Abstract
Background
A straight silicone stent can be used to treat proximal benign tracheal stenosis in non-surgical candidates. However, stent migration is a common complication when placed at a particular location and can lead to major complications. This case series of laryngotracheal stenosis reports a fixation method for straight silicone stents in the subglottic trachea (Stage 3 of the McCaffrey classification).
Methods
The medical charts of these patients scheduled for straight silicone stent placement with suture fixation between 2014 and 2020 at the CHU UCL Namur Hospital (Belgium) were retrospectively reviewed. The procedure was performed using a rigid bronchoscope. Details of the procedure were obtained from medical records.
Results
This case series included six patients (males: 4, females: 2). The median patient age was 59 years. Two suture fixations were placed following previous silicone stent migration episodes, whereas the others were placed proactively to avoid this risk. All fixations were performed by the device Freka® Pexact II ENFIt®, originally developed for gastropexy in endoscopic gastrostomy. The sutures were subcutaneously buried.
Conclusions
During the 6-month follow-up period, complications such as fixation issues and stent migration were reported despite the off-label use of the treatment. The straight silicone stent fixation technique used in this case series was simple and effective for securing the stent in upper benign tracheal stenosis.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Background
Central airway stenosis has recently received attention with the proposed standardized classification system reported by Freitag et al. [1] and has declined in terms of type, location, and degree.
Upper tracheal stenosis carries a particular burden of morbidity, in part due to its impact on ventilation, as long as it interferes with laryngeal and swallowing functions [2]. They have benign etiologies (endotracheal intubation, tracheostomy, trauma, inflammatory disorders, etc.), malignant disorders (thyroid carcinoma), and sometimes idiopathic disorders [3].
The management of upper tracheal stenosis is particularly challenging for several reasons.
First, stenosis location is a critical point, particularly in relation to the glottic and subglottic areas, which are remarkably close. McCaffrey, in a study on laryngotracheal diseases [4], defined different stages based on the extent of subglottic area involvement, considering it a significant predictor of outcome regarding stenosis diameter and length. In this case series, all the patients had McCaffrey stage 3 subglottic stenosis with a slow bottleneck-type transition.
Second, if tracheal resection with reconstruction is first recommended when possible and can provide definitive treatment, many cases are unresectable for multiple reasons (e.g., anatomic limitations and poor general health). In non-surgical cases, interventional bronchoscopic procedures such as dilation (with a balloon or rigid bronchoscope/tracheoscope), laser resection, mitomycin C, or topical steroid application have been proposed as alternatives to open surgery, with good outcomes and lasting benefits, including a permanent solution [5, 6].
Third, in the case of stenosis recurrence, silicone stents have multiple advantages, including safety, firmness, variety of lengths and diameters, removability, and low cost [7, 8]. However, complications have been reported, with particular attention being paid to migration, particularly when placed in the proximal trachea [8,9,10,11].
Fourth, the endoscopic management of tracheal stenosis requires close collaboration between interventional pulmonologists, thoracic surgeons, ear-nose-throat specialists (ENT), and anesthesiologists. This is reserved for tertiary hospitals with a dedicated interest in this topic.
In this study, we used straight stents and a fixation device (Freka® Pexact II ENFIt®), originally developed for fixation of the stomach to the abdominal wall, for an endoscopic gastrostomy with absorbable sutures buried subcutaneously. This study aimed to retrospectively evaluate the feasibility and tolerance of secured silicone stents placed in the proximal trachea using this device.
Methods
The medical charts of patients with laryngotracheal stenosis who underwent rigid bronchoscopy and tracheal stent placement with suture fixation using the proposed technique between 2014 and 2020 at CHU UCL Namur Hospital (Godinne Site, Belgium) were retrospectively reviewed in 2022. The patients were initially considered inoperable for several reasons (cardiac and respiratory function) and underwent several (at least three) rigid bronchoscopic dilation sessions completed with the local application of mitomycin C or submucosal injection of triamcinolone acetonide.
This study (approval and clinical trial number 47/2021) was approved by the local institutional review board (Comité d’éthique hospitalo-facultaire central, Site de Godinne – UNamur). Age, sex, comorbidities, characteristics of airway lesions (etiology, type, and location), previous interventional bronchoscopic procedures, stent characteristics, symptoms before and after intervention, complications, and clinical outcomes were recorded from computerized records and intraoperative protocols. Patients underwent a physical examination, laboratory tests, chest computed tomography, flexible bronchoscopy, and/or pulmonary function tests before the stenting operation. All procedures were performed in the operating room under total intravenous anaesthesia (propofol and remifentanil) and deep neuromuscular blockade (characterized by no train-of‐four responses) with rocuronium. Patients were intubated with a rigid tracheoscope/bronchoscope (Efer-Dumon®, La Ciotat, France or Shapshay Karl Storz® GmbH & Co. KG, Tuttlingen, Germany) and ventilated using high-frequency jet ventilation (HFJV) by the Monsoon™ III Jet Ventilator (Acutronic Medical System AG, Hirzel, Switzerland).
