Abstract
Background
Undiagnosed, or diagnosed and untreated osteoporosis (OP) increases the likelihood that falls result in hip fractures, decreased quality of life (QOL), and significant medical expenditures among older adults. We tested whether a tailored dual energy x-ray absorptiometry (DXA) test result letter and an accompanying educational bone-health brochure affected patient satisfaction, QOL, or OP knowledge.
Methods
The Patient Activation after DXA Result Notification (PAADRN) study was a double-blinded, pragmatic, randomized trial which enrolled patients from 2012 to 2014. We randomized 7,749 patients presenting for DXA at three health care institutions in the United States who were ≥ 50 years old and able to understand English. Intervention patients received a tailored letter four weeks after DXA containing their results, 10-year fracture risk, and a bone-health educational brochure. Control patients received the results of their DXA per the usual practices of their providers and institutions. Satisfaction with bone health care, QOL, and OP knowledge were assessed at baseline and 12- and 52-weeks after DXA. Intention-to-treat analyses used multiple imputation for missing data and random effects regression models to adjust for clustering within providers and covariates.
Results
At 12-weeks 6,728 (86.8 %) and at 52-weeks 6,103 participants (78.8 %) completed their follow-up interviews. The intervention group was more satisfied with their bone health care compared to the usual care group at both their 12- and 52-week follow-ups (standardized effect size = 0.28 at 12-weeks and 0.17 at 52-weeks, p < 0.001). There were no differences between the intervention and usual care groups in QOL or OP knowledge at either time point.
Conclusions
A tailored DXA result letter and bone-health educational brochure sent to patients improved patient satisfaction with bone-related health care.
Trial registration
Clinical Trials.gov Identifier: NCT01507662 First received: December 8, 2011.
Similar content being viewed by others
Background
Although only 10 % of Americans ≥ 50 years old are currently diagnosed with osteoporosis (OP) based on dual energy x-ray absorptiometry (DXA) testing [1], the lifetime risk of a low-impact fracture is 40 % for older women and 13 % for older men [2]. Hip fractures, related to OP, are associated with increased mortality and serious adverse effects on quality of life (QOL) and health care costs [3]. Many older adults know very little about their OP risks or the rationale for screening to identify this silent disease [4, 5]. As suggested by health behavior theories, like the Health Belief Model [6], knowledge of OP, its risks and how to prevent or improve OP, is a first step for patients in making bone health-related behavior changes (i.e. getting adequate amounts of calcium, vitamin D and weight-bearing exercise, taking appropriate pharmacotherapy, preventing falls).
Some randomized controlled trials (RCTs) have demonstrated that educational interventions improve OP-related knowledge [7–16]. Less is known about whether such interventions affect patient satisfaction or QOL [17, 18]. Most of these RCTs examining OP patient-education interventions have been multifaceted or lengthy [7, 12, 13, 15, 16], which are not scalable. To date, only one RCT has examined the effect of an OP education intervention on QOL, reporting a beneficial effect after both three months and one year [16]. To our knowledge only three studies have shown that patients receiving educational or quality improvement interventions were more satisfied with their bone-health care than usual care groups [16, 19, 20]. However, two of these studies were conducted only with older women with an OP diagnosis [16, 19]. The other study only saw improved satisfaction for timeliness in test result notification but not in other measures of satisfaction (e.g. understanding DXA results or treatment options) [20]. Because tailored interventions individualized to the patient’s characteristics are more effective and preferred by patients than standardized interventions [21, 22], we developed a tailored, pragmatic patient-activation intervention.
We reported that 90 % of older adults wanted to receive their DXA results by mail [23]. Therefore, we developed a DXA result letter and a bone-health educational brochure. We then designed and conducted a pragmatic RCT to assess the impact of this intervention on the pathways leading to appropriate pharmacotherapy, health behavior change, and satisfaction with bone-health care, QOL, OP knowledge, and cost-effectiveness (www.ClinicalTrials.gov Identifier NCT01507662). This article describes the effects of the intervention on three patient-reported outcomes: satisfaction with bone health care, QOL, and OP knowledge at 12 and 52 weeks post-baseline. We hypothesized that the intervention would improve bone-health care satisfaction and OP knowledge at both time points, but would not change QOL.
