Abstract
Purpose
Bone scintigraphy with 99mTc-labeled diphosphonates can identify prostate cancer bone metastases with high sensitivity, but relatively low specificity, because benign conditions such as osteoarthritis can also trigger osteoblastic reactions. We aimed to investigate the diagnostic performance of 99mTc-2,3-dicarboxy propane-1,1-diphosphonate (99mTc-DPD) uptake quantification by single-photon emission computed tomography coupled with computed tomography (SPECT/CT) for distinguishing prostate cancer bone metastases from spinal and pelvic osteoarthritic lesions.
Methods
We retrospectively assessed 26 bone scans from 26 patients with known prostate cancer bone metastases and 13 control patients with benign spinal and pelvic osteoarthritic changes without known neoplastic disease. Quantitative SPECT/CT (xSPECT, Siemens Symbia Intevo, Erlangen, Germany) was performed and standardized uptake values (SUVs) were quantified with measurements of SUVmax and SUVmean (g/mL) in all bone metastases for the prostate cancer group and in spinal and pelvic osteoarthritic changes for the control group. We used receiver operating characteristics (ROC) curves to determine the optimum SUVmax cutoff value to distinguish between bone metastases and benign spinal and pelvic lesions.
Results
In total, 264 prostate cancer bone metastases were analyzed, showing a mean SUVmax and SUVmean of 34.6 ± 24.6 and 20.8 ± 14.7 g/mL, respectively. In 24 spinal and pelvic osteoarthritic lesions, mean SUVmax and SUVmean were 14.2 ± 3.8 and 8.9 ± 2.2 g/mL, respectively. SUVmax and SUVmean were both significantly different between the bone metastases and osteoarthritic groups (p ≤ 0.0001). Using a SUVmax cutoff of 19.5 g/mL for prostate cancer bone metastases in the spine and pelvis, sensitivity, specificity, positive and negative predictive values were 87, 92, 99 and 49%, respectively.
Conclusion
This study showed significant differences in quantitative 99mTc-DPD uptake on bone SPECT/CT between prostate cancer bone metastases and spinal and pelvic osteoarthritic changes, with higher SUVmax and SUVmean in metastases. Using a SUVmax cutoff of 19.5 g/mL, high specificity and positive predictive value for metastases identification in the spine and pelvis were found, thus increasing accuracy of bone scintigraphy.
Similar content being viewed by others
Introduction
In developed countries, prostate cancer is the most frequently diagnosed cancer among men and the fifth leading cause of cancer death [1]. Bone is the main site of distant metastases [2], with a high yet underreported prevalence [3]. Standard initial local treatment options include watchful waiting, radiation therapy and radical prostatectomy. Recurrence is detected by serum elevation of prostate specific antigen (PSA). Accurate initial staging and restaging, namely the detection of bone metastases, is essential for choosing the most appropriate treatment for the patient.
99mTc-diphosphonates bone scintigraphy is the most widely available imaging modality worldwide to detect bone metastases in patients with prostate cancer. Bone scintigraphy uses DPD-labelled with 99mTc, which accumulate in remodeling bone by incorporation into the crystalline structure of calcium hydroxyapatite [3]. 99mTc-DPD bone uptake depends on bone osteoblastic activity, vascularization and environmental factors. Bone metastases of prostate cancer trigger an important osteoblastic reaction and substantially accumulate 99mTc-DPD. Planar bone scintigraphy has a high sensitivity but a relatively low specificity for characterizing bone metastases in prostate cancer patients. Indeed, benign conditions, such as degenerative joint and disk diseases, also trigger an increase in bone turnover and radiotracer accumulation. The 3D data acquired during bone scintigraphy, named single-photon emission computed tomography (SPECT), can be coupled with computed tomography (CT), a morphological imaging modality. It is well known that combined SPECT/CT increases the specificity of bone scintigraphy as the sites of increased 99mTc-DPD uptake can be correlated to morphological changes on CT images [4,5,6].
Recently, technological advances have allowed 99mTc-DPD uptake quantification (xSPECT/CT, Siemens Symbia Intevo). xSPECT has an accurate activity recovery within 10% of the expected value for objects > 10 mL, which is similar to PET/CT [7]. The aim of this study was to investigate the diagnostic performance of 99mTc-DPD uptake quantification for distinguishing bone metastases from benign spinal and pelvic osteoarthritic lesions in prostate cancer patients.
