will be obtained by the study doctor. At baseline, all the hand joints with a pain level of ≥40 on a 100 mm VAS will be documented on a hand map. The participants will be given a copy of this for reference when they are contacted by study staff by telephone. Participant will be asked to apply the study ointment on each of the pre-specified joints 3 times per day for 6 weeks. Participants are able to withdraw at any time during the trial; the time and reasons will be recorded. They will be requested to complete the questionnaires at 6 weeks.
Primary outcome
Pain reduction at 6 weeks will be measured according to the OARSI recommendations for the design and conduct of clinical trials for hand OA, which recommend the use of a single question pain VAS as the main outcome measure for hand pain [27]. VAS has been most frequently used for pain assessment in hand OA, with excellent reliability, good construct validity and sensitivity to change [28].
Secondary outcomes
Pain over 6 weeks
Participant will be asked weekly using a numerical rating scale (NRS) about their pain levels in general as well as in each of the study joints by referencing the hand map that they will have. Time to respond will be recorded.
Change in pain and function at 6 weeks
At baseline and 6 weeks, hand pain, physical function, and joint activity will be measured according to the OARSI recommendations [27] using the Functional Index for Hand OA (FIHOA) [29], Australian Canadian Osteoarthritis Hand Index (AUSCAN) [30], Michigan Hand Outcomes Questionnaire (MHQ) [31], and tender and swollen joint count [32].
Other measures
Success of participant blinding
Participants will be asked which treatment they believe they received.
Treatment adherence
We will weigh the ointment container before and after the study.
Adverse events, analgesic use, and co-interventions
These will be measured in a log-book and by structured questioning by the blinded assessor at each follow-up.
painDETECT
painDETECT, a validated questionnaire used to assess pain sensitization in OA [33] will be assessed at baseline and 6 weeks.
Descriptive data
Self-reported age, gender, height, weight, duration of symptoms, employment, medical history, medication use, education level, smoking, and alcohol will be collected using a questionnaire.
Sample size calculation
The mean VAS pain in a previous clinical trial of symptomatic hand OA similar to the proposed study was 54 mm (standard deviation 20 mm) [19]. The minimal clinically important difference to be detected in OA trials is a 15 mm change in VAS pain (out of 100 mm) [34]. We will require 40 participants per group to attain a power of 90% to detect the minimal clinically important difference (alpha 0.05, two-sided significance). Accounting for an estimated 20% loss to follow-up, we seek to recruit 100 participants, 50 in each group.
Statistical analyses
An intention-to-treat analysis, including all participants in their randomised groups regardless of their adherence to assigned treatments, will be performed in a blinded fashion. Pain reduction at 6 weeks will be analyzed using ANCOVA with adjustment for the baseline measure. The bootstrap approach will be used to provide 95% confidence intervals and p-values if the normality assumption is questionable. Differences between randomised groups in trajectories of pain over time will be examined using linear mixed-effects regression models with baseline score as the covariate, fixed factors for treatment, time, and treatment x time interaction, and with an autoregressive AR (1) covariance matrix for repeated measures of individuals over time. Time to respond (weeks) will be compared between randomised groups. If more than 5% of participants are missing their primary outcome, multiple imputation will be applied to account for missing data, with missing values imputed separately by treatment group, assuming data are missing at random. Additional adjustment for age, gender, body mass index, and severity of radiographic OA will be carried out in a sensitivity analysis if clinically important baseline imbalances between randomised groups are identified. Pre-specified subgroup analyses will be performed by testing a treatment by subgroup interaction in the models. This will include joint group, radiographic severity, pain level, and presence of central sensitization. Per-protocol analyses for the primary and secondary endpoints will be conducted as secondary analyses.
Data integrity and management
All data collected will be kept strictly confidential. Data will be collected using prespecified case report forms in REDCap. Paper copies of questionnaires (if participants prefer to complete the questionnaires on hard copy) will be stored in locked filing cabinets, with secured and restricted access. Electronic data will be stored in REDCap, and exported to password-protected servers with secured and restricted access after data collection, separating the identifying and non-identifying information. In the dataset, participants will be identified by study ID and randomisation code, with other information with the potential of identifying individuals removed. The codes linking data to identifying participant information will be kept separately from the study data, under password protection and with restricted access. After trial completion, case report forms will be securely archived, and electronic data will be saved on the secure server, password-protected and accessible only to study investigators. There are no planned interim analyses and stopping guidelines.
