Abstract
Background
SARS-CoV-2 infection has raised concerns about long-term health repercussions. Exercise ventilatory inefficiency (EVin) has emerged as a notable long-term sequela, potentially impacting respiratory and cardiovascular health. This study aims to assess the long-term presence of EVin after 34 months and its association with cardiorespiratory health in post-COVID patients.
Methods
In a longitudinal study on 32 selected post-COVID subjects, we performed two cardiopulmonary exercise tests (CPETs) at 6 months (T0) and 34 months (T1) after hospital discharge. The study sought to explore the long-term persistence of EVin and its correlation with respiratory and cardiovascular responses during exercise. Measurements included also V̇O2peak, end-tidal pressure of CO2 (PETCO2) levels, oxygen uptake efficiency slope (OUES) and other cardiorespiratory parameters, with statistical significance set at p < 0.05. The presence of EVin at both T0 and T1 defines a persisting EVin (pEVin).
Results
Out of the cohort, five subjects (16%) have pEVin at 34 months. Subjects with pEVin, compared to those with ventilatory efficiency (Evef) have lower values of PETCO2 throughout exercise, showing hyperventilation. Evef subjects demonstrated selective improvements in DLCO and oxygen pulse, suggesting a recovery in cardiorespiratory function over time. In contrast, those with pEvin did not exhibit these improvements. Notably, significant correlations were found between hyperventilation (measured by PETCO2), oxygen pulse and OUES, indicating the potential prognostic value of OUES and Evin in post-COVID follow-ups.
Conclusions
The study highlights the clinical importance of long-term follow-up for post-COVID patients, as a significant group exhibit persistent EVin, which correlates with altered and potentially unfavorable cardiovascular responses to exercise. These findings advocate for the continued investigation into the long-term health impacts of COVID-19, especially regarding persistent ventilatory inefficiencies and their implications on patient health outcomes.
Similar content being viewed by others
Introduction
Post-COVID condition refers to a range of symptoms and clinical findings that persist following the acute phase of SARS-CoV-2 infection [1]. In these patients, the cardiopulmonary exercise test (CPET) has highlighted a reduction of maximal exercise capacity and oxygen uptake (V̇O2peak) and has been helpful in elucidating the underlying pathophysiological mechanisms leading to exercise intolerance and unexplained perceived dyspnea [1, 2]. CPET has demonstrated that exercise hyperventilation and ventilatory inefficiency (Evin) are a contributor to numerous disabling signs and symptoms in post-COVID patients, such as persisting breathlessness and long-lasting exercise intolerance [3, 4].
Exercise ventilation efficiency is assessed by examining how minute ventilation (V̇E) correlates with the amount of carbon dioxide produced (V̇CO2). This relationship is quantified using three metrics: the slope of V̇E against V̇CO2 (V̇E/V̇CO2slope), the lowest value observed (nadir) for this ratio, and the carbon dioxide ventilatory equivalent at the first ventilatory threshold (V̇E/V̇CO2 at θL) [5]. These metrics are well-established for evaluating mismatches in ventilation and pulmonary perfusion during exercise in patients with heart and lung conditions [6]. High values of V̇E/V̇CO2 relationship commonly indicate EVin, which is a condition of breathing dysfunction related to excessive ventilation [5].
Ventilatory inefficiency is a global indicator of cardiorespiratory response to exercise and a well-recognized prognostic marker in chronic patients second only to V̇O2peak [7]. As pointed out by Weatherald et al., EVin is also a hallmark of pulmonary vascular diseases, such as pulmonary arterial hypertension and chronic thromboembolic pulmonary hypertension where it is an excellent prognostic marker [8].
Understanding the pathophysiological origins of EVin is essential to comprehending the exercise response in post-COVID syndrome. A significant amount of evidence indicates that a subset of asymptomatic COVID-19 survivors exhibits EVin, with prevalences reported at 29% and 17% at 6 and 12 months post-discharge, respectively [9,10,11]. Compared to those without exercise ventilatory inefficiency, those with ventilatory efficiency (Evef), post-COVID patients with Evin show lower values of end-tidal pressure of CO2 (PETCO2) throughout the exercise and display hypocapnia and respiratory alkalosis, which may correlate with an impairment in diffusing capacity (DLCO) [3, 4, 10, 12].
Moreover, evidence at 12 months following severe COVID-19 infections indicates that numerous patients, despite achieving normal V̇O2peak levels, exhibit signs of Evin, notably linked to signs of underlying pulmonary microvascular disease and increased dead space ventilation [13]. Such vascular complications are believed to stem from endothelial dysfunction and a hypercoagulable state, both of which are acute sequelae of the systemic inflammatory response to SARS-CoV-2 infection [13].