In cases of tracheal stenosis, assessment of the level and length of the stenosis is essential to deciding which stent is best to place. Herein, we used a straight-type Dumon® silicone stent (Novatech SA, La Ciotat, France) customized according to the stenosis.
Before stent placement, preparing and calibrating the tracheal lumen by microdebridement with, argon plasma coagulation, and dilation using a balloon or a rigid tracheoscope/bronchoscope of increasing diameters is crucial. After capturing the characteristics of airway stenosis, the stent diameter and length were endoscopically assessed and compared with computed tomography data. The stent was dropped under fluoroscopic guidance and adjusted just under the vocal folds using rigid forceps. The stent was fixed subcutaneously to the anterior neck after the dissection of subcutaneous tissue by the surgeon with nylon thread using Freka® Pexact II ENFIt®.
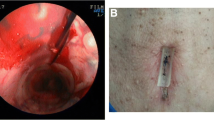
When subcutaneous dissection was performed, Pexact® passed through the trachea and the tracheal stent under endoscopic control. The gastropexy device consisted of two hollow needles. Upon insertion of the Pexact® device and ensuring proper positioning, the trigger was activated, resulting in a wire loop formation directly beneath the parallel hollow needle. The suture thread was pushed through the other hollow needle and into the tracheal stent through the wire loop using a thread wheel. By pressing the red release button, the wire loop was retracted into the puncture needle, capturing the suture thread at the tip. This allowed for the easy retrieval of the suture thread through the trachea and subsequent removal from the body. The suture thread was tied to the subcutaneous tissue to secure the stent to the anterior neck. Finally, the surgeon sutured the subcutaneous tissue and skin (Fig. 1). Postoperatively, all patients were prescribed prophylactic antibiotics with amoxicillin/clavulanic acid for 48 h to prevent infection and were administered nebulized acetylcysteine and physiological serum several times a day to prevent mucus plugging.
In our hospital, silicone stents were removed after approximately 6 months or earlier, as clinically indicated, by cutting the securing thread with endoscopic dedicated scissors. Subsequently, the stent can be easily extracted using rigid forceps.
Subcutaneous fixation of the tracheal stent using the Pexact® system. (a) The puncture is performed, and a wire loop is formed under the opposite parallel hollow needle. (b) Suture thread is pushed through the other hollow needle into the tracheal stent through the wire loop. (c) Suture thread is captured with the wire loop at the tip of the needle. (d) The wire loop for thread holding is retracted into the puncture needle, and the device is removed from the body. (e) The suture thread is tied in subcutaneous tissues to secure the stent on the anterior neck
Results
The study included six patients (males: 4, females: 2) with a mean age of 59 years (range 39–76 years). The initial airway pathology was benign (related to prior endotracheal intubation in most cases). The characteristics of the patients and their stenoses are summarized in Table 1. In this study, three patients had previously undergone stenting, and experienced stent migration. Two suture fixations were placed following previous migration episodes, whereas the others were placed proactively to avoid this risk.
All lesions were located in the proximal trachea at the junction of the proximal trachea and the glottic area as well with a slow bottleneck-type transition. The distance of the upper border of the lesion from the vocal cord range from 5 to 20 mm, and the stenosis length range from 10 to 40 mm.
The median length of the stents used was 5.5 cm (range 4–7 cm), and the diameter used was 18 mm in five cases and 14 mm in one case, as mentioned in Table 2. This table summarizes the intraoperative and postoperative information. All stents were Novatech Dumon TD tracheal stents (Novatech SA, La Ciotat, France).
The follow-up period was 6 months. Patients were monitored clinically and endoscopically approximately every 2 months except for one patient whose death occurred very shortly after the endoscopic procedure. The patient was discharged 24 h after the procedure and died abruptly at home the following day. According to the patient’s wife, the patient reported no complaints. The sudden death suggests an acute cardiovascular event.
In another patient – who did not tolerate a previous tracheotomy - removal of the stent on the third postoperative day was due to stent blockage caused by numerous secretions in the context of acute renal dysfunction, ischemic stroke, and lung infection.
No spontaneous stent migration or fixation-related complications, such as infection or suture breakdown, were reported in the remaining patients.
Three stents were removed after 6 months, including one following the development of granulation tissue and another following the recurrence of stenosis.
In one patient, in whom the stent was not removed after 6 months due to neurological reasons, the endoscopic evaluation (Fig. 2) conducted 15 months after stent insertion showed excellent local tolerance and unchanged tracheal positioning.
