Abstract
Background
Anti-synthetase syndrome (ASS) is a group of rare clinical subtypes within inflammatory myopathies, predominantly affecting adult females. Instances of critical illness associated with ASS in children are even rarer.
Case presentation
We report the case of a 7-year-old boy finally diagnosed with ASS, combined with pneumomediastinum. He presented with intermittent fever persisting for 12 days, paroxysmal cough for 11 days, chest pain, and shortness of breath for 4 days, prompting admission to our hospital. Pre-admission chest CT revealed diffuse pneumomediastinum, subcutaneous pneumatosis in the neck and bilateral chest wall, consolidation, atelectasis, and reticular nodular shadowing in both lungs, as well as pericardial effusion and bilateral pleural effusions. Laboratory tests revealed a positive result for serum MP immunoglobulin M (MP-IgM) and MP immunoglobulin G (MP-IgG). The patient was initially diagnosed with mycoplasma pneumoniae (MP) infection, and following 3 days of antibiotic treatment, the patient's tachypnea worsened. Positive results in muscle enzyme antibody tests included anti–PL-12 antibody IgG, anti–Jo-1 antibody IgG, and anti–RO-52 antibody IgG. Ultrasonography detected moderate effusions in the right shoulder, bilateral elbow, and knee joints. Corticosteroids pulse therapy was initiated on the 27th day following disease onset, and continued for 3 days, followed by sequential therapy for an additional 12 days. The child was discharged on the 43rd day, and subsequent follow-up revealed a significant improvement in consolidation and interstitial lesions in both lungs.
Conclusions
ASS in children may combine with rapidly progressive interstitial lung disease (RPILD) and pneumomediastinum. It is crucial to promptly identify concurrent immunologic abnormalities during the outbreak of MP, particularly when the disease exhibits rapid progression with ineffective conventional antibiotic therapy.
Similar content being viewed by others
Background
Anti-synthetase syndrome (ASS) is an autoimmune disorder characterized by inflammatory myopathies, arthritis, and cutaneous manifestations such as Raynaud’s phenomenon and Mechanic’s hands, along with interstitial lung disease (ILD) [1, 2]. ASS is one of the most prevalent subtypes of idiopathic inflammatory myopathies (IIMs), with distinctive serological markers being anti-tRNA synthetase antibodies (ARSs), including anti–Jo-1-ARS, anti–PL-7, anti–PL-12, anti-EJ, anti-KS, anti-Zo, anti-Tyr/YRS, and anti-OJ antibodies. Among these, anti–Jo-1-ARS is the most frequently observed in ASS patients [3].
When the lungs are affected, ASS manifests as nonspecific interstitial pneumonia characterized by diffuse ground-glass opacities and peripheral consolidation. Additional features encompass linear opacities, a honeycomb pattern, and traction bronchiectasis [4]. Pathological observations have revealed varying degrees of inflammation and fibrosis in the lung interstitium. Microscopically, this is characterized by infiltration of lymphocytes and plasma cells in the alveolar septa, which comprises collagen fibers exhibiting varying degrees of fibrosis mixed with chronic inflammation [5].
This study presents the case of an ASS child complicated with pneumomediastinum. The report underscores the importance for clinicians to pay attention to immunologic abnormalities when encountering a general infection that rapidly progresses to multi-system abnormalities during the outbreak of MP. Additionally, we conducted a literature review on ASS combined with pneumomediastinum, with the aim of enhancing understanding of ASS during infected states and preventing delays in treatment.
Case presentation
A 7-year-old male was admitted to our hospital exhibiting intermittent fever persisting for 12 days (with a peak temperature of 39.0 °C), paroxysmal cough ongoing for 11 days, and chest pain along with shortness of breath for the past 4 days. Before admission, the patient had undergone antibiotic treatment at other medical facilities, however, it proved ineffective. At the time of admission to our hospital, his body temperature was 36.9℃, respiratory rate was 65 breaths/min, pulse rate was 64 beats/min, and oxygen saturation was 98.0% with mask oxygenation. The patient had obvious symptoms of hypoxia, including tachypnea, three-concave sign, rough and decreased respiratory sounds in both lungs. There were rales in the left lung. Subcutaneous crepitation was detected in the neck. No hemorrhages or rashes were observed on the skin and mucous membranes throughout the body. The patient exhibited normal muscle strength and tension. There was no reported history of abnormal development, allergies, surgery, trauma, family medical background, or chronic infectious diseases.