Methods
Participants
Patients ≥ 50 years old presenting for DXA between February 2012 and August 2014 at the University of Iowa (UI), University of Alabama at Birmingham (UAB), and Kaiser Permanente of Georgia (KPGA) were invited to participate. We excluded non–English speakers and prisoners. Twenty dollar gift cards were provided after the baseline interview.
Design and randomization
We used a double-blind, parallel, pragmatic RCT [24]. After patients completed their DXA and baseline interviews they were randomized based on their providers using a computer program written in R [25]. Providers were first ranked within sites based on the number of DXAs they ordered in the previous two years, and then they were randomized within sites using blocks of three into three groups (1:1:1 allocation ratio). Patients of providers in the first group were assigned to the intervention arm, patients of providers in the second group were assigned to the usual care arm, and patients of providers in the third group were randomized to either the usual care or intervention arms (1:1 allocation ratio). We selected three provider randomization groups to assess potential spill-over effects on the main outcomes for usual care patients of providers in the third group.
Procedures
At baseline, research assistants (RAs) at each site used REDCap™ [26] computer assisted interviewing (CAI) software to interview patients up to four weeks before or three days after their DXA. All KPGA patients and half of the UI patients completed these interviews in person. All UAB patients and the remaining UI patients completed their baseline interviews over the telephone. Three RAs at UI mailed study materials to intervention patients.
Intervention
Intervention materials included a letter describing results of their DXA (lowest T-score and interpretation [OP, low BMD or normal]), a graphic portrayal of their 10-year probability for a major osteoporotic fracture (using FRAX®; https://www.shef.ac.uk/FRAX/), and a bone-health educational brochure. These materials have been described elsewhere [27, 28].
Outcomes
Twelve weeks and 52 weeks after their DXA, Iowa Social Science Research Center interviewers telephoned patients at all three sites to conduct follow-up interviews using WinCATI 4 · 2 and 5 · 0 CAI software (Sawtooth Technologies, Northbrook, IL). Interview questions have been described elsewhere [24].
OP Care Satisfaction. This five-item scale assesses patient satisfaction with notification and understanding DXA results, understanding OP treatments, receiving adequate information to make an informed decision, and overall satisfaction with bone-health care. Response options ranged from strongly agree to strongly disagree, with summary scores ranging from 5 (least satisfied) to 25 (most satisfied). Four of these items were used in a previous study [20], with a fifth item related to overall satisfaction added for the current study. Because this was the first use of the satisfaction with OP care scale after its development and initial publication, we used exploratory factor and reliability analyses to explore its psychometric properties. Those results revealed a simple factor structure that was unidimensional with principal factor loadings for each item ranging from 0.53 to 0.77, and an internal consistency reliability (alpha) coefficient of 0.77. At baseline, these items were only asked of patients with prior DXAs because they were irrelevant for DXA naïve patients. All patients were asked these questions at the 12- and 52-week interviews.
Quality of Life. We used three QOL measures. The first was the SF-1 (“In general, would you say your health is excellent, very good, good, fair, or poor”), which was scored as 95, 90, 80, 30, and 15 to reflect the underlying health utilities [29]. The second QOL measure was the EQ-5D-3 L which has five items assessing difficulties with mobility, self-care, activities of daily living, pain, and mood (α = 0 · 70) [30, 31]. Responses (no difficulties, some, or were completely impaired) were converted to health utilities ranging from 0 (death) to 1 (best health state) [30, 31]. The third QOL measure was the EuroQol Visual Analog Scale (VAS) which asks patients to rate their health from 1 (worst health state they can imagine) to 100 (best health state they can imagine) [30, 31].
