Abstract
Background
Both osteoarthritis (OA) and cardiovascular diseases (CVD) are prevalent conditions which often co-exist. Vascular involvement in the pathogenesis of these diseases, as well as increased cardiovascular risk in OA patients give occasion to investigate arterial stiffness in OA. The aim of this study was to establish associations between OA and arterial stiffness.
Methods
The characteristics of arterial stiffness were measured with Sphygmocor and HDI devices in 48 patients (age 63 ± 7 years (mean ± SD)) with end-stage OA awaiting knee and hip replacement and in 49 age and gender matched controls (61 ± 7 years). Independent Student’s t-test or the Mann-Whitney U test was used to compare means between the groups. Correlation between variables was determined using Pearson’s or Spearman’s correlation analysis and stepwise multiple regression analysis.
Results
Carotid-femoral pulse wave velocity (car-fem PWV) was increased in the patients with OA compared to the controls (9.6 ± 2.4 and 8.4 ± 1.9 m/s, p = 0.015 respectively). High-sensitivity C-reactive protein and white blood cells count were significantly higher in the OA patients compared with the controls (1.80 ± 1.10 and 1.48 ± 1.32 mg/l, p = 0.042; 6.5 ± 1.5 and 5.6 ± 1.9 109/l, p = 0.001 respectively). In multiple regression analysis age (p < 0.001), mean arterial blood pressure (p = <0.001) and OA status (p = 0.029) were found to be independent predictors of car-fem PWV.
Conclusions
This study showed that patients with OA had increased aortic stiffness compared to non-OA controls. The potential link between arterial stiffening and OA suggests that vascular alterations are involved in OA pathogenesis and could be responsible for increased cardiovascular risk in end-stage OA patients.
Similar content being viewed by others
Background
Osteoarthritis (OA) is a chronic progressive disease that affects the whole joint: cartilage, bone, joint capsule, synovial membrane, ligaments and periarticular muscles. Due to overweight and aging populations, the disease has a huge and growing socioeconomic impact [1]. Knee OA alone affects more than 250 million people worldwide [2]. OA patients have increased cardiovascular risk [3–5]. In a prospective study, Barbour et al. [4] found that radiographic hip OA increased cardiovascular mortality by 25 % and all-cause mortality by 43 %. Similarly, in a prospective longitudinal study, Rahman et al. [6] found OA to be associated with higher risk of ischemic heart disease. However, it remains to be elucidated whether OA and cardiovascular diseases (CVD) are causally related, or whether this association is due to shared risk factors.
Arterial stiffness is an established marker of increased cardiovascular risk [7, 8] but it has rarely been studied in OA patients. There is evidence that hand OA, a more systemic form of OA, is related to increased arterial stiffness [9]. In contrast, a study of 206 patients with symptomatic knee OA found no association between arterial stiffness and bone marrow lesions, a surrogate marker for OA [10]. However, the data about possible interactions between OA and arterial stiffness are contradictory and need further research.
Many mechanisms responsible for OA pathogenesis are also involved in development of vascular damage. Common risk factors include aging, obesity and limited physical activity, as well as high exposure to non-steroidal anti-inflammatory drugs that increase CVD risk. Also, excessive oxidative stress has been found to occur in patients with OA [11, 12]. Oxidative stress has a significant role in development of OA by promoting inflammation, cellular senescence and apoptosis [13]. Chronic low-grade inflammation is crucial in deterioration of OA joints [14, 15]. Analogously, arterial stiffness and endothelial dysfunction have also been found to be associated with oxidative stress [16, 17] and inflammation [18, 19]. Obesity is considered a risk factor for both OA and CVD. Adipose tissue produces cytokines and adipokines that damage cartilage and arteries [20, 21]. Thus, there is a potential pathogenetic link between OA and increased arterial stiffness via inflammation and oxidative stress. The aim of this present study was to elucidate, besides conventional cardiovascular risk factors, also the role of arterial stiffness in patients with OA.