Discussion
Silicone stents are usually considered for treating recurrent tracheal stenosis in non-surgical patients or patients undergoing surgery, tracheostomy, or Montgomery T-tubes. Proximal or caudal migration is a critical complication, especially in patients having difficulty reaching interventional bronchoscopy referral centers. Migration has been reported in 5–21% of cases, with worse results when the stent location involves the proximal part of the trachea [2, 9,10,11].
In this study of benign recurrent stenosis, the stenosis was remarkably close to the caudal glottic area with partial involvement according to the McCaffrey classification. This likely contributes to the potential instability of stent positioning, given the mobility of the subglottic area due to factors such as respiratory movement, coughing, and swallowing [8,9,10,11].
For years, several techniques have been described to fix the stent and avoid migration (e.g., stent- and technique-based and external fixation strategies). However, no standardized method exists for this purpose. The fixation technique by the Pexact® system used in this case series, similar to the case series reported by Huang in 2018 [12], is a modality considered to limit migration chances and was herein applied proactively. We intended to confirm its feasibility at our institution and its tolerance during the follow-up period.
In 2004, Miwa et al. described a silicone fixation stent using a fixation system designed for gastropexy similar to ours [13]. Here, we report the same advantages of the present fixation device: ease of placement and accuracy of the procedure in real-time under endoscopic control. The thread was placed in the transverse tracheal plane, which was judged to be more suitable than the vertical plane, given the limited space between the vocal cords and the proximal part of the stenosis. In contrast to the findings of Miwa et al., all tracheal stenoses in our study were benign, and we performed a subcutaneous suture to avoid skin necrosis and infection. At our institution, subcutaneous dissection and suturing require collaboration with thoracic and ENT surgeons, which could be a limiting factor in other institutions. Stent fixation using this technique in our hospital requires an additional 20 min of stent placement time.
Several studies have described sutures securing the skin with an external foreign body [3, 12, 14,15,16], which could be complicated by skin infection or necrosis. For example, Huang [12] used a silicon pad to limit the suture stress on the skin. In 2018, Andreetti et al. [9] used absorbable rather than nonabsorbable sutures buried in the subcutaneous tissue to prevent the risk of subcutaneous infection. For the same reason, prophylactic antibiotics were administered to patients to avoid infection, as suggested in previous studies [16, 17]. Subcutaneous sutures result in small scars of a few millimeters, which are more cosmetically acceptable compared to those requiring buttons or plugs. This prevents accidental tearing and offers easier motility and comfort during deglutition and neck movements. In our study, cutaneous tolerance was excellent, without local infection, poor healing, or skin necrosis. No additional care is required. Moreover, the suturing was performed with a colored thread that easily allowed endoscopic cutting of the stitch at the time of removal.
All the fixation techniques mentioned herein were considered safe and successful by the authors. Well-known stent-related issues, such as mucus plugging or granulation tissue (noted in one of our patients), were also reported by Majid [14], whose patients were monitored for a mean of 11.2 months. In our patients, the only one who kept the stent for longer than six months (e.g., for neurological reasons) exhibited excellent stent tolerance, as illustrated in Fig. 2. External fixation took an extra time between 1 and 10 min [10, 13], depending on the procedure. In our study, an additional 20 min was necessary, which we do not consider as a significant issue.
No migration was reported in this retrospective study, considering that two patients had previous migration with an unfixed stent. However, the follow-up time was limited to a maximum of 6 months, after which retrieval was considered, given the benign condition of the stenosis. In one patient, the development of granulation tissue between the vocal cords and the proximal part of the stent led to withdrawal 6 months after placement. No mucus stasis was noted in general, and the patients were instructed to take an aerosol saline solution three times a day.
In countries where the Pexact® device is not available, the “hitch stitch” method with sutures passed through two catheters was recently illustrated by Mehta [10], with a procedure considered successful in 97.6% (41/42) of their patients. A simple and inexpensive method uses two 16-gauge intravenous cannulas through which a thread is inserted with a loop fashioned for the second cannula, allowing an external stent to be fixed at the end [6].The management of laryngotracheal stenosis requires multidisciplinary assessments. At our institution, we work in close collaboration with thoracic and ENT specialists. Having a pre-established treatment strategy enables us to proactively address any potential complications that may arise before and after the procedure.
This study had a few limitations, such as its retrospective design and limited sample size. However, the feasibility of this technique at our institution and acceptable tolerance following stent placement encouraged us to integrate it into the management of high tracheal stenosis, including cases with partial glottic involvement.
Conclusions
A straight tracheal silicone stent is a good option for the management of laryngotracheal stenosis in patients who, after multidisciplinary consultation, are not candidates for surgery.
In this case series with high tracheal stenosis partially involving the subglottic area, we report, over a period of 6-month, the feasibility of the fixation technique using the Pexact® device with subcutaneous securing and its advantages, including simplicity, safety, and efficiency.