OP Knowledge. We used the 10-item “Osteoporosis and You” scale [5, 32] to measure. The five responses ranged from strongly agree (SA) to strongly disagree (SD), which were collapsed into “correct” or “incorrect” responses. Correct responses (SA or A for true or SD or D for false statements) were coded “1” with incorrect responses coded “0”. We summed the responses into a total score (α = 0 · 68). We also examined the subscales (biological, lifestyle, consequences, and prevention and treatment).
Statistical considerations and analysis
PAADRN was powered for guideline concordant treatment as the clinical endpoint. Therefore, for the current analysis, we calculated the statistical power that we would have to detect a standardized effect size of 0.10 with p < 0.05 and attrition from baseline as high as 20 %. Those calculations indicated that we would have 91.3 % power to detect such differences. We used multiple imputation techniques to account for missingness (lost to follow-up, patient refused a specific question or responded “don’t know”). We imputed each item separately and constructed the outcomes based on the imputed values. Our primary analysis was based on intention-to-treat (ITT).
We first compared the outcome measures between the intervention and control groups at baseline and at the two follow-ups using t-tests. We then used linear random effects regression methods to adjust for patient clustering within provider and for pre-specified covariates. For patient satisfaction, we first examined differences between intervention and control groups at 12-weeks and 52-weeks (baseline patient satisfaction was only asked for those with prior DXAs). Among those with prior DXAs, we adjusted for baseline satisfaction in separate models. For QOL and OP knowledge we examined differences between baseline and 12-weeks, and baseline and 52-weeks. We used Bonferroni methods to adjust for testing at two time points (12- and 52-weeks) and for using three QOL measures. We examined minimally important differences (MIDs) defined distributionally as improvements ≥ 0.5 standard deviations (SD) [33]. For the EQ-5D utility score we also used an anchor-based approach to predict utility scores for the pairwise SF-1 comparisons (adjusting for age, gender, and race).
We also investigated pre-specified heterogeneity of treatment (HTE) effects. These included median splits on preferred approaches to health care decision-making and treatments [34], those with prior DXAs vs. those without, those on OP-medications at baseline vs. those who were not, those with a history of OP or osteopenia at baseline vs. those who did not, site (UAB vs. KPGA vs. UI), age (<65 vs. 65-75 vs. > 75), men vs. women, Whites vs. non-Whites, education (high school or less vs. some college vs. graduate school), self-rated health (poor vs. fair vs. good vs. very good vs. excellent), having COPD, depression, or prior fracture at baseline (vs. not), FRAX risk (low vs. moderate vs. high), current smoker vs. former smoker vs. never smoked, heavy vs. moderate alcohol consumption, and median splits on weight-bearing exercise.
In sensitivity analyses we used case-wise deletion instead of multiple imputation. Because those results were entirely consistent with the results presented below that used multiple imputation, we only report the latter here. With the Bonferroni adjustments, all p-values were 2-tailed with ≤ 0.025 deemed statistically significant for patient satisfaction and OP-related knowledge, and < 0.0083 deemed statistically significant for the three measures of QOL. Analyses were performed using SAS 9 · 4 (SAS Institute Inc., Cary, NC).
Results
Participant enrollment and characteristics
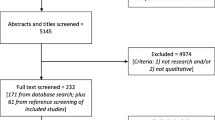
There were 20,397 potentially eligible patients, of whom 7,782 agreed to participate, were interviewed at baseline, and were then randomized to either the intervention or usual care groups (Fig. 1). Of these, 33 patients were randomized in error and were removed from the study, leaving 7,749 patients. Of these, 6,728 (86.8 %) completed the 12-week and 6,107 (78.8 %) completed the 52-week follow-up interviews. All 7,749 randomized participants were included in the analysis using intent-to-treat principles.
Table 1 presents baseline characteristics for all 7,749 participants. The mean age of our participants was 66 years, 84 % were women, 77 % were White, and 67 % had previously undergone DXA. The intervention and usual care groups were similar in age, sex, race, education, and self-reported health. The usual care group, however, was more likely to have had OP prior to baseline (p = 0.001), and to have had an index DXA indicating low bone mineral density (BMD) or OP (p = 0.001).