An invasive CPET study documents that symptomatic long-COVID patients with reduced exercise capacity have a blunted peripheral oxygen extraction [14]. However, in asymptomatic patients, exercise limitations are less clear and still need to be clarified. In addition to V̇O2peak and V̇E/V̇CO2 relationship, impairments of the respiratory and cardiovascular response to exercise, could be also evaluated through the oxygen pulse (O2 pulse), aerobic efficiency slope (V̇O2/Wslope) and oxygen uptake efficiency slope (OUES) which also estimates the cardiovascular risk in certain populations [15, 16]. O2 pulse is the ratio between oxygen uptake and heart rate (HR): it reflects the amount of oxygen extracted by the tissue per heartbeat and could be used as a non-invasive estimator of stroke volume, or peripheral oxygen utilization [7].
Despite these parameters being less strong indicators for evaluating overall survival in the general population, some recent long-term longitudinal studies show that low O2 pulse at peak and OUES have been associated with increased cardiovascular and all-cause mortality in certain populations [15,16,17] These data need to be further confirmed by other similar longitudinal studies: however, evidence shows that post-COVID patients have a reduced aerobic capacity and O2 pulse independent from V̇O2peak levels [18]. While this data could not be interpreted in terms of long-term implications, they could be a subclinical sign of altered cardiovascular response due to the infection in these patients [19].
The enduring clinical significance of EVin and the altered cardiovascular response to exercise in post-COVID patients remains an area of ongoing investigation [13, 20]. The persistence of these conditions after 1 year following hospital discharge underscores the need for pathophysiological investigations and sustained longitudinal studies.
Our study aims to explore the persistence of EVin in post-COVID patients and to unravel its potential long-term repercussions on respiratory and cardiovascular health.
Our first hypothesis is that EVin may persist chronically after COVID-19 infection. Evidence suggests that it could be a sign of acute SARS-CoV-2 infection and a subclinical impairment of exercise response which involves both the cardiovascular and the respiratory systems and this leads to our second hypothesis. We also hypothesized that EVin is a sign of a broader dysfunction in the cardiorespiratory response, which may also correlate with signs of an increased cardiovascular risk.
Methods
Selection of patients
We evaluated the resting and exercise ventilatory and cardiovascular responses in a cohort of selected post-COVID patients at 34 months from hospitalization, comparing data with a previous evaluation performed 6 months after discharge. Data were collected from the RESPICOVID initiative, a prospective observational study conducted at the Respiratory Medicine Unit of the University of Verona and Azienda Ospedaliera Universitaria Integrata of Verona (Italy), involving patients hospitalized for COVID-19 pneumonia during the first two waves of the pandemic emergency in Italy. A dedicated outpatient clinic has been organized, and all subjects discharged were considered. The present longitudinal analysis with repeated measures has been designed to evaluate the long-term persistence of ventilatory inefficiency in subjects enrolled in the RESPICOVID-2 study [11]. Only subjects who performed both CPETs (at T0 and T1) were considered. Figure 1 shows the study flow diagram.
Study flow diagram
Abbreviations: BMI defines body mass index; CPET, cardiopulmonary exercise test. *Patient were not able to perform maximal CPET due to musculoskeletal symptoms. **Patients were excluded due to personal unavailability, refusal to continue with the study, or the emergence of new musculoskeletal conditions that limited their ability to exercise
To better define the EVin and cardiovascular response to exercise, we excluded any potential physiological or pathological variable influencing exercise adaptations [6]. We have then excluded subjects meeting the following criteria: (a) age exceeding 65 years; (b) concurrent presence of respiratory and non-respiratory chronic diseases (including the suspected clinical presentation of new-onset), respiratory failure, or need for long-term oxygen therapy; (c) a body mass index (BMI) ≥ 35 kg/m2; (d) an inability to perform a CPET with a peak respiratory exchange ratio (RER) < 1.05 (to exclude poor motivation); and (e) psychiatric disorders in order to avoid psychogenic hyperventilation. Among chronic diseases, only stable systemic arterial hypertension was accepted.
Measurements
All measures were prospectively collected beginning in July 2020, approximately 6 months after the subjects’ discharge (T0), and repeated until March 2023, 34 months after the discharge (T1). Only subjects with both CPET measures (T0 and T1) were considered for the analysis. Preliminary data about measures performed at T0 have been reported previously [11]. The local Ethics Committee approved the study protocol (no. 2785CESC), which was performed according to the Good Clinical Practice recommendations and the requirements of the Declaration of Helsinki. Written informed consent was obtained from all subjects.
Lung function
Lung function procedures were performed according to international recommendations [21,22,23]. A flow-sensing spirometer connected to a computer for data analysis (Jaeger MasterScreen PFT System) was used to measure lung function. Forced vital capacity (FVC), forced expiratory volume in the first second (FEV1), and total lung capacity (TLC) were recorded. FEV1/FVC ratio was taken as the index of airflow obstruction. The single-breath method measured the diffusion capacity for carbon monoxide (DLCO). FEV1, FVC, TLC, and DLCO were expressed as percentages of the predicted values [22, 23].