This study uses REDCap for data collection, facilitating telehealth options. For participants who use the telehealth option for the screening/baseline visit, we will seek consent electronically (eConsent). REDCap has a feature that implements consent forms through an online survey which can be accessed on a computer, mobile phone, or tablet. The completed eConsent PDFs are stored in REDCap in a File Repository under “PDF Survey Archive”.
Monitoring
The study investigators will monitor the study execution, ensuring that trial execution is consistent with the study protocol. Regular meetings will ensure efficient study completion and monitoring of adverse events. Study investigators will perform annual self-auditing of study execution according to the School of Public Health and Preventive Medicine, Monash University. We will not need a data safety monitoring board as this agent is Therapeutic Goods Administration approved with well-known safety profile.
Dissemination
Trial results, regardless of statistical significance, will be published in peer-reviewed journals and presented at national and international conferences. Upon publication of the primary manuscript, participants will be informed of their group allocation and provided with the results.
Discussion
This randomised controlled trial is conducted to determine whether topical Diprosone OV ointment administered 3 times daily reduces pain over 6 weeks in participants with symptomatic hand OA.
Synovial pathology of hand OA has shown histological changes that are consistent with those in rheumatoid arthritis and manifest the stage of disease at the time of biopsy [35]. There are increased levels of pro-inflammatory cytokines, reduced levels of anti-inflammatory cytokines, infiltration of mononuclear cells and adaptive immune cell responses within OA fluid and tissues [36,37,38]. Local inflammation has been shown to play a role in causing pain and disease progression in hand OA [1], suggesting inflammation could be a potential treatment target. Randomised controlled trials have demonstrated that oral and intra-articular injections of corticosteroids may reduce pain over short-term in hand OA [19, 20, 24], providing proof of concept that targeting inflammation in hand OA is effective for pain relief. However, there is the concern of significant adverse events associated with systemic corticosteroids [22, 23]. Topical corticosteroid would provide a safe, alternative therapeutic approach to reducing pain in hand OA, which will be tested in the proposed clinical trial.
This study will provide high-quality evidence to determine whether topical corticosteroid reduces pain over 6 weeks in patients with hand OA, with major clinical and public health importance by informing clinical practice guidelines for a potentially effective treatment option for hand OA and reducing the burden of the disabling disease.
Availability of data and materials
Data sharing is not applicable to this article as no datasets were generated or analysed during the current study.
Abbreviations
- AUSCAN:
-
Australian Canadian Osteoarthritis Hand Index
- CONSORT:
-
Consolidated Standards of Reporting Trials
- FIHOA:
-
Functional Index for Hand Osteoarthritis
- MHQ:
-
Michigan Hand Outcomes Questionnaire
- NRS:
-
Numerical rating scale
- NSAIDs:
-
Non-steroidal anti-inflammatory drugs
- OA:
-
Osteoarthritis
- OARSI:
-
Osteoarthritis Research Society International
- VAS:
-
Visual analogue scale
References
Bijlsma JW, Berenbaum F, Lafeber FP. Osteoarthritis: an update with relevance for clinical practice. Lancet. 2011;377:2115–26.
Michon M, Maheu E, Berenbaum F. Assessing health-related quality of life in hand osteoarthritis: a literature review. Ann Rheum Dis. 2011;70:921–8.
Qin J, Barbour KE, Murphy LB, Nelson AE, Schwartz TA, Helmick CG, et al. Lifetime risk of symptomatic hand osteoarthritis: the Johnston County osteoarthritis project. Arthritis Rheum. 2017;69:1204–12.
Dahaghin S, Bierma-Zeinstra SM, Ginai AZ, Pols HA, Hazes JM, Koes BW. Prevalence and pattern of radiographic hand osteoarthritis and association with pain and disability (the Rotterdam study). Ann Rheum Dis. 2005;64:682–7.
Dillon CF, Hirsch R, Rasch EK, Gu Q. Symptomatic hand osteoarthritis in the United States: prevalence and functional impairment estimates from the third U.S. National Health and nutrition examination survey, 1991-1994. Am J Phys Med Rehabil. 2007;86:12–21.
Zhang W, Doherty M, Leeb BF, Alekseeva L, Arden NK, Bijlsma JW, et al. EULAR evidence-based recommendations for the diagnosis of hand osteoarthritis: report of a task force of ESCISIT. Ann Rheum Dis. 2009;68:8–17.
Kloppenburg M, Kroon FP, Blanco FJ, Doherty M, Dziedzic KS, Greibrokk E, et al. 2018 update of the EULAR recommendations for the management of hand osteoarthritis. Ann Rheum Dis. 2019;78:16–24.