Patient satisfaction with OP health care
Intervention patients had significantly greater (better) levels of patient satisfaction with their bone-health care than the usual care group at both 12-weeks (1.0 points, standardized effect size = 0.28, p < 0.001; Table 2) and 52-weeks (0.6 points, standardized effect size = 0.21, p < 0.001). Adjustments for clustering within providers and the covariates did not alter these differences (1.02 points at 12-weeks and 0.63 at 52-weeks, p < 0.0005; Table 3). Patients in the intervention group had 58 % greater odds of having an MID improvement (AOR = 1.58, p < 0.0005) at 12-weeks and 34 % greater odds at 52-weeks (AOR = 1.34, p < 0.005). We observed comparable results in all of the HTE comparison groups (not shown).
Additionally, we repeated the analyses at the item level. In the pooled unadjusted and adjusted analyses at both 12- and 52–weeks, the intervention group reported significantly (p ≤ 0.002) higher satisfaction for each of the five satisfaction items (data not shown). When stratified by prior DXA use, the unadjusted results were significant (p ≤ 0.03) for each of the five satisfaction items with one exception (data not shown); at 52-weeks among prior DXA users the intervention did not have a significant effect (p = 0.14) on the item related to their overall-satisfaction with bone-health care.
Quality of life
Intervention and control participants had similar mean scores on all three QOL measures at baseline (SF-1, p = 0.925; EQ-5D-3 L, p = 0.676; and, EuroQol VAS, p = 0.590; Table 2). Mean scores for all three QOL measures did not differ between groups at either 12- or 52-weeks (Table 2). The changes from baseline to the two follow-ups are not significant for all three QOL measures (data not shown). No significant differences were observed in the random effects models, either, for any of the three QOL measures (Table 3). Similarly, no significant differences were observed for MID improvements for any of the three QOL measures. In additional analyses at the item level, no significant (p > 0.05) effects of the intervention were observed for any of the QOL items (data not shown).
OP Knowledge
The intervention and usual care groups had the same mean scores for OP knowledge (7.5, SD 1.9; Table 2). OP knowledge increased significantly by 0.3 points for both the intervention and usual care groups between baseline and the 12- and 52-week follow-ups (p < 0.001), but there was no difference in the amount of the increase between the two groups (p =0.759 at 12-weeks and p = 0.479 at 52-weeks; Table 2). Adjustment for patient clustering within provider, and for the covariates did not alter these findings (Table 3). In additional analyses at the item level, no significant (p > 0.05) effects of the intervention were observed for any of the OP knowledge items (data not shown).
Discussion
There is growing interest in engaging patients in their own healthcare [35, 36]. Tailoring health communication to be more patient-centered is becoming more common. Yet, it is unclear whether greater access to tailored, DXA testing communication results in any measurable improvements in patient reported outcomes. We designed a, pragmatic, multi-site, RCT to evaluate the effects of a tailored DXA result letter accompanied by a bone-health educational brochure on patient satisfaction with OP care, QOL, and OP knowledge. Our results revealed significantly improved patient satisfaction with OP care in the intervention group compared to the usual care group. Intervention patients had 58 % greater odds of improving by at least an MID (0.5 SD) at 12-weeks (p < 0.0005) and 34 % greater odds off improving by at least an MID at 52-weeks (p < 0.005). However, we found no differences in terms of QOL or OP knowledge between the intervention and usual care groups.
These findings are important because, to our knowledge, this is the first positive study to include a comparison group, and to include patients with and without OP. Patient satisfaction is recognized as an important dimension of healthcare quality. Medicare now evaluates patient satisfaction [37] which will soon be used in determining preventive service reimbursements for doctors and hospitals. The intervention materials were pilot tested to ensure comprehension as well as patient preferences for information and design [27, 28]. Tailoring test result communications to patients may improve their satisfaction with other types of testing as well.