Cardiopulmonary exercise test
According to the ATS/ACCP Statement, for the CPET measures, we used a cycle ergometer (E100, Cosmed Srl, Rome, Italy) with a ramp protocol of 10 to 25 watts increment every minute and based on the predicted peak power output, to achieve an exercise time between 8 and 12 min [24]. Patients were monitored 3 min before the ramp protocol (rest phase) and 5 min after (cool down phase). Subjects were asked to avoid caffeine, alcohol, cigarettes, and strenuous exercise 24 h before the day of testing and avoid eating for the 2 h before the test. Subjects suspended β-blockers before testing but could take their current antihypertensive therapies. During the test, subjects were asked to maintain a pedal frequency of 65 per minute and were continuously monitored [24]. Subjects were continuously monitored with a 12-lead electrocardiogram (ECG) and a pulse oximeter; blood pressure was measured every two minutes. Stopping criteria consisted of symptoms, such as unsustainable perceived dyspnoea or leg fatigue, chest pain, a significant ST-segment depression at ECG, or a drop in systolic blood pressure or oxygen saturation ≤ 84% [24]. Cardio-respiratory measures were sampled continuously with a breath-by-breath method using a gas analysis system (Quark CPET, Cosmed Srl, Rome, Italy). Oxygen uptake was expressed in mL/kg/min and as a percentage of predicted. The ventilatory response during exercise was through the relationship of V̇E against V̇CO2 obtained every 10 s, excluding data above the respiratory compensation point (RCP). We gathered data of V̇E/V̇CO2 slope and Y-intercept (V̇E/V̇CO2 intercept) values obtained from the regression function. V̇E/V̇CO2 was also been evaluated at nadir (V̇E/V̇CO2 nadir) and the first ventilatory threshold (V̇E/V̇CO2 at θL) [7].
For the definition of the EVin, we used the regression equation of V̇E/ V̇CO2 slope for healthy subjects [5]. Related to our small sample and to avoid false positive results, we considered three standard deviations as the upper limit to define EVin [5]. Then, we considered subjects having a lower range of V̇E/V̇CO2 slope (EVef) and subjects with over the upper limit of V̇E/V̇CO2 slope (EVin). Subjects having EVin at T0 and T1 were defined as persisting ventilatory inefficiency subjects (pEVin).
The end-tidal pressure of CO2 (PETCO2, in mmHg) was measured as the mean of PETCO2 during the 3-minute rest period and the last 20 s of the test and was recorded at any time during CPET (at rest, at θL, at the respiratory compensation point - RCP, and at peak of exercise).
The cardiovascular response to exercise was expressed by HR, O2 pulse, OUES, V̇O2/Wslope and HR after 1 min of recovery (heart rate recovery, HRR). O2 pulse was calculated by dividing instantaneous V̇O2 by HR [7]. The OUES describes the relationship between V̇O2 and V̇E during incremental exercise, via a log transformation of V̇E, and was expressed in L/min as the gradient of the linear relationship of log10 V̇E to V̇O2 [25]. V̇O2/Wslope was calculated as the slope of oxygen uptake as a function of Watts [7, 25]. OUES thus represents the absolute rate of increase in oxygen uptake per 10-fold increase in minute ventilation. HRR in bpm was defined as the reduction in the HR from the peak exercise level to the rate 1 min after the end of exercise [26].
At the end of the exercise, dyspnoea and leg fatigue were measured by a Borg 6–20 rate perceived exertion (RPE) scale [27]. Perceived peak dyspnoea and fatigue data have been described as RPE and peak workload ratio. We considered a test as maximal if subjects had a plateau of the V̇O2 for more than 20 s, a Respiratory Exchange Ratio (RER) > 1.15, and a Borg RPE score > 18 [24].
Self-reported questionnaire
The modified Medical Research Council (mMRC) questionnaire was administered to measure perceived breathlessness, with a range from 0 (shortness of breath with strenuous exercise) to 4 (too breathless to leave the house) [28]. The Italian version of the International Physical Activity Questionnaire (IPAQ) was administered to measure the daily physical activity of the subjects estimating, the three levels of the metabolic equivalent of task (METs): inactive, minimally active, and health- enhancing physical activity (HEPA) active [29].
Statistical analysis
A preliminary Shapiro-Wilk test was performed. Data are reported as percentages for categorical variables, as mean (SD) or median [IQR-interquartile range] for continuous variables with a normal or non-normal distribution, respectively. Categorical variables were compared using the Chi-square test or the Fisher exact test. According to the distribution of continuous variables, the independent t-test or the non-parametric Mann-Whitney test were used to compare EVef and pEVin groups, while the paired t-test, or the non-parametric Wilcoxon signed-rank test were used to compare the differences between T1 and T0. Relationships between variables were assessed using Pearson’s correlation coefficient (r).