Hochberg MC, Altman RD, April KT, Benkhalti M, Guyatt G, McGowan J, et al. American College of Rheumatology 2012 recommendations for the use of nonpharmacologic and pharmacologic therapies in osteoarthritis of the hand, hip, and knee. Arthritis Care Res. 2012;64:465–74.
Kloppenburg M. Hand osteoarthritis-nonpharmacological and pharmacological treatments. Nat Rev Rheumatol. 2014;10:242–51.
Keen HI, Wakefield RJ, Grainger AJ, Hensor EM, Emery P, Conaghan PG. An ultrasonographic study of osteoarthritis of the hand: synovitis and its relationship to structural pathology and symptoms. Arthritis Rheum. 2008;59:1756–63.
Kortekaas MC, Kwok WY, Reijnierse M, Watt I, Huizinga TW, Kloppenburg M. Pain in hand osteoarthritis is associated with inflammation: the value of ultrasound. Ann Rheum Dis. 2010;69:1367–9.
Wittoek R, Carron P, Verbruggen G. Structural and inflammatory sonographic findings in erosive and non-erosive osteoarthritis of the interphalangeal finger joints. Ann Rheum Dis. 2010;69:2173–6.
Haugen IK, Boyesen P, Slatkowsky-Christensen B, Sesseng S, van der Heijde D, Kvien TK. Associations between MRI-defined synovitis, bone marrow lesions and structural features and measures of pain and physical function in hand osteoarthritis. Ann Rheum Dis. 2012;71:899–904.
Kortekaas MC, Kwok WY, Reijnierse M, Kloppenburg M. Inflammatory ultrasound features show independent associations with progression of structural damage after over 2 years of follow-up in patients with hand osteoarthritis. Ann Rheum Dis. 2015;74:1720–4.
Kortekaas MC, Kwok WY, Reijnierse M, Stijnen T, Kloppenburg M. Brief report: Association of Inflammation with development of erosions in patients with hand osteoarthritis: a prospective ultrasonography study. Arthritis Rheum. 2016;68:392–7.
Haugen IK, Slatkowsky-Christensen B, Boyesen P, Sesseng S, van der Heijde D, Kvien TK. MRI findings predict radiographic progression and development of erosions in hand osteoarthritis. Ann Rheum Dis. 2016;75:117–23.
Mathiessen A, Slatkowsky-Christensen B, Kvien TK, Hammer HB, Haugen IK. Ultrasound-detected inflammation predicts radiographic progression in hand osteoarthritis after 5 years. Ann Rheum Dis. 2016;75:825–30.
Spies CM, Strehl C, van der Goes MC, Bijlsma JW, Buttgereit F. Glucocorticoids. Best Pract Res Clin Rheumatol. 2011;25:891–900.
Kroon FPB, Kortekaas MC, Boonen A, Bohringer S, Reijnierse M, Rosendaal FR, et al. Results of a 6-week treatment with 10 mg prednisolone in patients with hand osteoarthritis (HOPE): a double-blind, randomised, placebo-controlled trial. Lancet. 2019;394:1993–2001.
Kvien TK, Fjeld E, Slatkowsky-Christensen B, Nichols M, Zhang Y, Prøven A, et al. Efficacy and safety of a novel synergistic drug candidate, CRx-102, in hand osteoarthritis. Ann Rheum Dis. 2008;67:942–8.
Wenham CY, Hensor EM, Grainger AJ, Hodgson R, Balamoody S, Doré CJ, et al. A randomized, double-blind, placebo-controlled trial of low-dose oral prednisolone for treating painful hand osteoarthritis. Rheumatology (Oxford). 2012;51:2286–94.
Sarnes E, Crofford L, Watson M, Dennis G, Kan H, Bass D. Incidence and US costs of corticosteroid-associated adverse events: a systematic literature review. Clin Ther. 2011;33:1413–32.
Waljee AK, Rogers MA, Lin P, Singal AG, Stein JD, Marks RM, et al. Short term use of oral corticosteroids and related harms among adults in the United States: population based cohort study. BMJ. 2017;357:j1415.
Kroon FP, Rubio R, Schoones JW, Kloppenburg M. Intra-articular therapies in the treatment of hand osteoarthritis: a systematic literature review. Drugs Aging. 2016;33:119–33.
Moher D, Hopewell S, Schulz KF, Montori V, Gotzsche PC, Devereaux PJ, et al. CONSORT 2010 explanation and elaboration: updated guidelines for reporting parallel group randomised trials. BMJ. 2010;340:c869.
Chan AW, Tetzlaff JM, Gøtzsche PC, Altman DG, Mann H, Berlin JA, et al. SPIRIT 2013 explanation and elaboration: guidance for protocols of clinical trials. BMJ. 2013;346:e7586.