Methods
Study population
Patients, with primary knee or hip OA, who were eligible for total joint replacement at Tartu University Hospital, Estonia in 2014–2015, were included. The OA was assessed using the American College of Rheumatology’s criteria for knee and hip OA [22, 23]. Our study includes both knee and hip OA and focuses on systemic changes that accompany OA. Subjects with pre-existing hypertension were included. Patients with any acute or chronic inflammatory disease, diabetes, coronary artery disease, cardiac arrhythmias or known valve pathology, peripheral atherosclerotic disease, malignancies or renal insufficiency (eGFR < 60 ml/min/1.73 m2) were excluded.
Age and sex matched controls with no prior joint problems were selected from family medical practices. The family medical practices are in the catchment area of Tartu University Hospital. Selection was made, using the group matching strategy, on the basis of clinical examination and not on plain radiography, which is known to be insensitive to early OA [24] and correlates poorly with patients’ disability (pain, function) and disease severity [25]. The exclusion criteria for the control group were (based on interviews, clinical examinations and blood tests): any concomitant acute or chronic inflammatory disease, a visit to their family practitioner due to hip or knee joint complaints, any persistent knee or hip joint pain, diabetes, coronary artery disease, cardiac arrhythmia, cerebrovascular or peripheral artery disease, malignancies and renal insufficiency.
The study was conducted according to the Helsinki declaration and received approval from the Ethics Committee of the University of Tartu. Written informed consent was obtained from all participants.
Study protocol
Blood samples were collected from the antecubital fossa between 07:00–11:00 after an overnight fast and abstinence from tobacco. Study participants’ height, weight, waist and hip circumference were recorded and joint-specific evaluation was performed. The Harris Hip Score (HHS) was used for hip joint evaluation and the Hospital for Special Surgery (HSS) Knee Score was used for knee joint evaluation. Both of these tests have a scale of 0–100, where 100 is the best possible outcome. After 15 min of rest in a temperature controlled quiet room in a supine position, participants’ blood pressure and pulse wave velocity (PWV) were recorded and pulse wave analysis was made. All pulse wave parameters were measured at least twice and the average was calculated.
Hemodynamic measurements
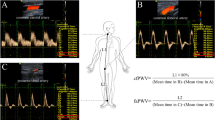
Blood pressure was measured using a digital oscillometric device (A&D UA-767; A&D Company Ltd., Tokyo, Japan) and the mean of three readings was recorded. Mean arterial pressure was obtained by integration of the radial pressure waveform using the SphygmoCor software (SCOR Px, 7.0; AtCor Medical, Sydney, Australia). Arterial stiffness was measured using pulse wave analysis and PWV. Left radial artery waveforms were recorded with a high fidelity micromanometer (SPT-301BH; Millar Instruments, Tx., USA). Corresponding ascending aortic waveforms were then generated using a transfer function to calculate central hemodynamics and the augmentation index (AIx). The AIx was corrected for a heart rate of 75 beats per minute. Carotid-femoral (car-fem) and carotid-radial (car-rad) PWV were measured by sequentially recording ECG-gated carotid and femoral or radial artery waveforms using a Sphygmocor device (SphygmoCor; AtCor Medical, Sydney, Australia) and the foot-to-foot method [26]. The pulse waveform was recorded from the right radial artery with a Cardiovascular Profiling Instrument (HDI/Pulse Wave CR-2000; Hypertension Diagnostics Inc., Eagan, USA) which measures large (C1) and small (C2) artery elasticity.
Laboratory analysis
Plasma glucose, complete blood count, triglycerides, total cholesterol, LDL-cholesterol and HDL-cholesterol, creatinine and urea were measured by standard laboratory methods, using certified assays, in a local clinical laboratory.