This technique involves some constraints, such as the extra time needed to place the device and a subcutaneous suture, requiring collaboration between interventional pulmonologists, thoracic and ENT specialists, and anesthesiologists.
The suture, buried subcutaneously, avoids potential infections and skin necrosis and provides long-term fixation with good esthetics.
Nevertheless, this technique offers good outcomes, potentially reducing the need for repeated endoscopic procedures related to recurrent stenosis and stent migration as well.
Data availability
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- ENT:
-
Ear-nose-throat specialists
- HFJV:
-
High-frequency jet ventilation
References
Freitag L, Ernst A, Unger M, Kovitz K, Marquette CH. A proposed classification system of central airway stenosis. Eur Respir J. 2007;30(1):7–12.
Wood DE, Liu YH, Vallieres E, Karmy-Jones R, Mulligan MS. Airway stenting for malignant and benign tracheobronchial stenosis. Ann Thorac Surg. 2003;76(1):167–72. discussion 73 – 4.
Colt HG, Harrell J, Neuman TR, Robbins T. External fixation of subglottic tracheal stents. Chest. 1994;105(6):1653–7.
McCaffrey TV. Classification of laryngotracheal stenosis. Laryngoscope. 1992;102(12 Pt 1):1335–40.
Brichet A, Verkindre C, Dupont J, Carlier ML, Darras J, Wurtz A, et al. Multidisciplinary approach to management of postintubation tracheal stenoses. Eur Respir J. 1999;13(4):888–93.
Dhooria S, Agarwal R. External fixation of a subglottic tracheal silicone stent. Ann Am Thorac Soc. 2014;11(3):467–8.
Folch E, Keyes C. Airway stents. Ann Cardiothorac Surg. 2018;7(2):273–83.
Wood DE. Airway stenting. Chest Surg Clin N Am. 2001;11(4):841–60.
Andreetti C, Menna C, D’Andrilli A, Ibrahim M, Venuta F, Santini M, et al. A modified technique to simplify external fixation of the subglottic silicone stent. Interact Cardiovasc Thorac Surg. 2018;27(6):878–80.
Mehta RM, Singla A, Shah A, Loknath C. The Hitch stitch: an effective method of preventing Migration in High Tracheal stenosis. Respiration. 2017;93(2):106–11.
Zakaluzny SA, Lane JD, Mair EA. Complications of tracheobronchial airway stents. Otolaryngol Head Neck Surg. 2003;128(4):478–88.
Huang J, Zhang Z, Zhang T. Suture fixation of tracheal stents for the treatment of upper trachea stenosis: a retrospective study. J Cardiothorac Surg. 2018;13(1):111.
Miwa K, Takamori S, Hayashi A, Fukunaga M, Shirouzu K. Fixation of silicone stents in the subglottic trachea: preventing stent migration using a fixation apparatus. Ann Thorac Surg. 2004;78(6):2188–90.
Majid A, Fernandez-Bussy S, Kent M, Folch E, Fernandez L, Cheng G, et al. External fixation of proximal tracheal airway stents: a modified technique. Ann Thorac Surg. 2012;93(6):e167–9.
Lin X, Ye M, Li Y, Chen C. A novel simple external fixation for securing silicone stent in patients with upper tracheal stenosis. J Thorac Dis. 2018;10(3):E194–8.
Huang G, He J, Li S. Efficacy of external fixation of a straight silicone stent in the treatment of subglottic stenosis. Ann Palliat Med. 2021;10(1):194–201.
Musani AI, Jensen K, Mitchell JD, Weyant M, Garces K, Hsia D. Novel use of a percutaneous endoscopic gastrostomy tube fastener for securing silicone tracheal stents in patients with benign proximal airway obstruction. J Bronchol Interv Pulmonol. 2012;19(2):121–5.
Acknowledgements
The authors are grateful for the assistance of Axel Baily with the figures.
Funding
The authors declare that no funds, grants, or other support were received during the preparation of this manuscript.
Author information
Authors and Affiliations
Contributions
Material preparation, data collection and analysis were performed by S.M. and L.Pi. The first draft of the manuscript was written by S.M. All authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study was performed in line with the principles of the Declaration of Helsinki and approval was granted by the Institutional Ethics Committee (Comité d’éthique hospitalo-facultaire central, Site de Godinne – UNamur; approval number: 47/2021). Informed consent was waived due to the retrospective nature of the study in agreement with the Institutional Ethics Committee (Comité d’éthique hospitalo-facultaire central, Site de Godinne – UNamur).
Consent for publication
not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Meyer, S., d’Odémont, JP., Putz, L. et al. Subcutaneous fixation model for complex stenting of recurrent laryngotracheal stenosis. BMC Pulm Med 24, 383 (2024). https://doi.org/10.1186/s12890-024-03197-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12890-024-03197-1