All analyses were performed using IBM SPSS, version 17.0 (IBM Corp., Armonk, NY, USA), with p-values of < 0.05 considered statistically significant.
Results
We evaluated the same thirty-two post-COVID subjects at T0 (median time from discharge 184 days) and T1 (median 1015 days). At T0, of 32 subjects, 8 had EVin (25%), while at T1 5 subjects (16%) had a pEVin. Subjects with pEVin, in comparison to subjects with EVef, had significantly higher values of a baseline of V̇E/V̇CO2 slope, V̇E/V̇CO2 nadir, and V̇E/V̇CO2 at θL with lower values of V̇E/V̇CO2 intercept. No other variables, including those related to COVID-19 hospitalization, differed between subjects with pEVin and subjects with EVef. Baseline variables were reported in Table 1. Supplementary Table 1 reports the characteristics of EVin and pEVin patients.
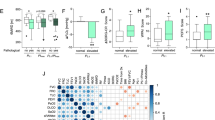
In all subjects, comparing T1 vs. T0 (Table 2), there was an increment of BMI, DLCO % predicted, V̇O2 at peak % predicted, and O2 pulse at peak, with a reduction of FEV1 and FVC (both % predicted), V̇E/V̇CO2 at θL and V̇E at rest. In EVef, selective changes between T1 and T0 were evident in the following variables: BMI, DLCO % predicted, O2 pulse at peak, V̇E/V̇CO2 at θL and V̇E at rest. No selective changes were evident in subjects with pEVin.
PETCO2 was significantly lower in patients with EVin than EVef at any time point of the exercise (at rest, at θL, at RCP and peak) at T1, while at T0 were different at rest, at RCP, and peak (Fig. 2).
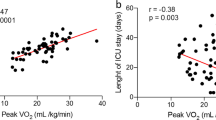
At T1, PETCO2 at rest (r 0.366; p = 0.039 and r 0.353; p = 0.048), such as at θL (r 0.532; p = 0.002 and r 0.586; p < 0.001), at RCP (r 0.514; p = 0.004 and r 0.565; p = 0.001), and peak (r 0.427; p = 0.015 and r 0.480; p = 0.005) were significantly and respectively correlated with O2 pulse at peak and OUES (Table 3).
Discussion
Our study starts from the hypothesis that EVin may be a persistent ventilo-perfusory alteration after COVID-19, which is a well-known phenomenon observed after 6 to 12 months after infection recovery [3, 4, 10, 11]. In a selected cohort of post-COVID patients, at almost three years of follow-up, we demonstrated that a pEVin is present in 16% of subjects. These subjects showed the phenomenon of exercise hyperventilation, documented by lower levels of PETCO2, and variables related to hospitalization do not seem to have a role in this alteration. However, even if not statistically significant, it seems that pEvin population presents a higher percentage of ICU admission (40 vs. 11%) and needs oxygen therapy, but this data will need to be confirmed by longitudinal studies with a larger sample size. Our patient cohort, comprising individuals with both EVef and EVin, exhibited consistently normal maximal exercise capacity, as well as normal levels of FEV1, FVC, TLC at both 6 months (T0) and 34 months after discharge (T1). This persistent exercise hyperventilation correlates with an exacerbated cardiovascular response to exercise, which was the second hypothesis of this study.
Ventilatory inefficiency and hyperventilation
A reduction in maximal exercise capacity and V̇O2peak has been reported as the main CPET feature of symptomatic post-COVID patients [1]. However, most of the asymptomatic post-COVID patients, despite maintaining preserved lung functionality, maximal exercise capacity and V̇O2peak, exhibit EVin [10, 11]. Research has indicated that exercise ventilatory inefficiency may be a significant feature also in apparently healthy COVID-19 survivors: however, its clinical role has not yet been fully elucidated, as well as its pathophysiological cause [20].
In healthy subjects, EVin is uncommon and anthropometric as well as anxiety-related variables may influence it [6, 31]On the contrary, EVin in cardiopulmonary chronic conditions is a very common alteration and may be caused mainly by two reasons: (1) An altered arterial partial carbon dioxide pressure (PaCO2) set-point and chemosensitivity (usually a consequence of chronic hypoxemia), and (2) an abnormally high dead space fraction during exercise caused by a ventilatory-perfusion mismatch, which could involve the ventilation, or the pulmonary perfusion [8, 30].