Radiographic evaluation
Standard weight-bearing antero-posterior knee and hip joint radiographs were taken in the OA group, no radiographs were taken in the control group. Osteoarthritis was assessed according to the Kellgren-Lawrence grading system, where: 0 = no changes; 1 = doubtful joint space narrowing; 2 = definite osteophytes and doubtful joint space narrowing; 3 = definite ostephytes, joint space narrowing, sclerosis and possible deformity; 4 = marked joint space narrowing, large osteophytes, severe sclerosis and definite bone deformity [27]. The radiographs were evaluated independently by two raters blinded to the clinical data and a consensus score was used.
Statistical analysis
The statistical analysis software SPSS 22.0 (SPSS Inc., Chicago IL, USA) for windows was used for data analysis. The Shapiro- Wilk test was used to ascertain whether variables were normally distributed. Continuous variables are presented as means with ± standard deviation. Dichotomous variables are presented as prevalence in number. A two tailed Student’s t-test was used for detecting differences between the groups for normally distributed data and logarithmic transformation or Mann-Whitney U test was used for non-parametric data. Pearson’s correlation coefficient and Spearman’s rho were used to identify associations between continuous variables within the study groups. A Chi-square test or Fischer’s exact test was used to compare group proportions where appropriate. Multiple linear regression analyses with the use of a forward and backward stepwise variable selection procedure, wereperformed to investigate the independent associations between variables. The authors set the goal to detect a difference of 1 m/s in car-fem PWV. The standard deviation for the study group was estimated at 2.0. Statistical power calculations showed that group size had to be n = 41 for either group with an Alpha error level of 5 % and a Beta error level of 20 %. In order to further minimize Beta error level the number of subjects was increased to 48 + 49. Interrater reliability analysis, with the use of intraclass correlation coefficient (ICC), was performed to determine consistency among the raters of radiographic severity of OA.
Results
The study population consisted of a total of 97 participants of whom 48 were with hip or knee osteoarthritis and 49 were healthy volunteers. The general anthropometric characteristics of the participants are presented in Table 1. Both groups had approximately the same age, BMI, male to female ratio, waist to hip ratio and number of active smokers. The OA group included 23 subjects and the control group included 19 subjects with hypertension, of whom 10 in either group were using hypertension medications. There was a clear difference in HHS and HSS Knee Score between the groups: both were significantly higher in the control group. The median radiographic Kellgren-Lawrence score in the OA group was 3 (range 2–4). The ICC for the raters was found to be ICC = 0.73, 95 % CI (0.53-0.85).
Hemodynamics
There was a significant difference between the OA patients and the controls in central arterial stiffness parameter. Car-fem PWV was significantly higher in the OA group compared to controls (Table 2). Central blood pressure and peripheral blood pressure did not differ significantly between the study groups. There was no significant difference in pulse pressure amplification, or in C1 and C2 levels between the OA and non-OA groups. There was significant difference in car-fem PWV between the hip and the knee OA patients (9.2 ± 2.0 and 10.6 ± 2.7 m/s, p = 0.042).
Laboratory analysis
The results of laboratory analysis are presented in Table 2. Triglyceride level was significantly higher in the OA patients compared to the controls. The levels of LDL and HDL-cholesterol, glucose, urea and eGFR did not differ between the groups. High-sensitivity C-reactive protein (hsCRP) and white blood cells count were significantly higher in the OA group and the difference in platelet count was of borderline significance.
In the OA patients, serum urea level correlated positively with car-fem PWV (Fig. 1) and central systolic blood pressure (Fig. 2). There were no associations between parameters of arterial stiffness and urea in the control group. In multiple regression analysis (Table 3), where car-fem PWV was set as the dependent variable, age (p < 0.001), mean arterial blood pressure (p = <0.001) and OA status (p = 0.029) were found to be independent predictors while urea was not significant (p = 0.233).