As important as the effect on satisfaction with OP care is, failure to improve QOL or OP knowledge in our study also must be considered. We did hypothesize that the patient-activation intervention would not affect overall QOL because OP care is a small component of the healthcare received by older adults who may have several comorbidities. Indeed, OP would likely have minimal effects on QOL in the short-term until a fracture occurs, at which point profound effects on QOL. Thus, our results are consistent with prior studies of OP patient education interventions on QOL [11, 38], in which only one reported a significant improvement among Malaysian women taking bisphosphonates [16]. Moreover, trials of OP therapies, which demonstrate reduced fracture rates, seldom are powered to detect an effect on QOL for some of the reasons noted.
The absence of an effect of the patient-activation intervention on OP knowledge is surprising and contrary to our expectations. Prior OP-education interventions have reported significant improvements in knowledge [7–16]. In contrast, we found that OP knowledge significantly increased in both intervention and usual care groups, but that the magnitude of these improvements was the same. This may be due to the measure of OP knowledge. First, the reliability of the OP knowledge measure was only marginally acceptable (α = 0.68) [39]. Second, this was the first RCT to use the “Osteoporosis and You” measure, and the two prior observational studies assessing its psychometric properties included younger women and not men [5, 32]. Third, significant practice effects (about half of an additional correct answer) were observed and these may have created a ceiling effect that constrained our ability to detect short-term differences. Finally, the null effect may be due to the fact that neither the patient-activation letter nor the educational brochure were targeted to the “Osteoporosis and You” measure, although an ad hoc analysis of the four items most closely reflecting the intervention did not reveal an effect either (data not shown). Although several other measures of OP knowledge were available when our study began, we eliminated them because they were too long and cumbersome [40, 41] or were designed for younger women who did not have OP [42]. Improved measures of OP knowledge are needed, particularly among those known to have OP.
Despite its strengths, our RCT had limitations. First, the patient satisfaction with OP health care scale had not been used in RCTs designed to improve bone health. Second, we did not use an OP-specific QOL measure, which might have been more responsive to our patient-activation intervention. Lastly, given the clinical centers used, our study population may not have been representative of all osteoporosis patients.
Conclusion
In conclusion, because of increases in the number and percentage of older Americans at risk for OP and hip fractures, there is a growing need for better OP health care and patient knowledge about the prevention, treatment, and consequences of this disease that remains silent until fracture. We developed a pragmatic and tailored patient-activation intervention that improved OP care satisfaction. Future research and quality improvement projects should examine whether patient satisfaction scores in other clinical domains or in general would increase when providing patients with their test results in a tailored manner.
Abbreviations
- BMD:
-
Bone mineral density
- CAI:
-
Computer assisted interviewing
- DXA:
-
Dual energy x-ray absorptiometry
- HTE:
-
Heterogeneity of treatment
- ITT:
-
Intention-to-treat
- KPGA:
-
Kaiser permanente of Georgia
- OP:
-
Osteoporosis
- PAADRN:
-
Patient activation after DXA result notification
- QOL:
-
Quality of life
- RAs:
-
Research assistants
- RCT:
-
Randomized controlled trial
- SA:
-
Strongly agree
- SD:
-
Standard deviation
- SD:
-
Strongly disagree
- UAB:
-
University of Alabama at Birmingham
- UI:
-
University of Iowa
- VAS:
-
EuroQol visual analog scale
References
Wright NC, Looker AC, Saag KG, Curtis JR, Delzell ES, Randall S, Dawson-Hughes B. The recent prevalence of osteoporosis and low bone mass in the United States based on bone mineral density at the femoral neck or lumbar spine. J Bone Miner Res. 2014;29(11):2520–6.
US Department of Health and Human Services. Bone health and osteoporosis: a report of the Surgeon General. In: Office of the Surgeon General. Rockville: US Department of Health and Human Services; 2004.