Hyperventilation is a frequent manifestation of subjects recovering from COVID-19, and it is frequently associated with ventilatory inefficiency; both have been reported as a possible mechanism of persisting disabling signs and symptoms limiting exercise capacity due to an increase in the cost of ventilation [3, 4, 31]. The exact cause of this hyperventilation remains unknown. As a consequence of SARS-CoV-2 infection, an imbalance in the ventilatory control has been hypothesized as a mechanism, related to either heightened activation of activator systems (including automatic and cortical ventilatory control, peripheral afferents, and sensory cortex) or suppression of inhibitory systems (endorphins) [3]. In COVID-19 survivors, there is also a close relationship between hypocapnia resulting from resting hyperventilation and residual DLCO, which are the most common functional abnormalities in the early convalescence phase [12, 32]. Compared with non-severe cases, patients with severe COVID-19 had a higher impairment in DLCO, which likely indicates a restrictive pattern and a decrease in TLC [32]. Although the ventilatory response was unrelated to disease severity, in survival cohorts, higher values of V̇E/V̇CO2 slope have been found in a follow-up of seven months as a predictor in developing pulmonary fibrosis [33]. Our study reports a close association between exercise hyperventilation and EVin as a permanent and distinctive sign of a proportion of asymptomatic survivors after 34 months (Fig. 2). Even if this phenomenon has been documented, the pathophysiological mechanism is still unclear. The clinical significance of hyperventilation and Evin in cardiorespiratory conditions may be related to a perpetual altered PaCO2 set-point, chemosensitivity and dysautonomia [3, 4]. This reason may explain Evin in asymptomatic post-COVID subjects without signs of clinical impairment and maintained exercise capacity, as described in our cohort. Most reports fail to demonstrate that this factor is independent of cardiorespiratory and endothelial damage, which led to an alteration of the ventilatory-perfusion mismatch. Some papers demonstrate a relationship between Evin and residual lung function impairment in DLCO, especially in symptomatic long-COVID subjects [6, 12, 30].However, other papers fail to demonstrate a correlation between DLCO, Evin, hyperventilation and a clear ventilo-perfusory mismatch [10, 11, 20]. We previously demonstrated the association between a DLCO impairment and ventilatory inefficiency in post-COVID patients [11]. Now, we document a selective improvement of diffusion capacity only in EVef subjects (Table 2), compared to pEvin subjects. Even if our study was not designed to explain the physiopathology of pEvin, the DLCO behavior of our pEVin subjects after 3 years is a novel finding that merit notice as an indirect sign of subclinical damage of the cardio-respiratory system leading to an increase of dead space ventilation during exercise [2].
In line with the assessments made in a shorter period after one year of discharge, the EVin prevalence in our survivors (16%) is similar to that described by Ingul CB and colleagues (17%), with similar considerations about hyperventilation (PETCO2) [10]. Of note, Ingul CB and colleagues found a close relationship between the perceived dyspnea and EVin: this relationship is not confirmed in our asymptomatic patients’ cohort, in which the level of dyspnea is very low (median mMRC 1) [10]. While perceived dyspnea is typically multifaceted in nature, our methodology, which involved the selection of subjects without comorbidities and variables that might affect the ventilatory efficiency—like a subject’s weight, or a history of anxiety-related breathlessness — could have impacted these findings [6, 34]. For instance, Ingul’s study included a cohort with 29% obese patients, in contrast to our study, which comprised only three out of 32 subjects (approximately 9%) being obese (data not shown) [10]. Persistent viral presence, long-term inflammation, microclots, and hypoxia may contribute to developing symptoms in obese subjects [35]. Moreover, obesity, related to the alteration of mechanical lung function, may affect the subject’s dyspnoea perception a priori [36].
Cardiovascular response to exercise in patients with pEVin
COVID patients are at risk for cardiovascular disease during the acute phase of the infection [19]. Due to the damage of pulmonary endothelium and microclots during the disease, we cannot exclude long-term cardiovascular complications in these patients. EVin is a well-recognized hallmark of pulmonary vascular disease and increased dead-space ventilation [30]. Despite normal V̇O2peak levels in subjects recovered from severe COVID after one year of follow-up, dead space ventilation correlates with D-Dimer plasma concentrations during hospital stay [13].