Discussion
The results of this case-control study demonstrate that OA patients have increased central arterial stiffness compared to non-OA controls. The study gives a comprehensive overview of arterial stiffness measured, with the use of different validated methods in end-stage OA patients and controls. The study used both patient related outcome measure and (SF36) and physician related outcome measures. To the best of our knowledge, this is the first study to demonstrate increased level of car-fem PWV- gold standard measure of arterial stiffness- in hip and knee OA patients.
Stiffening of the arteries causes an increase in systolic and a decrease in diastolic pressure [28]. Rise in systolic pressure increases the cardiac afterload while lower diastolic pressure reduces coronary perfusion [29]. Arterial stiffness is one of the main determinants of cardiovascular risk and has been found to be an independent risk factor for the development of CVD and increased mortality in many epidemiological studies [30–32]. Implementation of arterial stiffness assessment into clinical practice helps identify patients with higher cardiovascular risk [32]. Car-fem PWV is the gold standard method for quantifying arterial stiffness and has been accepted as an intermediate endpoint for cardiovascular events [8]. The AIx is a parameter that mainly describes wave reflections [33]. In healthy vessels the reflected waves augment diastolic pressure in the aortic root and hence increase coronary blood flow; however, in the case of stiffened arteries, waves arrive earlier in systole and increase end-systolic pressure [33]. Measurements of central blood pressure have been found to be a better predictor of future cardiovascular events than brachial blood pressure [34]. Furthermore, there is evidence that central blood pressure is more closely related to end-organ damage than peripheral blood pressure [35–37]. The C2 describes primarily wave reflections that are produced by small arteries and branching points [37] and has been shown to independently predict mortality and future cardiovascular events [31, 38].
In our study we found significantly higher car-fem PWV in the OA patients compared to the apparently healthy controls of approximately the same age and with similar BMI. Since there were no differences in blood pressure or the proportion of hypertensive subjects, the effect of hypertension on arterial stiffness was similar in both study groups. Arterial stiffness parameters have rarely been studied in OA patients. Saleh et al. [9] found association between hand OA and car-fem PWV but it was largely attributable to the confounding effect of age. Goldsmith et al. [10] found no difference in arterial stiffness between subjects with and without knee bone marrow lesions. The discrepancy between the above results and those of the current study could be explained by the fact that our patients had a more advanced stage of OA. Due to pain and functional disability, the level of physical activity is limited in the advanced stages of OA. Since physical activity is associated with arterial stiffness [39], increased pain and higher functional disability status might explain increased arterial stiffness in OA patients. In support of our hypothesis, a recent study has also found elevated stiffness of the aorta in end-stage OA patients [40]. Many factors, such as reduced physical activity, inflammation and oxidative stress, which are present across end-stage OA patients might be responsible for the stiffer arteries. Obesity is a known risk factor of both OA and CVD; Adipokines (white adipose tissue derived hormones) are associated with CVD and have also been shown to play an important role in OA development and progression, which allows to link OA with obesity. Aging is another potential link between OA and CVD. Accumulation of advanced glycation end-products is a feature of aging that is present in both diseases [41, 42].
Although OA has generally been considered a non-inflammatory condition, the role of inflammation is increasingly being recognised [43–45]. We found increased level of hsCRP in OA patients. The hsCRP is a marker of low-grade systemic inflammation and has been shown to increase in OA [43]. In addition, inflammation and elevated levels of hsCRP have been directly associated with arterial stiffness [16] and increased cardiovascular risk [31]. Oxidative stress has also been found to be related to arterial stiffness and endothelial dysfunction [16]. Analogously, there is evidence to suggest that oxidative stress is involved in the pathogenesis of OA by causing chondrocyte senescence and apoptosis [13]. We found a significantly higher level of oxidative stress index in patients with OA compared to healthy controls (paper in preparation), which is in line with previous results and with the hypothesis that oxidative stress might be at least partly responsible for the increased arterial stiffness in OA.