Materials and methods
Patient selection
We retrospectively analyzed 26 bone scans from 26 male patients (mean age 74 ± 10 years; range 55–92 years) with confirmed prostate cancer on biopsy or based on biological data and imaging follow-up, referred for evaluation of bone metastases between January 2016 and December 2018. The second group consisted of 13 male patients (70 ± 15 years; range 32–83 years) without any known neoplastic disease, referred for investigation of various benign musculoskeletal disorders. Patient data, including body mass index (BMI), administered 99mTc-DPD activity, creatinine levels, PSA levels and time interval between radiotracer injection and image acquisition were retrieved.
SPECT/CT image analysis
All patients underwent whole-body planar imaging with the low-energy high-resolution collimator with a scanning speed of 12 cm/min and quantitative xSPECT/CT (Siemens Symbia Intevo, Erlangen, Germany) on regions with high uptake on planar scintigraphy. The xSPECT was acquired in average at 3 h35 ± 54 min in the bone metastases group and 3 h50 ± 50 min in the osteoarthritic group, after intravenous injection of 10 MBq/kg of 99mTc-DPD (this radiotracer has been in use in our center for over 2 decades, as we believe the bone/soft tissue ratio is better) with a mean patient dose of 777 ± 113 MBq and 733 ± 101 MBq, respectively. Images were acquired with 3 degrees rotation/step and 12 s/projection with a 256 × 256 matrix. Reduced dose CT was acquired with 130 kV and 25 reference mAs modulation (Siemens Care Dose, Symbia Intevo, Erlangen, Germany). Images were reconstructed to generate SPECT data allowing SUVbw quantification on post-processed images and measurement of SUVmax and SUVmean (g/mL) using xSPECT reconstruction algorithm.
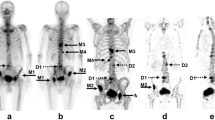
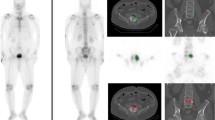
For each patient in the bone metastases group, SUVmax and SUVmean of all prostate cancer bone metastases visible on SPECT and CT were measured (Fig. 1a). For each patient in the control group, SUVmax and SUVmean were measured in the active degenerative changes of the lumbar spine and pelvis on SPECT/CT (Fig. 1b). For all patients, the SUVmean of lumbar vertebrae was measured in a 4 to 5 cm3 region of interest (ROI), with no metastatic or osteoarthritic lesion visible on SPECT and CT.
a Osteoblastic lesion of the pelvis in a 57-year-old male patient known for prostate cancer, showing a high SUVmax of 28 g/mL and SUVmean of 17 g/mL. b Lumbar spine osteoarthritic changes of the L4-L5 facet joints in a 83-year-old male patient with hip pain, showing SUVmax of 15 and 13 g/mL and SUVmean of 9.0 and 7.8 g/mL in the left and right facet joints, respectively
Statistical analysis
Statistical differences between bone metastases and osteoarthritic groups regarding age, BMI, creatinine levels, lumbar vertebrae SUVmax and SUVmean, time interval between radiotracer injection and image acquisition, were calculated using the Wilcoxon rank-sum test. Mean values, standard deviations (SD) of SUVmax and SUVmean in both metastatic and osteoarthritic groups were calculated and statistical differences assessed by the Wilcoxon rank-sum test. Subgroup analysis taking into account the metastases location was performed in the bone metastases group. Differences in SUVmax and SUVmean between subgroups were assessed using one-way ANOVA. We also used receiver operating characteristics (ROC) curves to determine the best-fit cutoff values of SUVmax between metastatic and osteoarthritic lesions, with computation of the respective sensitivity, specificity, positive and negative predictive values. All statistical analyses were performed using STATA (version 15.1; STATA Corp., College Station, Texas, USA). P-values less than 0.05 were considered as statistically significant.