During a long-term follow-up, asymptomatic post-COVID cohorts failed to show a clear cardiac involvement [37] while invasive measurement during exercise in patients with exertional dyspnea shows that the main exercise limitation regards peripheral oxygen extraction [14]. However, at six months of discharge, higher values of V̇E/V̇CO2 slope have been linked to diminished HRR, suggesting that subjects with EVin may have cardiac autonomic dysfunction [26, 30, 39, 40]. An altered cardiac autonomic function may be one of the determinants of reduced peripheral extraction during exercise, and it is a general predictor of mortality in adults without a heart disease history [26]. Some studies confirm that normotensive post-COVID patients present a significantly higher blood pressure response in the post-exercise recovery, with an achieved lower O2 pulse at peak than controls without a history of COVID-19 [18]. The O2 pulse may have a non-specific interpretation due to its relationship with stroke volume and peripheral oxygen utilization. Recent data show that low levels of O2 pulse during exercise may be related to an increase in cardiovascular and all-cause mortality in some populations [15]. This leads to speculating that reduced O2 pulse peak values in COVID-19-recovered subjects could be a significant measure of health outcome. Low O2 pulse at peak is a consequence of a reduced V̇O2peak during short-term follow-up. Already at 6 months of follow-up up to a year after hospital discharge for COVID-19, O2 pulse and V̇O2peak increased significantly [1, 11]. In our longer follow-up, we document a significant global improvement of the O2 pulse from 6 to 34 months, despite no significant increase in VO2peak. The same was true for V̇O2/Wslope, which generally increased as a sign of recovery of the hemodynamic response and the peripheral oxygen utilization. Of note, the selective increase of V̇O2/Wslope in pEVin patients has not a clear interpretation but may be related to the high variability of the few patients considered as pEVin group. In the context of the relationship between the cardiovascular response and the hyperventilation pattern, we demonstrate a significant correlation between the V̇O2/Wslope, O2 pulse at peak, OUES and PETCO2 (Table 3), but only O2 pulse at peak and OUES with PETCO2 at rest.
Similarly to O2 pulse, OUES values represent an individual’s cardiorespiratory reserve and indicate how effectively oxygen is extracted and utilized by the body [25]. The prognostic potential of OUES has been examined in some clinical populations, such as patients with heart failure and very recently, the determination of OUES on healthy males has proved its prediction in all-cause mortality [17, 38]. Our data about the correlation between the hyperventilation and OUES, similarly for O2 pulse, define this variable as potentially prognostic for COVID survivors.
Data about the exercise training on parameters of cardiovascular response in patients with chronic obstructive pulmonary disease (COPD) report OUES - but also O2 pulse - as susceptible to changes, as a sign of an enhancement of ventilatory function upon exercise [39]. In the context of post-COVID patients, although in a single survivor patient from critical COVID-19 illness, and the data requires scientific confirmation, home-based exercise training has been demonstrated to produce a remarkable increment not only of V̇O2 peak but also of the OUES, with a consensual reduction in V̇E/V̇CO2 and exertional dyspnea [40].
Our study’s strength is related to evaluating the EVin for a very long time from COVID-19 discharge (pEVin). Although we report a small number of patients (an explicit limitation), this was related to a selective approach excluding patients having a condition potentially influencing the exercise ventilation assessment. We included a healthy population with normal exercise capacity and pulmonary function tests. This may also be considered a study strength because we excluded any potential cause of ventilatory inefficiency. Finally, we lack same-time data concerning the structural pulmonary (by thorax computed tomography scan) and cardiac (by echocardiography) damage. There is a possibility that these data could have confirmed a coexistent organic residual alteration.
In conclusion, our longitudinal data analysis on COVID-19 survivors, performed at 34 months from discharge, confirms the persistence of exercise ventilatory inefficiency in 16% of subjects. These subjects exhibit a hyperventilation status that correlates closely with an altered and unfavorable cardiovascular response to exercise. These observations underscore the importance of prolonged follow-up studies in individuals recovering from COVID-19.
Data availability
All data generated or analyzed during this study are included in this published article.
References
Davis HE, Mccorkell L, Vogel JM, Topol EJ. Long COVID: major findings, mechanisms and recommendations. Nat Reviews Microbiol |. 2023;21:133–46.
Mancini DM, Brunjes DL, Lala A, Trivieri MG, Contreras JP, Natelson BH. Use of Cardiopulmonary Stress Testing for Patients With Unexplained Dyspnea Post-Coronavirus Disease. 2021. https://doi.org/10.1016/j.jchf.2021.10.002
Motiejunaite J, Balagny P, Arnoult F, Mangin L, Bancal C, Vidal-Petiot E, et al. Hyperventilation as one of the mechanisms of persistent dyspnoea in SARS-CoV-2 survivors. Eur Respir J. 2021;58:2101578.
Motiejunaite J, Balagny P, Arnoult F, Mangin L, Bancal C, d’Ortho M-P et al. Hyperventilation: a possible explanation for long-lasting Exercise intolerance in mild COVID-19 survivors? Front Physiol. 2021;11.
Sun X-GG, Hansen JE, Garatachea N, Storer TW, Wasserman K. Ventilatory Efficiency during Exercise in Healthy Subjects. https://doi.org/101164/rccm2202033. 2012;166:1443–8.
Laveneziana P, Naeije R, Stickland MK, Phillips DB, Collins SÉ. Measurement and interpretation of Exercise Ventilatory Efficiency. Front Physiol | www Frontiersin org. 2020;1.