A number of studies have demonstrated higher CVD prevalence among OA patients [4, 6]. One plausible explanation for the association of CVD with OA is that vascular pathology plays a role in development of OA. Vascular damage to the subchondral bone has been proposed as a possible initiator of OA [46]. Blood supply to the subchondral bone region may be disturbed by microemboli and venous stasis. Highly vascularized epiphysis is mainly supplied with blood via the epiphyseal artery, which makes this region of high nutrient demand particularly susceptible to perfusion insufficiencies. In support of this hypothesis, Chang et al. [47] found evidence that osteoblasts and chondrocytes from osteoarthritic joints suffer from hypoxia. Furthermore, they also found that hypoxia induced production of matrix metalloproteinase 9 and proangiogenic factors and caused reduction in osteoblast mineralized bone nodule formation, which are all characteristic of OA. Whilst the hypothesis of vascular involvement in OA pathology is gaining support, the potential role of increased arterial stiffness in OA patients’ increased cardiovascular risk remains unclear.
We found car-fem PWV to be positively correlated with urea in OA patients. Since urea is the end-product of amino acid metabolism, this association highlights the possible role of altered amino acid metabolism in OA patients. Changes in amino acid profiles and impaired kidney function have been shown to contribute to increased arterial stiffness [48, 49]. On the basis of eGFR levels, which were approximately the same across both groups in this study, there was no difference in the kidney function. After adjusting for the effects of age and mean pressure, the association between urea and car-fem PWV was no longer significant. Higher applied forces (higher blood pressure) in aorta and aging is a strong independent predictors of car-fem PWV [50]. It is therefore important to adjust for mean pressure and age when evaluating predictors of car-fem PWV. In the present study, the association between urea level and car-fem PWV was largely due to the confounding effects of age and mean arterial blood pressure. Additional analysis exploring the effects of body composition, smoking status, functional status and use of anti inflammatory drugs on arterial stiffness in OA patients should be within the scope of future studies.
This study had several limitations that need to be recognised. First, because of the cross-sectional design of this study, it was not possible to establish causal associations between OA and arterial stiffness. Second, since no radiographs were taken in the control group, some of the controls might have had asymptomatic OA and a type II error might have been introduced. Third, this study had a relatively small sample size and hence limited statistical power; concequently, the results need to be verified by larger studies. Fourth, this study did not account for effects of the body composition, smoking status, functional status or use of anti inflammatory drugs, which might influence arterial stiffness.
Conclusions
In conclusion, our results provide evidence that patients with end-stage OA have increased central arterial stiffness. Arterial stiffness might be important regarding the development of OA and increased cardiovascular risk. However, causal association between OA and vascular stiffness needs to be confirmed by larger-scale studies.
References
Litwic A, Edwards M, Dennison E, Cooper C. Epidemiology and burden of osteoarthritis. Br Med Bull. 2013;105:185–99.
Vos T, Flaxman AD, Naghavi M, Lozano R, Michaud C, Ezzati M, et al. Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990–2010: a systematic analysis for the global burden of disease study 2010. Lancet. 2012;380:2163–96.
Hochberg M. Mortality in osteoarthritis. Clin Exp Rheumatol. 2008;26:120.
Barbour KE, Lui LY, Nevitt MC, Murphy LB, Helmick CG, Theis KA, et al. Hip osteoarthritis and the risk of all-cause and disease-specific mortality in older women: population-based cohort study. Arthritis Rheumatol. 2015;67:1798–805.
Gordon M, Rysinska A, Garland A, Rolfson O, Aspberg S, Eisler T, Garellick G, Stark A, Hailer NP, Sköldenberg O. Increased long-term cardiovascular risk after total hip arthroplasty: a nationwide cohort study. Medicine (Baltimore). 2016;95:e2662. doi:10.1097/MD.0000000000002662.
Rahman MM, Kopec JA, Anis AH, Cibere J, Goldsmith CH. Risk of cardiovascular disease in patients with osteoarthritis: a prospective longitudinal study. Arthritis Care Res. 2013;65:1951–8.