Wolinsky FD, Fitzgerald JF, Stump TE. The effect of hip fracture on mortality, hospitalization, and functional status: a prospective study. Am J Public Health. 1997;87(3):398–403.
Doheny MO, Sedlak CA, Estok PJ, Zeller R. Osteoporosis knowledge, health beliefs, and DXA T-scores in men and women 50 years of age and older. Orthop Nurs. 2007;26(4):243–50.
Cadarette SM, Gignac MA, Beaton DE, Jaglal SB, Hawker GA. Psychometric properties of the “Osteoporosis and You” questionnaire: osteoporosis knowledge deficits among older community-dwelling women. Osteoporos Int. 2007;18(7):981–9.
Bandura A. Social foundations of thought and action. Englewood Cliffs, NJ: Prentice-Hall; 1986.
Babatunde OT, Himburg SP, Newman FL, Campa A, Dixon Z. Theory-driven intervention improves calcium intake, osteoporosis knowledge, and self-efficacy in community-dwelling older Black adults. J Nutr Educ Behav. 2011;43(6):434–40.
Schulman JE, Williams S, Khera O, Sahba T, Michelson J, Fine K. Effective osteoporosis education in the outpatient orthopaedic setting. J Bone Joint Surg Am. 2007;89(2):301–6.
Kulp JL, Rane S, Bachmann G. Impact of preventive osteoporosis education on patient behavior: immediate and 3-month follow-up. Menopause (New York, NY). 2004;11(1):116–9.
Qi BB, Resnick B, Smeltzer SC, Bausell B. Self-efficacy program to prevent osteoporosis among Chinese immigrants: a randomized controlled trial. Nurs Res. 2011;60(6):393–404.
Majumdar SR, McAlister FA, Johnson JA, Weir DL, Bellerose D, Hanley DA, Russell AS, Rowe BH. Critical impact of patient knowledge and bone density testing on starting osteoporosis treatment after fragility fracture: secondary analyses from two controlled trials. Osteoporos Int. 2014;25(9):2173–9.
Oh EG, Yoo JY, Lee JE, Hyun SS, Ko IS, Chu SH. Effects of a three-month therapeutic lifestyle modification program to improve bone health in postmenopausal Korean women in a rural community: a randomized controlled trial. Res Nurs Health. 2014;37(4):292–301.
Wu F, Laslett LL, Wills K, Oldenburg B, Jones G, Winzenberg T. Effects of individualized bone density feedback and educational interventions on osteoporosis knowledge and self-efficacy: a 12-years prospective study. J Clin Densitom. 2014;17(4):466–72.
Montori VM, Shah ND, Pencille LJ, Branda ME, Van Houten HK, Swiglo BA, Kesman RL, Tulledge-Scheitel SM, Jaeger TM, Johnson RE, et al. Use of a decision aid to improve treatment decisions in osteoporosis: the osteoporosis choice randomized trial. Am J Med. 2011;124(6):549–56.
Francis KL, Matthews BL, Van Mechelen W, Bennell KL, Osborne RH. Effectiveness of a community-based osteoporosis education and self-management course: a wait list controlled trial. Osteoporos Int. 2009;20(9):1563–70.
Lai PS, Chua SS, Chan SP. Impact of pharmaceutical care on knowledge, quality of life and satisfaction of postmenopausal women with osteoporosis. Int J Clin Pharm. 2013;35(4):629–37.
Cram P, Rosenthal GE, Ohsfeldt R, Wallace RB, Schlechte J, Schiff GD. Failure to recognize and act on abnormal test results: the case of screening bone densitometry. Jt Comm J Qual Patient Saf. 2005;31(2):90–7.
Shibli-Rahhal A, Vaughan-Sarrazin MS, Richardson K, Cram P. Testing and treatment for osteoporosis following hip fracture in an integrated U.S. healthcare delivery system. Osteoporos Int. 2011;22(12):2973–80.