Wasserman K, Hansen JE, Sue DY, Stringer WW, Sietsema KE, Sun XG et al. Principles of exercise testing and interpretation: Including pathophysiology and clinical applications: Fifth edition. 2011.
Weatherald J, Farina S, Bruno N, Laveneziana P. Cardiopulmonary exercise testing in pulmonary hypertension. Ann Am Thorac Soc. 2017;14 July:S84–92.
Szekely Y, Lichter Y, Sadon S, Lupu L, Taieb P, Banai A, et al. Cardiorespiratory Abnormalities in patients recovering from Coronavirus Disease 2019. J Am Soc Echocardiogr. 2021;34:1273–e12849.
Ingul CB, Edvardsen A, Follestad T, Trebinjac D, Ankerstjerne OAW, Brønstad E, et al. Changes in cardiopulmonary exercise capacity and limitations 3–12 months after COVID-19. Eur Respir J. 2023;61:2200745.
Dorelli G, Braggio M, Gabbiani D, Busti F, Caminati M, Senna G, et al. Importance of cardiopulmonary exercise testing amongst subjects recovering from covid-19. Diagnostics. 2021;11:1–8.
Crisafulli E, Gabbiani D, Magnani G, Dorelli G, Busti F, Sartori G, et al. Residual lung function impairment is Associated with Hyperventilation in patients recovered from hospitalised COVID-19: a cross-sectional study. J Clin Med. 2021;10:1–6.
Noureddine S, Roux-Claudé P, Laurent L, Ritter O, Dolla P, Karaer S, et al. Evaluation of long-term sequelae by cardiopulmonary exercise testing 12 months after hospitalization for severe COVID-19. BMC Pulm Med. 2023;23:1–13.
Singh I, Joseph P, Heerdt PM, Cullinan M, Lutchmansingh DD, Gulati M, et al. Persistent Exertional Intolerance after COVID-19: insights from Invasive Cardiopulmonary Exercise Testing. Chest. 2022;161:54–63.
Laukkanen JA, Araújo CGS, Kurl S, Khan H, Jae SY, Guazzi M, et al. Relative peak exercise oxygen pulse is related to sudden cardiac death, cardiovascular and all-cause mortality in middle-aged men. Eur J Prev Cardiol. 2018;25:772–82.
Peterman JE, Harber MP, Chaudhry S, Arena R, Kaminsky LA. Peak oxygen pulse and mortality risk in healthy women and men: the Ball State Adult Fitness Longitudinal Lifestyle Study (BALL ST). Prog Cardiovasc Dis. 2021;68:19–24.
Peterman JE, Novelli DS, Fleenor BS, Whaley MH, Kaminsky LA, Harber MP. Oxygen uptake efficiency slope as a predictor of Mortality Risk. J Cardiopulm Rehabil Prev. 2023;43:282–9.
Miętkiewska-Szwacka K, Domin R, Kwissa M, Żołyński M, Niziński J, Turska E, et al. Effect of COVID-19 on blood pressure Profile and Oxygen Pulse during and after the Cardiopulmonary Exercise Test in healthy adults. J Clin Med. 2023;12:4483.
Harrison SL, Buckley BJR, Rivera-Caravaca JM, Zhang J, Lip GYH. Cardiovascular risk factors, cardiovascular disease, and COVID-19: an umbrella review of systematic reviews. Eur Heart J Qual Care Clin Outcomes. 2021. https://doi.org/10.1093/ehjqcco/qcab029
Crisafulli E, Dorelli G, Sartori G, Dalle Carbonare L. Exercise ventilatory inefficiency may be a relevant CPET-feature in COVID-19 survivors. Int J Cardiol. 2021;343:200.
Miller MR, Hankinson J, Brusasco V, Burgos F, Casaburi R, Coates A et al. Standardisation Spirometry. https://doi.org/10.1183/09031936.05.00034805
Quanjer PH, Tammeling GJ, Cotes JE, Pedersen OF, Peslin R, Yernault JC. Lung volumes and forced ventilatory flows. Report Working Party standardization of lung function tests, European Community for Steel and Coal. Official Statement of the European Respiratory Society. The European respiratory journal. Supplement. 1993;16:5–40.
Cotes JE, Chinn DJ, Quanjer PH, Roca J, Yernault J-C. Standardization of the measurement of transfer factor (diffusing capacity). Eur Respir J. 1993;6(Suppl 16):41–52.
Weisman IM, Marciniuk D, Martinez FJ, Sciurba F, Sue D, Myers J, et al. ATS/ACCP Statement on Cardiopulmonary Exercise Testing. Am J Respir Crit Care Med. 2003;167:211–77.
Baba R. The Oxygen Uptake Efficiency Slope and its value in the Assessment of Cardiorespiratory Functional Reserve. Congestive Heart Fail. 2000;6:256–8.