Results
There were no significant differences between the bone metastases and osteoarthritic groups regarding age, BMI, creatinine levels, time between radiotracer injection and xSPECT imaging and lumbar vertebrae SUVmax and SUVmean (Table 1). The average lumbar vertebrae SUVmax and SUVmean of all patients were 8.8 ± 2.3 and 6.9 ± 1.9 g/mL, respectively. The PSA level in the metastatic group was 206 ± 573 μg/L.
A total number of 265 prostate cancer bone metastases (221 osteoblastic, 5 osteolytic, 35 mixed and 4 non-classified) were analyzed, showing a mean SUVmax and SUVmean of 35 ± 25 and 21 ± 15 g/mL, respectively. In the osteoarthritic group, 24 active focal osteoarthritic changes (20 spinal and 4 pelvic) were analyzed and showed a mean SUVmax and SUVmean of 14.2 ± 3.8 and 8.9 ± 2.2 g/mL, respectively. SUVmax and SUVmean were both significantly different between bone metastatic and osteoarthritic lesions (p < 0.0001) (Fig. 2a).
In the bone metastases group, there were 87 lesions in the pelvis (SUVmax 44 ± 25 g/mL and SUVmean 26 ± 15 g/mL), 84 lesions in the spine (SUVmax 39 ± 28 g/mL and SUVmean 24 ± 17 g/mL), 28 lesions in the scapular girdle (SUVmax 27 ± 12 g/mL and SUVmean 16 ± 7.2 g/mL), 54 lesions in the ribs (SUVmax 18 ± 8.9 g/mL and SUVmean 10.6 ± 5.1 g/mL), and 12 lesions in the extremities (SUVmax 33 ± 26 g/mL and SUVmean 20 ± 16 g/mL). SUVmax and SUVmean of metastatic lesions in the spine and pelvis were significantly higher than in osteoarthritic lesions (p < 0.0001), whereas there was no significant difference between SUVs of osteoarthritic changes and metastatic lesions in the ribs, scapular girdle, and the extremities (p = 0.53, p = 1.0 and p = 0.3, respectively) (Fig. 2b).
In the bone metastases group, 16 patients had previous therapy before relapse. All 16 patients had hormone therapy, one patient had Xofigo (223Radium dichloride) and immunotherapy prior to bone scan and another patient had chemotherapy. SUVmax and SUVmean of the bone metastases were not significantly lower in patients having had previous systemic therapy compared to patients without previous treatment (SUVmax 32 ± 22 versus 40 ± 29 g/mL, p = 0.18, and SUVmean 20 ± 14 versus 23 ± 17 g/mL, p = 0.3) (Fig. 3a). Interestingly, this difference became significant when only spinal and pelvic bone metastases were considered: SUVmax 37 ± 24 versus 50 ± 30 g/mL (p = 0.01) and SUVmean 23 ± 14 versus 30 ± 18 g/mL (p = 0.03), respectively (Fig. 3b).
ROC curves showed that both SUVmax and SUVmean had very good diagnostic accuracy for differentiating between spinal and pelvic bone metastases and osteoarthritic changes (AUC 0.947 and 0.943, respectively) (Fig. 4). The optimum cutoff value of SUVmax for defining spinal and pelvic prostate cancer bone metastases was 19.5 g/mL. Using this cutoff value, we found a sensitivity, specificity, positive and negative predictive values of 87% [95% CI: 81–91%], 92% [73–99%], 99% [95–100%] and 49% [34–64%], respectively. This cutoff value remained identical even in patients who had prior therapy, with a sensitivity, specificity, positive and negative predictive values of 86% [95% CI: 78–91%], 92% [73–99%], 98% [93–100%] and 56% [40–72%], respectively.
Discussion
In this study, we showed that quantification in bone scintigraphy could help in distinguishing prostate cancer bone metastases from spinal and pelvic osteoarthritic changes, and therefore increase bone scan specificity. Using an optimum SUVmax cutoff of 19.5 g/mL for defining spinal and pelvic bone metastases on SPECT/CT, bone scan yielded a sensitivity, specificity, positive and negative predictive values of 87, 92, 99 and 49%, respectively.