Chest radiography conducted prior to admission revealed diffuse consolidation of both lungs, pneumomediastinum, and subcutaneous emphysema. To further elucidate the extent of pneumomediastinum and characterize the lung lesions, a chest CT examination was performed on the patient. Chest CT disclosed subcutaneous pneumatosis in the neck and bilateral chest wall, pneumomediastinum, interstitial emphysema in both lungs, diffuse consolidation, reticular nodular shadowing in both lungs, atelectasis in the upper lobes of both lungs, and in the lower lobe of the left lung, in addition to pericardial effusion and bilateral pleural effusions. Laboratory tests revealed a positive result for serum MP immunoglobulin M (MP-IgM) and immunoglobulin G (MP-IgG). Metagenomic next-generation sequencing (mNGS) of bronchoalveolar lavage fluid indicated the presence of MP, Human gammaherpesvirus 4, and human metapneumovirus (Table 1). Tests for Streptococcus pneumoniae and group A streptococcus antigens returned negative results. PCR results for EV71 + CA16 + enterovirus and COVID-19 nucleic acid were also negative. Other relevant laboratory tests are detailed in Table 2. Noninvasive assisted ventilation was administered initially, followed by anti-infection medication (intravenous cefoperazone sulbactam and erythromycin) and anti-inflammation treatment (intravenous methylprednisolone, 2 mg/kg/day, b.i.d.).
On day 13 of illness, due to evident shortness of breath, the child was transferred to the PICU, where he underwent antibody testing to help identify autoimmune disease as CT indicated interstitial lesions in both lungs. The autoantibody profile, encompassing 11 items (serum), revealed anti–Jo-1 antibody (+ +). Myositis antibody spectrum analysis showed anti–PL-12 antibody IgG ( +), anti–Jo-1 antibody IgG ( +), and anti–RO-52 antibody IgG ( +). We did not perform tests on antibodies to melanoma differentiation-associated gene 5 (MDA5) because of restricted examination scope. Sputum culture showed no abnormalities.
On day 18, after a slight stabilization of his condition, the child was transferred to the Department of Respiratory Medicine. Although the child did not have obvious joint swelling or pain, based on the positive results of several myositis antibodies, he was considered to have an autoimmune disease, and was recommended to undergo a joint examination to confirm the joint involvement. Ultrasound revealed synovial bursa effusion in the right shoulder joint, bilateral elbow joints, and knee joints.
On day 26, following a multidisciplinary consultation and discussion, ASS was considered. Pulse treatment (methylprednisolone, 500 mg/day, intravenously guttae, q.d.), was administered for 3 days, followed by sequential oral prednisolone (30 mg/day, q.d.) on days 30–34, which was then reduced to 25 mg/day on day 35. On day 35, oral tacrolimus (1 mg, q12H) was added (Fig. 1).
Chest radiographs were reexamined on day 42, revealing the absorption of pneumomediastinum, bilateral cervical and axillary subcutaneous pneumoperitoneum, reduction of lesions in both lungs, and nearly complete absorption of left pleural effusion. Following treatment, the child's condition stabilized, and he was discharged from the hospital. At the time of discharge, he showed no signs of fever, cough, shortness of breath, wheezing, or cyanosis, and the pneumomediastinum had resolved. One-week post-discharge, the chest CT scan revealed residual interstitial lesions and consolidation in both lungs, indicating improvement compared to the CT scan on day 18 of illness (Fig. 2). During a follow-up visit 1 month later, inflammatory indexes showed no abnormalities. The patient received oral administration of tacrolimus and prednisone acetate tablets, along with fluticasone propionate inhaler prescription. Regular follow-ups were scheduled in the 3rd, 4th, and 5th months to monitor the blood concentration of tacrolimus, adjust the tacrolimus dosage, lower the prednisone dosage, and undergo regular hospital admission for gammaglobulin transfusion (1 g/kg) for immunotherapy regulation.
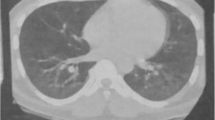
Radiologic findings of the patient. A-C On day 18 of illness, CT shows subcutaneous pneumatosis of the neck and bilateral chest wall, pneumomediastinum, consolidation, and reticular nodular shadow in both lungs, along with pericardial effusion and bilateral pleural effusion. D-E On day 23, subcutaneous air is essentially absorbed, pneumomediastinum is reduced compared with earlier, and consolidation and interstitial lesions in both lungs are present. F-G On day 30, pneumomediastinum is fully absorbed, and consolidation and pleural effusion are less pronounced than earlier. H-I One week after discharge, CT shows reticular nodular shadow, and both lungs exhibit residual consolidation, which have significantly resolved
Discussion
Pneumomediastinum, characterized by the entry of air into the mediastinum through connective tissue spaces within the pleura [6, 7], can be asymptomatic with a small gas accumulation. However, sudden onset or a significant influx of air compressing the organs within the mediastinum can result in respiratory and circulatory disorders, and in severe cases, be life-threatening.
The current case represents the first report of anti-synthetase syndrome in a child combined with pneumomediastinum. Previous studies revealed the similar phenotype of ASS in juveniles and adults, although this subtype is much less frequent in childhood than in adulthood. Important features such as Raynaud phenomenon, mechanics hands and ILD seem to occur at a lower frequency in childhood-onset disease than in adult-onset disease [8].