Vlachopoulos C, Aznaouridis K, Stefanadis C. Prediction of cardiovascular events and all-cause mortality with arterial stiffness: a systematic review and meta-analysis. J Am Coll Cardiol. 2010;30;55:1318-27. doi:10.1016/j.jacc.2009.10.061.
Laurent S, Boutouyrie P, Asmar R, Gautier I, Laloux B, Guize L, et al. Aortic stiffness is an independent predictor of all-cause and cardiovascular mortality in hypertensive patients. Hypertension. 2001;37:1236–41.
Saleh AS, Najjar SS, Muller DC, Shetty V, Ferrucci L, Gelber AC, et al. Arterial stiffness and hand osteoarthritis: a novel relationship? Osteoarthr Cartil. 2007;15:357–61.
Goldsmith GM, Aitken D, Cicuttini FM, Wluka AE, Winzenberg T, Ding CH, et al. Osteoarthritis bone marrow lesions at the knee and large artery characteristics. Osteoarthr Cartil. 2014;22:91–4.
Davies CM, Guilak F, Weinberg JB, Fermor B. Reactive nitrogen and oxygen species in interleukin-1-mediated DNA damage associated with osteoarthritis. Osteoarthr Cartil. 2008;16:624–30.
Ziskoven C, Jäger M, Zilkens C, Bloch W, Brixius K, Krauspe R. Oxidative stress in secondary osteoarthritis: from cartilage destruction to clinical presentation? Orthop Rev. 2010;2:e23.
Altay MA, Ertürk C, Bilge A, Yaptı M, Levent A, Aksoy N. Evaluation of prolidase activity and oxidative status in patients with knee osteoarthritis: relationships with radiographic severity and clinical parameters. Rheumatol Int. 2015;21 [Epub ahead of print].
Kapoor M, Martel-Pelletier J, Lajeunesse D, Pelletier JP, Fahmi H. Role of proinflammatory cytokines in the pathophysiology of osteoarthritis. Nat Rev Rheumatol. 2011;7:33–42.
Sokolove J, Lepus CM. Role of inflammation in the pathogenesis of osteoarthritis: latest findings and interpretations. Ther Adv Musculoskelet Dis. 2013;5:77–94.
Kals J, Kampus P, Kals M, Pulges A, Teesalu R, Zilmer K, Kullisaar T, Salum T, Eha J, Zilmer M. Inflammation and oxidative stress are associated differently with endothelial function and arterial stiffness in healthy subjects and in patients with atherosclerosis. Scand J Clin Lab Invest. 2008;68:594–601.
Lessiani G, Santilli F, Boccatonda A, Iodice P, Liani R, Tripaldi R, Saggini R, Davì G. Arterial stiffness and sedentary lifestyle: role of oxidative stress. Vascul Pharmacol. 2015;2 [Epub ahead of print].
Mahmud A, Feely J. Arterial stiffness is related to systemic inflammation in essential hypertension. Hypertension. 2005;46:1118–22.
Kals J, Zagura M, Serg M, Kampus P, Zilmer K, Unt E, Lieberg J, Eha J, Peetsalu A, Zilmer M. β2-microglobulin, a novel biomarker of peripheral arterial disease, independently predicts aortic stiffness in these patients. Scand J Clin Lab Invest. 2011;71:257–63.
Thijssen E, van Caam A, van der Kraan PM. Obesity and osteoarthritis, more than just wear and tear: pivotal roles for inflamed adipose tissue and dyslipidaemia in obesity-induced osteoarthritis. Rheumatology (Oxford). 2015;54:588–600. doi:10.1093/rheumatology/keu464.
Martin SS, Qasim A, Reilly MP. Leptin resistance: a possible interface of inflammation and metabolism in obesity-related cardiovascular disease. J Am Coll Cardiol. 2008;7(52):1201–10. doi:10.1016/j.jacc.2008.05.060.