To the best of our knowledge, only few studies previously reported on the quantification of 99mTc-DPD uptake in bone metastases of prostate cancer patients. Beck et al. showed that the mean SUVpeak of metastatic lesions in breast and prostate cancer patients was 20.4 ± 20.8 g/mL [8]. This difference is at least partly due to the fact that SUVpeak is usually lower than SUVmax. Moreover, patients included in their study were both prostate and breast cancer patients combined, with a majority of the latter. Since breast cancer metastases are both osteoblastic and osteolytic, their uptake is probably lower than that of prostate cancer metastases, which are mostly osteoblastic as in our patient population. SUVs were further higher in our study than those reported by Umeda et al., who found a lower threshold of 7 g/mL of SUVmax, above which the tumor burden of metastatic prostate cancer patients was determined [9]. Kuji et al. reported a high accuracy of quantitative SPECT/CT for the diagnosis of bone metastases in 170 prostate cancer patients [10]. They used a different reconstruction algorithm based on CT zonal mapping and included only the three hottest lesions explaining a slightly higher SUVmax and SUVmean (SUVmax of 35 ± 25 g/mL in our study versus 41 ± 34 g/mL in the study by Kuji et al., and a mean SUVmean of 21 ± 15 g/mL versus 24.6 ± 21.2 g/mL, respectively). We had similar quantitative results compared to Kuji et al. regarding radiotracer uptake by spinal osteoarthritic changes with a mean SUVmax of 14.2 ± 3.8 g/mL compared to 16.7 ± 6.7 g/mL, and a mean SUVmean of 8.9 ± 2.2 g/mL compared to 9.5 ± 3.9 g/mL, respectively. Interestingly, SUVs were comparable although the radiotracers used for the bone scan were slightly different: 99mTc-DPD versus 99mTc-methylene diphosphonate (99mTc-MDP) in the Kuji et al. study. Thus, SUVs seem not only comparable from one center to another but also comparable regardless of which type of diphosphonate is used. This is further reinforced by comparable lumbar vertebrae SUVmean in our study compared to the vertebral SUVmean in the study by Cachovan et al. (SUVmean 6.9 ± 1.9 in our study versus 5.91 ± 1.54 in the Cachovan et al. study) [11].
In our study, SUVmax and SUVmean of metastatic lesions varied depending on the lesion location, with lowest values in the ribs and scapular girdle, which is similar to the observations from Beck and al [8]. This is probably at least partly due to the size of the lesions, which tend to be smaller in the ribs and scapular girdle with partial volume effect, as described for PET [12]. Different vascularization and osteoblastic reaction in these different anatomical regions may also play a role. There was no significant difference in SUVmax and SUVmean between metastatic lesions in the ribs, scapular girdle and extremities, and osteoarthritic lesions; hence, no cutoff value could be obtained to distinguish between them. For lesions in the spine and pelvis, we found an optimum SUVmax cutoff of 19.5 g/mL with a very high sensitivity and positive predictive value. We believe this cutoff could be of added diagnostic value enabling physicians to decide with a high accuracy that a focal 99mTc-DPD uptake in the spine or pelvis above 19.5 g/mL is most likely to be metastatic in a patient with known prostate cancer.
In this study, SUVmax was significantly lower in spinal and pelvic bone metastases of patients relapsing after prior systemic therapy compared to patients with no previous systemic treatment. This is not surprising as previous treatment may induce sclerosis, reduced vascularization, or other tumor environment changes, which can all influence 99mTc-DPD uptake. Understanding the mechanism of reduced uptake in these lesions may help better understand the bone tumor microenvironment of prostate metastases [13]. Interestingly, the SUVmax cutoff of 19.5 g/mL still had a high positive predictive value for spinal and pelvic bone metastases in patients having received prior treatment for bone metastases.
There are of course other nuclear medicine modalities available for the detection and characterization of bone metastases in prostate cancer patients. 68Ga-prostate specific membrane antigen (PSMA), 18F-choline and 18F-sodium fluoride (NaF) PET/CT all have very good accuracies for the diagnosis of prostate cancer bone metastases [14,15,16,17]. The most promising modality seems to be 68Ga-PSMA PET/CT [18, 19]. Nonetheless, in many countries, 68Ga-PSMA, 18F-Choline and 18F-NaF PET are not widely available for primary staging due to cost and reimbursement issues, as impact on patient management and cost-effective studies are not yet available [20]. A study comparing PSA cutoff value for ordering 18F-NaF PET or bone scintigraphy in patients with newly diagnosed prostate cancer showed no major difference between both modalities [20]. Furthermore, a recent study by Arvola et al. showed a strong correlation between SUVs from 99mTc-HDP SPECT/CT and 18F-NaF PET/CT [21]. The authors concluded that SPECT is an applicable tool for clinical quantification of bone metabolism in osseous metastases in breast and prostate cancer patients.