Cole CR, Blackstone EH, Pashkow FJ, Snader CE, Lauer MS. Heart-rate recovery immediately after Exercise as a predictor of Mortality. N Engl J Med. 1999;341:1351–7.
Borg G. Psychophysical scaling with applications in physical work and the perception of exertion. Work Environ Health. 1990;16:441–554.
Standardized Questionaries on Respiratory Symptoms. Br Med J. 1960;2:1665.
Mannocci A, Thiene D, Di, Cimmuto A, Del, Masala D, Boccia A, Vito E, De, et al. International Physical Activity Questionnaire: validation and assessment in an Italian sample. Italian J Public Health. 2010;7:369–76.
Weatherald J, Sattler C, Garcia G, Laveneziana P. Ventilatory response to exercise in cardiopulmonary disease: the role of chemosensitivity and dead space. Eur Respir J. 2018;51:1700860.
Taverne J, Salvator H, Leboulch C, Barizien N, Ballester M, Imhaus E, et al. High incidence of hyperventilation syndrome after COVID-19. J Thorac Dis. 2021;13:3918–22.
Huang Y, Tan C, Wu J, Chen M, Wang Z, Luo L, et al. Impact of coronavirus disease 2019 on pulmonary function in early convalescence phase. Respir Res. 2020;21:163.
Liu M, Lv F, Huang Y, Xiao K. Follow-Up study of the Chest CT Characteristics of COVID-19 survivors seven months after recovery. Front Med (Lausanne). 2021;8:636298.
Parshall MB, Schwartzstein RM, Adams L, Banzett RB, Manning HL, Bourbeau J, et al. An official American thoracic Society Statement: update on the mechanisms, Assessment, and management of Dyspnea. Am J Respir Crit Care Med. 2012;185:435–52.
Xiang M, Wu X, Jing H, Novakovic VA, Shi J. The intersection of obesity and (long) COVID-19: Hypoxia, thrombotic inflammation, and vascular endothelial injury. Front Cardiovasc Med. 2023;10.
Dixon AE, Peters U. The effect of obesity on lung function. Expert Rev Respir Med. 2018;12:755–67.
Cassar MP, Tunnicliffe EM, Petousi N, Lewandowski AJ, Xie C, Mahmod M, et al. Symptom Persistence despite Improvement in Cardiopulmonary Health – insights from longitudinal CMR, CPET and lung function testing post-COVID-19. EClinicalMedicine. 2021;41:101159.
Arena R, Brubaker P, Moore B, Kitzman D. The oxygen uptake efficiency slope is reduced in older patients with heart failure and a normal ejection fraction. Int J Cardiol. 2010;144:101–2.
Ramponi S, Tzani P, Aiello M, Marangio E, Clini E, Chetta A. Pulmonary Rehabilitation improves Cardiovascular Response to Exercise in COPD. Respiration. 2013;86:17–24.
Longobardi I, do Prado DML, Goessler KF, Meletti MM, de Oliveira Júnior GN, de Andrade DCO, et al. Oxygen uptake kinetics and chronotropic responses to exercise are impaired in survivors of severe COVID-19. Am J Physiol Heart Circ Physiol. 2022;323:H569–76.
Acknowledgements
We are grateful to the Italian Minister of the University and Research for supporting the development of the study “Long-COVID syndrome: pathophysiology of the impaired exercise tolerance” (PRIN 2022LBBKHX).
Funding
This study received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
Substantial contributions to the conception or design of the study and the acquisition, analysis, or interpretation of data: GD, GS, GF, NR, NB, MB, MF, MV, LDC, CC, BG, EC. Drafting the study or revising it critically for important intellectual content: GD, GS, GF, NR, NB, MB, MF, MV, LDC, CC, BG, EC. Final approval of the version to be published: GD, GS, GF, NR, NB, MB, MF, MV, LDC, CC, BG, ECAgreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved: GD, GS, GF, NR, NB, MB, MF, MV, LDC, CC, BG, EC.All authors take responsibility for all aspects of the reliability and freedom from bias of the data presented and their discussed interpretation.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Written informed consent was obtained before the first visit and the protocol was approved by the local ethics committee, Comitato etico per la Sperimentazione Clinica (CESC). The study was performed in accordance with the Declaration of Helsinki and Good Clinical Practice guidelines. Clinical trial registration number: 2785CESC.
Consent for publication
All patients gave informed consent.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Dorelli, G., Sartori, G., Fasoli, G. et al. Persisting exercise ventilatory inefficiency in subjects recovering from COVID-19. Longitudinal data analysis 34 months post-discharge. BMC Pulm Med 24, 258 (2024). https://doi.org/10.1186/s12890-024-03070-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12890-024-03070-1