Altman R, Alarcon G, Appelrouth D, Bloch D, Borenstein D, Brandt K, et al. The American College of Rheumatology criteria for the classification and reporting of osteoarthritis of the hip. Arthritis Rheum. 1991;34:505–14.
Altman R, Asch E, Bloch D, Bole G, Borenstein D, Brandt K, et al. The American College of Rheumatology criteria for the classification and reporting of osteoarthritis of the knee. Arthritis Rheum. 1986;29:1039–49.
Peat G, Thomas E, Duncan R, Wood L. Is a “false-positive” clinical diagnosis of knee osteoarthritis just the early diagnosis of pre–radiographic disease? Arthritis Care Res. 2010;62:1502–6.
Cubukcu D, Sarsan A, Alkan H. Relationships between pain function and radiographic findings in osteoarthritis of the knee: a cross-sectional study. Arthritis. 2012;2012:984060.
Wilkinson IB, Fuchs SA, Jansen IM, Spratt JC, Murray GD, Cockcroft JR, et al. Reproducibility of pulse wave velocity and augmentation index measured by pulse wave analysis. J Hypertens. 1998;16:2079–84.
Kellgren JH, Lawrence JS. Radiological assessment of osteo-arthrosis. Ann Rheum Dis. 1957;16:494–502.
Nichols WW, O'Rourke MF. McDonald’s blood flow in arteries: theoretic, experimental and clinical principles. 5th ed. London: Edward Arnold; 2005.
Mattace-Raso FU, van der Cammen TJ, Hofman A, van Popele NM, Bos ML, Schalekamp MA, et al. Arterial stiffness and risk of coronary heart disease and stroke: the Rotterdam study. Circulation. 2006;113:657–63.
Boutouyrie P, Tropeano AI, Asmar R, Gautier I, Benetos A, Lacolley P, et al. Aortic stiffness is an independent predictor of primary coronary events inhypertensive patients: a longitudinal study. Hypertension. 2002;39:10–5.
Kals J, Lieberg J, Kampus P, Zagura M, Eha J, Zilmer M. Prognostic impact of arterial stiffness in patients with symptomatic peripheral arterial disease. Eur J Vasc Endovasc Surg. 2014;48:308–15.
Townsend RR, Wilkinson IB, Schiffrin EL, Avolio AP, Chirinos JA, Cockcroft JR, et al. Recommendations for improving and standardizing vascular research on arterial stiffness: a scientific statement from the American Heart Association. Hypertension. 2015;66:698–722.
Nichols WW, Edwards DG. Arterial elastance and wave reflection augmentation of systolic blood pressure: deleterious effects and implications for therapy. J Cardiovasc Pharmacol Ther. 2001;6:5–21.
Vlachopoulos C, Aznaouridis K, O'Rourke MF, Safar ME, Baou K, Stefanadis C. Prediction of cardiovascular events and all-cause mortality with central hemodynamics: a systematic review and meta-analysis. Eur Heart J. 2010;31:1865–71.
Pini R, Cavallini MC, Palmieri V, Marchionni N, Di Bari M, Devereux RB, et al. Central but not brachial blood pressure predicts cardiovascular events in an unselected geriatric population: the ICARe dicomano study. J Am Coll Cardiol. 2008;51:2432–9.
Roman MJ, Devereux RB, Kizer JR, Lee ET, Galloway JM, Ali T, et al. Central pressure more strongly relates to vascular disease and outcome than does brachial pressure: the strong heart study. Hypertension. 2007;50:197–203.
Duprez DA, De Buyzere MM, De Bruyne L, Clement DL, Cohn JN. Small and large artery elasticity indices in peripheral arterial occlusive disease (PAOD). Vasc Med. 2001;6:211–4.
Wan Z, Liu X, Wang X, Liu F, Liu W, Wu Y, et al. Small artery elasticity predicts future cardiovascular events in chinese patients with angiographic coronary artery disease. Angiology. 2014;65:298–302.