Therefore, quantitative bone scintigraphy seems to increase bone SPECT/CT accuracy, allowing bone scintigraphy to remain competitive in the era of new multimodality imaging of bone metastases in prostate cancer patients. We believe that the SUVmax cutoff of 19.5 mg/L for spinal and pelvic lesions could further increase bone scan specificity.
The main limitations of our study are the small subject population and the lack of histological confirmation for all prostate cancer bone metastases. However, as authors reported that histological confirmation may be avoided in the case of typical morphological imaging findings and patterns of radiotracer uptake [22], lesions were thus diagnosed as metastatic on conventional SPECT/CT and were subsequently analyzed for tracer uptake quantification. In addition, since we proceeded with a lesion-based analysis, the relatively small number of patients did not allow correlating further the level of uptake with the different histological grades of prostate cancer.
Conclusion
This study demonstrated significant differences in 99mTc-DPD uptake on bone scan between prostate cancer bone metastases and spinal and pelvic osteoarthritic changes based on quantitative data analysis, with significantly higher SUVmax and SUVmean in metastases. Using an optimum SUVmax cutoff of 19.5 g/mL for defining spinal and pelvic bone metastases on SPECT/CT, bone scan yielded a sensitivity, specificity, positive and negative predictive values of 87, 92, 99 and 49%, respectively. Hence, adding quantitative data analysis to bone scan interpretation can help to characterize more confidently malignant versus benign spinal and pelvic focal bone lesions, and thus increase the overall diagnostic performance of bone scintigraphy. The main limitations of this study remain the small subject population and lack of histological confirmation of the metastatic lesions.
Availability of data and materials
The datasets analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- 95%CI:
-
95% confidence interval
- 99mTc-DPD:
-
99mTc-2,3-dicarboxy propane-1,1- diphosphonate
- 99mTc-MDP:
-
99mTc-methylene diphosphonate
- NaF:
-
Sodium fluoride
- PSA:
-
Prostate specific antigen
- PSMA:
-
Prostate specific membrane antigen
- ROC:
-
Receiver operating characteristics
- SD:
-
Standard deviation
- SPECT/CT:
-
Single-photon emission computed tomography coupled with computed tomography
- SUV:
-
Standardized uptake value
- xSPECT:
-
Quantitative xSPECT
References
Torre LA, et al. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65(2):87–108.
Lin SC, et al. Endothelial-to-osteoblast conversion generates osteoblastic metastasis of prostate cancer. Dev Cell. 2017;41(5):467–80 e3.
Kanishi D. 99mTc-MDP accumulation mechanisms in bone. Oral Surg Oral Med Oral Pathol. 1993;75(2):239–46.
Helyar V, et al. The added value of multislice SPECT/CT in patients with equivocal bony metastasis from carcinoma of the prostate. Eur J Nucl Med Mol Imaging. 2010;37(4):706–13.
Strobel K, et al. Characterization of focal bone lesions in the axial skeleton: performance of planar bone scintigraphy compared with SPECT and SPECT fused with CT. AJR Am J Roentgenol. 2007;188(5):W467–74.
Saha S, et al. SPECT-CT: applications in musculoskeletal radiology. Br J Radiol. 2013;86(1031):20120519.
Gnesin S, et al. Phantom validation of Tc-99m absolute quantification in a SPECT/CT commercial device. Comput Math Methods Med. 2016;2016:4360371.
Beck M, et al. Longitudinal analysis of bone metabolism using SPECT/CT and (99m)Tc-diphosphono-propanedicarboxylic acid: comparison of visual and quantitative analysis. EJNMMI Res. 2016;6(1):60.
Umeda T, et al. Evaluation of bone metastatic burden by bone SPECT/CT in metastatic prostate cancer patients: defining threshold value for total bone uptake and assessment in radium-223 treated patients. Ann Nucl Med. 2018;32(2):105–13.