Waalen J, Bruning AL, Peters MJ, Blau EM. A telephone-based intervention for increasing the use of osteoporosis medication: a randomized controlled trial. Am J Manag Care. 2009;15(8):e60–70.
Cram P, Schlechte J, Christensen A. A randomized trial to assess the impact of direct reporting of DXA scan results to patients on quality of osteoporosis care. J Clin Densitom. 2006;9(4):393–8.
Noar SM, Benac CN, Harris MS. Does tailoring matter? Meta-analytic review of tailored print health behavior change interventions. Psychol Bull. 2007;133(4):673–93.
Ryan P, Lauver DR. The efficacy of tailored interventions. J Nurs Scholarsh. 2002;34(4):331–7.
Cram P, Schlechte J, Rosenthal GE, Christensen AJ. Patient preference for being informed of their DXA scan results. J Clin Densitom. 2004;7(3):275–80.
Edmonds SW, Wolinsky FD, Christensen AJ, Lu X, Jones MP, Roblin DW, Saag KG, Cram P. The PAADRN Study: A design for a randomized controlled practical clinical trial to improve bone health. Contemp Clin Trials. 2013;34(1):90–100.
R Core Team. A language and environment for statistical computing. In: R Foundation for Statistical Computing. Vienna: R Development Core Team; 2015.
Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377–81.
Edmonds SW, Solimeo SL, Lu X, Roblin DW, Saag KG, Cram P. Developing a bone mineral density test result letter to send to patients: a mixed-methods study. Patient Preference Adherence. 2014;8:827–41.
Edmonds SW, Cram P, Lu X, Roblin DW, Wright NC, Saag KG, Solimeo SL. Improving bone mineral density reporting to patients with an illustration of personal fracture risk. BMC Med Inform Decis Mak. 2014;14:101.
Diehr P, Patrick DL, Spertus J, Kiefe CI, McDonell M, Fihn SD. Transforming self-rated health and the SF-36 scales to include death and improve interpretability. Med Care. 2001;39(7):670–80.
Euroqol Group. EuroQol--a new facility for the measurement of health-related quality of life. Health Policy (Amsterdam, Netherlands). 1990;16(3):199-208.
Brooks R. EuroQol: the current state of play. Health policy (Amsterdam, Netherlands). 1996;37(1):53–72.
Brenneman SK, Blau EM, Chen Y, Abbott TA. Validation of a patient questionnaire, 'Osteoporosis and You', designed to assess osteoporosis-related attitudes, knowledge and behavior. J Bone Miner Res. 2002;17 Suppl 1:S466.
Norman GR, Sloan JA, Wyrwich KW. Interpretation of changes in health-related quality of life: the remarkable universality of half a standard deviation. Med Care. 2003;41(5):582–92.
Krantz DS, Baum A, Wideman M. Assessment of Preferences for self-treatment and information in health care. J Pers Soc Psychol. 1980;39(5):977–90.
Epstein RM, Street Jr RL. The values and value of patient-centered care. Ann Fam Med. 2011;9(2):100–3.
Hibbard JH, Greene J. What the evidence shows about patient activation: better health outcomes and care experiences; fewer data on costs. Health Aff (Project Hope). 2013;32(2):207–14.
HCAHPS: Patients’ Perspectives of Care Survey [http://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-instruments/HospitalQualityInits/HospitalHCAHPS.html]. Accessed 23 July 2015.
Yuksel N, Majumdar SR, Biggs C, Tsuyuki RT. Community pharmacist-initiated screening program for osteoporosis: randomized controlled trial. Osteoporos Int. 2010;21(3):391–8.
Nunnally JC. Psychometric theory. 2nd ed. New York, NY: McGraw-Hill; 1978.
Kim KK, Horan ML, Gendler P. Osteoporosis Knowledge Test, Osteoporosis Health Belief Scale, and Osteoporosis Self-Efficacy Scale. Allendale, MI: Grand Valley State University; 1991.
Pande KC, de Takats D, Kanis JA, Edwards V, Slade P, McCloskey EV. Development of a questionnaire (OPQ) to assess patient's knowledge about osteoporosis. Maturitas. 2000;37(2):75–81.