Kloppenburg M, Maheu E, Kraus VB, Cicuttini F, Doherty M, Dreiser RL, et al. OARSI clinical trials recommendations: design and conduct of clinical trials for hand osteoarthritis. Osteoarthr Cartil. 2015;23:772–86.
Visser AW, Boyesen P, Haugen IK, Schoones JW, van der Heijde DM, Rosendaal FR, et al. Instruments measuring pain, physical function, or Patient's global assessment in hand osteoarthritis: a systematic literature search. J Rheumatol. 2015;42:2118–34.
Dreiser RL, Maheu E, Guillou GB, Caspard H, Grouin JM. Validation of an algofunctional index for osteoarthritis of the hand. Rev Rhum Engl Ed. 1995;62:43s–53s.
Bellamy N, Campbell J, Haraoui B, Gerecz-Simon E, Buchbinder R, Hobby K, et al. Clinimetric properties of the AUSCAN osteoarthritis hand index: an evaluation of reliability, validity and responsiveness. Osteoarthr Cartil. 2002;10:863–9.
Chung KC, Pillsbury MS, Walters MR, Hayward RA. Reliability and validity testing of the Michigan hand outcomes questionnaire. J Hand Surg [Am]. 1998;23:575–87.
Doyle DV, Dieppe PA, Scott J, Huskisson EC. An articular index for the assessment of osteoarthritis. Ann Rheum Dis. 1981;40:75–8.
Hochman JR, Gagliese L, Davis AM, Hawker GA. Neuropathic pain symptoms in a community knee OA cohort. Osteoarthr Cartil. 2011;19:647–54.
Tubach F, Ravaud P, Martin-Mola E, Awada H, Bellamy N, Bombardier C, et al. Minimum clinically important improvement and patient acceptable symptom state in pain and function in rheumatoid arthritis, ankylosing spondylitis, chronic back pain, hand osteoarthritis, and hip and knee osteoarthritis: results from a prospective multinational study. Arthritis Care Res. 2012;64:1699–707.
Belhorn LR, Hess EV. Erosive osteoarthritis. Semin Arthritis Rheum. 1993;22:298–306.
Goldring MB. Anticytokine therapy for osteoarthritis. Expert Opin Biol Ther. 2001;1:817–29.
Smith MD, Triantafillou S, Parker A, Youssef PP, Coleman M. Synovial membrane inflammation and cytokine production in patients with early osteoarthritis. J Rheumatol. 1997;24:365–71.
Myers SL, Brandt KD, Ehlich JW, Braunstein EM, Shelbourne KD, Heck DA, et al. Synovial inflammation in patients with early osteoarthritis of the knee. J Rheumatol. 1990;17:1662–9.
Acknowledgements
Not applicable.
Funding
The study is funded by an Investigator Grant from the National Health and Medical Research Council of Australia (NHMRC, APP1194829), and a Project Grant from Arthritis Australia. YW is the recipient of NHMRC Translating Research into Practice Fellowship (APP1168185). SMH is the recipient of NHMRC Early Career Fellowship (APP1142198). YZL is the recipient of NHMRC Clinical Postgraduate Scholarship (APP1133903) and Royal Australasian College of Physicians Woolcock Scholarship. MME is the recipient of Bangabandhu Science and Technology Fellowship from Ministry of Science and Technology, Government of the People’s Republic of Bangladesh. AEW is the recipient of the Royal Australian College of Physicians Fellows Career Development Fellowship. FMC is the recipient of NHMRC Investigator Grant (APP1194829). The funder of the study had no role in the study design and conduct of the study; collection, management, analysis and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Author information
Authors and Affiliations
Contributions
Concept and design: YW, SMH, DG, FMC; Study execution and data acquisition: YW, SMH, DG, YZL, MME, SH, AEW, FMC; Drafting of the manuscript: YW, FMC; Critical revision of the manuscript for important intellectual content and approval of the final manuscript: YW, SMH, DG, YZL, MME, SH, AEW, FMC; Obtaining of funding: YW, SMH, AEW, FMC. All authors approved the final manuscript for submission.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Ethics approval has been obtained from Alfred Hospital Ethics Committee (117/20), Monash University Human Research Ethics Committee (24219). Participants will be included after they have provided written informed consent.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Wang, Y., Hussain, S.M., Gan, D. et al. Topical corticosteroid for treatment of hand osteoarthritis: study protocol for a randomised controlled trial. BMC Musculoskelet Disord 22, 1036 (2021). https://doi.org/10.1186/s12891-021-04921-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12891-021-04921-2