Kuji I, Yamane T, Seto A, Yasumizu Y, Shirotake S, Oyama M. Skeletal standardized uptake values obtained by quantitative SPECT/CT as an osteoblastic biomarker for the discrimination of active bone metastasis in prostate cancer. Eur J Hybrid Imag. 2017;1(1):2.
Cachovan M, et al. Quantification of 99mTc-DPD concentration in the lumbar spine with SPECT/CT. EJNMMI Res. 2013;3(1):45.
van der Vos CS, et al. Quantification, improvement, and harmonization of small lesion detection with state-of-the-art PET. Eur J Nucl Med Mol Imaging. 2017;44(Suppl 1):4–16.
Wong SK, et al. Prostate cancer and bone metastases: the underlying mechanisms. Int J Mol Sci. 2019;20(10):2587.
Pyka T, et al. Comparison of bone scintigraphy and (68) Ga-PSMA PET for skeletal staging in prostate cancer. Eur J Nucl Med Mol Imaging. 2016;43(12):2114–21.
Garcia JR, et al. Diagnostic performance of bone scintigraphy and (11) C-choline PET/CT in the detection of bone metastases in patients with biochemical recurrence of prostate cancer. Rev Esp Med Nucl Imagen Mol. 2015;34(3):155–61.
Even-Sapir E, et al. The detection of bone metastases in patients with high-risk prostate cancer: 99mTc-MDP planar bone scintigraphy, single- and multi-field-of-view SPECT, 18F-fluoride PET, and 18F-fluoride PET/CT. J Nucl Med. 2006;47(2):287–97.
Beheshti M, et al. Evaluation of prostate cancer bone metastases with 18F-NaF and 18F-Fluorocholine PET/CT. J Nucl Med. 2016;57(Suppl 3):55S–60S.
Rathke H, et al. Intra-individual comparison of Tc-99m-MDP bone scan and the PSMA-ligand Tc-99m-MIP-1427 in patients with osseous metastasized prostate cancer. J Nucl Med. 2018;59:1373–79.
Zacho HD, et al. (68) Ga-PSMA PET/CT for the detection of bone metastases in prostate cancer: a systematic review of the published literature. Clin Physiol Funct Imaging. 2018;38:911–92.
Cook GJ, Azad G, Padhani AR. Bone imaging in prostate cancer: the evolving roles of nuclear medicine and radiology. Clin Transl Imaging. 2016;4(6):439–47.
Arvola S, et al. Comparison of standardized uptake values between (99m)Tc-HDP SPECT/CT and (18) F-NaF PET/CT in bone metastases of breast and prostate cancer. EJNMMI Res. 2019;9(1):6.
Goyal P, et al. Elastofibroma dorsi. Proc (Bayl Univ Med Cent). 2017;30(3):340–2.
Acknowledgments
We thank Christine Geldhof for managing the ethical committee requirements and all nuclear medicine technologists involved in the patient data acquisitions.
Declarations
Compliance with Ethical Standards, Consent and Permissions.
Funding
This study benefited from no funding.
Author information
Authors and Affiliations
Contributions
MJ, MNL and JOP designed the study. FT and MJ analyzed the bone scans and retrieved the data. MJ, MNL and JOP performed the statistical analysis. MJ, FT and MNL drafted the manuscript. FB, JOP and NS critically revised the manuscript. All authors read and approved the final version of manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The procedure followed was in accordance with the ethical standards of the institutional research committee. The local Ethics Research Committee of the State of Vaud approved this research protocol (CER-VD #2017–01492) and, considering the retrospective nature of the study, waived the need for obtaining patient informed consent. Patients who had refused to sign the general hospital informed consent form were excluded from the study. Patients who signed the general hospital informed consent or did not receive it were included in this retrospective study.
Consent for publication
Both patients whose images appear in the figures of this paper have signed the general hospital informed consent.
Competing interests
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Tabotta, F., Jreige, M., Schaefer, N. et al. Quantitative bone SPECT/CT: high specificity for identification of prostate cancer bone metastases. BMC Musculoskelet Disord 20, 619 (2019). https://doi.org/10.1186/s12891-019-3001-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12891-019-3001-6