Winzenberg TM, Oldenburg B, Frendin S, Jones G. The design of a valid and reliable questionnaire to measure osteoporosis knowledge in women: the Osteoporosis Knowledge Assessment Tool (OKAT). BMC Musculoskelet Disord. 2003;4:17.
Acknowledgements
We thank Sylvie Hall, BA (UI), Rebecca Burmeister, MPH (UI), Mollie Giller, MPH (UI), April Miller RT (UI), CBDT, Amna Rizvi-Toner, BA, BS (UI), Brandon Van Cleave (UI), Kara Wessels, BA (UI), Roslin Nelson (KPGA), Kathy Pines (KPGA), Aimee Khamar (KPGA), and Brandi Robinson, MPH (KPGA), and all of the staff at the Iowa Social Science Research Center for recruiting and interviewing all study participants. All except Ms. Miller were compensated from grant funds for their time. We also thank Sylvie Hall, BA (MPH), Mollie Giller, MPH (UI), Ryan Outman, MS (UAB), Marina Reynolds, BSN (UI), Brandi Robinson, MPH (KPGA), and Jessica Williams, PhD (UAB) for coordinating and facilitating recruitment of study participants. We also thank Xin Lu, MS, and Thuy Nguyen, MS (UI) for managing trial data. Finally, we thank the 7,749 patients who participated in PAADRN.
Jeffrey R. Curtis, MD, MPH, Sarah L. Morgan, RD, MD, CCD, FACP, Janet A. Schlechte, MD, PhD, David J. Zelman, MD
Funding
This work was supported by R01 AG033035 (Cram/Wolinsky) from the NIA at NIH. Dr. Cram is supported by a K24 AR062133 award from NIAMS at the NIH. Dr. Saag is supported by a K24 AR052361 award from the NIAMS at the NIH. The NIA had no role in the design and conduct of the study; collection, management, analysis, and interpretation of data; preparation, review, or approval of the manuscript; or, decision to submit the manuscript for publication.
Availability of data and materials
Data will be shared at the conclusion of the study funding period (March 2017). Researchers wishing to view this data before that time may email the first author.
Authors’ contributions
SE served as the project coordinator of the PAADRN study, participated in the design and coordination of the study, oversaw data collection and management, and drafted the manuscript. PC was the co-principal investigator, conceived of the study, led in its design, and helped to draft the manuscript. YL was the lead data analyst and helped to draft the manuscript. MJ participated in the design of the randomization, oversaw the statistical analysis, and helped draft the manuscript. DR was a site principal investigator, participated in its design and coordination and helped to draft the manuscript. KS was a site principal investigator, participated in its design and coordination and helped to draft the manuscript. NW DR was an investigator, participated in its design and coordination and helped to draft the manuscript. FW was a co-principal investigator, led in its design and coordination and drafted the manuscript. All authors read and approved the final manuscript.
Competing interests
The authors declare that they have no competing interests. We have no conflicts of interests to report on this work. KS has received consulting fees from Amgen, Eli Lilly, Merck, and Novartis and grant funding from Amgen, Merck, Novartis, and Eli Lilly unrelated to this work.
Consent for publication
Not applicable.
Ethics and consent to participate
The University of Iowa Human Subjects Office, the University of Alabama at Birmingham’s Office of the IRB, and the Kaiser Permanente of Georgia Institutional Review Board approved the study procedures. All participants provided either written (University of Iowa) or verbal (University of Alabama and Kaiser Permanente) consent as required by each site’s IRB.
Author information
Authors and Affiliations
Consortia
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Edmonds, S.W., Cram, P., Lou, Y. et al. Effects of a DXA result letter on satisfaction, quality of life, and osteoporosis knowledge: a randomized controlled trial. BMC Musculoskelet Disord 17, 369 (2016). https://doi.org/10.1186/s12891-016-1227-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12891-016-1227-0