Ashor AW, Lara J, Siervo M, Celis-Morales C, Mathers JC. Effects of exercise modalities on arterial stiffness and wave reflection: a systematic review and meta-analysis of randomized controlled trials. PLoS ONE. 2014;9:e110034.
Belen E, Karaman O, Caliskan G, Atamaner O, Aslan O. Impaired aortic elastic properties in primary osteoarthritis. Vascular. 2015;0:1–8.
Li Y, Wei X, Zhou J, Wei L. The age-related changes in cartilage and osteoarthritis. BioMed Res Int. 2013;2013:916530.
Goldin A, Beckman JA, Schmidt AM, Creager MA. Advanced glycation end products: sparking the development of diabetic vascular injury. Circulation. 2006;114:597–605.
Pearle AD, Scanzello CR, George S, Mandl LA, Dicarlo EF, Peterson M, Sculco TP, Crow MK. Elevated high-sensitivity C-reactive protein levels are associated with local inflammatory findings in patients with osteoarthritis. Osteoarthr Cartil. 2007;15:516–23.
Houard X, Goldring MB, Berenbaum F. Homeostatic mechanisms in articular cartilage and role of inflammation in osteoarthritis. Curr Rheumatol Rep. 2013;15:375.
Liu-Bryan R, Terkeltaub R. Emerging regulators of the inflammatory process in osteoarthritis. Nat Rev Rheumatol. 2015;11:35–44.
Findlay DM. Vascular pathology and osteoarthritis. Rheumatology (Oxford). 2007;46:1763–8.
Chang J, Jackson SG, Wardale J, Jones SW. Hypoxia modulates the phenotype of osteoblasts isolated from knee osteoarthritis patients, leading to undermineralized bone nodule formation. Arthritis Rheumatol. 2014;66:1789–99.
Jung S, Kim OY, Kim M, Song J, Lee SH, Lee JH. Age-related increase in alanine aminotransferase correlates with elevated levels of plasma amino acids, decanoylcarnitine, Lp-PLA2 Activity, oxidative stress, and arterial stiffness. J Proteome Res. 2014;13:3467–75.
Chue CD, Townend JN, Steeds RP, Ferro CJ. Arterial stiffness in chronic kidney disease: causes and consequences. Postgrad Med J. 2010;86:560–6.
Mceniery CM, Wilkinson IB, Avolio AP. Age, hypertension and arterial function. Clin Exp Pharmacol Physiol. 2007;34:665–71.
Acknowledgements
We thank Pirja Sarap, who contributed to the radiographic evaluation of the OA patients.
Funding
This study was supported by grants of the Estonian Science Foundation [No. 9094] by Personal Institutional Research Funding (GMVBS1169), by Institutional Research Funding No IUT20-42 from the Estonian Ministry of Education and by the European Union through the European Regional Development Fund (Project No. 2014–2020.4.01.15-0012).
Availability of data and materials
All the data that supports this study is contained within the manuscript. Requests for further detail on the dataset may be submitted to the corresponding author.
Authors’ contributions
K. T., A. M., M. Z. and J. K. contributed to the conception and design of the study. K. T., M. Z., J. K., K. P. and A. M. participated in the acquisition and/or analysis of data. All authors contributed to data interpretation, drafting and revising the manuscript, plus approved the submitted version of the manuscript.
Competing interests
The authors have declared no conflicts of interest.
Consent to publish
Not applicable.
Ethics approval and consent to participate
The study was conducted according to the Helsinki declaration and received approval from the Ethics Committee of the University of Tartu. Written informed consent was obtained from all participants.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Tootsi, K., Märtson, A., Zilmer, M. et al. Increased arterial stiffness in patients with end-stage osteoarthritis: a case-control study. BMC Musculoskelet Disord 17, 335 (2016). https://doi.org/10.1186/s12891-016-1201-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12891-016-1201-x