Our retrospective analysis of ASS cases combined with pneumomediastinum, previously reported in adults (Table 3), strongly indicates a correlation of ASS with ILD and pulmonary infection [9,10,11]. This underscores the pivotal role of infection in precipitating severe complications of ASS-associated ILD. In our case report, pneumomediastinum was potentially associated with ASS-associated rapidly progressive ILD (RPILD), with infection being a crucial factor in its progression. Remarkably, the MP and other virus infection are not the trigger for the onset of ASS. The patient in this particular case had an underlying preexisting condition of ASS, which had not been previously identified.
Metagenomic next-generation sequencing (mNGS) of bronchoalveolar lavage fluid revealed the presence of MP, Human gammaherpesvirus 4, and human metapneumovirus. Notably, the number of sequences detected for MP was significantly higher than that for other pathogens. Considering the clinical manifestations in children, MP infection remains a significant factor to consider when explaining the rapid progression of interstitial lung disease; however, it is important not to exclude the possibility of infection with other viruses.
Numerous studies have confirmed that abnormalities in the immune mechanism play a significant role in the development of ASS-ILD, with a focus on the production of anti-synthesis enzyme antigen–antibody interactions [12, 13]. In vitro experiments have shown the presence of histidyl-tRNA synthetase (anti–Jo-1)–reactive CD4 + T cells in bronchoalveolar lavage fluid, indicating immune activation against anti–Jo-1 antibody in the lungs of ASS patients [14]. Antigen stimulation triggers innate and adaptive immune responses, leading to the production of antibodies that can be transferred to various tissues in the body.
Pneumomediastinum and subcutaneous pneumatosis are commonly observed in IIM patients with ILD [5, 7]. The mechanism underlying pneumomediastinum in IIMs remains unclear, with some scholars proposing that it may be induced by vasculitis and pulmonary fibrosis [7, 15]. Vasculitis can lead to necrosis of the alveolar and bronchial walls, disrupting the mucosal barrier and allowing air to enter the mediastinum. Pulmonary fibrosis, on the other hand, may result in lung atelectasis near the mediastinum, forming pulmonary bullae; rupture of these bullae can puncture the pleura, enabling air to enter the mediastinum and causing pneumomediastinum. Moreover, rapidly progressing lung infections, such as MP, can cause mucosal damage to alveolar walls, resulting in pneumomediastinum and pneumothorax.
Approximately 33% [16] of IIM patients can develop RPILD. ASS, accounting for 7.8% of IIMs, is associated with ILD in > 90% of cases [17], and ILD significantly contributes to increased prevalence and mortality in these patients [17, 18], and ASS patients with ILD often experience more severe disease with rapid progression. ILD associated with IIMs exhibit a high ICU admission rate of 26.7% and a mortality rate of 28.9% [16], emphasizing the importance of early diagnosis and aggressive treatment to prevent serious outcomes.
In the current case, the child did not exhibit significant manifestations of myositis; however, despite the absence of cutaneous features of connective tissue disease, myositis is not present in approximately 53% of ASS patients [2]. Positive results for anti–PL-12, anti–Jo-1, and anti–Ro-52 antibodies were reported in this case. Studies have shown that anti–Ro-52 antibodies in ASS patients are associated with RPILD [19], whereas anti–PL-12 antibodies are strongly associated with ILD development [20]. In a previous study, arthritis was the most common initial symptom in the anti–Jo-1 antibody-positive group [21], and in alignment with literature, our patient presented with multiple joint effusions. Additionally, this case highlights the importance of promptly considering the possibility of combined autoimmune-associated nonspecific ILD and, if necessary, conducting tests for IIM-associated antibodies when evaluating rapidly progressing infections with uncommon extrapulmonary complications.
Conclusion
The occurrence of ASS in children is uncommon, and when infection is present, the disease may progress rapidly or even lead to pneumomediastinum. In the context of an epidemic of MP, if a child demonstrates rapid progression and uncommon extrapulmonary complications, pediatricians should consider the possibility of immune-related diseases and promptly conduct tests for relevant antibodies.
Availability of data and materials
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.
References
Acharya S, Meador R, Plocharczyk E, Tiwari A. Gupta SS Isolated interstitial lung disease associated with anti-synthetase syndrome. QJM An International Journal of Medicine. 2022;115(6):408–9.
Bartoloni E, Gonzalez-Gay MA, Scirè C, Castaneda S, Gerli R, Lopez-Longo FJ, Martinez-Barrio J, Govoni M, Furini F, Pina T, et al. Clinical follow-up predictors of disease pattern change in anti-Jo1 positive anti-synthetase syndrome: Results from a multicenter, international and retrospective study. Autoimmun Rev. 2017;16(3):253–7.
Sun S, Chen Z, Zhang D, Xu W, Wu W, Sun F, Gu L, Chen J, Li J, Li T, et al. Description and Analysis of a Novel Subtype of the Anti-Synthetase Syndrome Characterized by Frequent Attacks of Fever and Systemic Inflammation in a Single-Center Cohort Study. Front Immunol. 2021;12:729602.
Alfraji N, Mazahir U, Chaudhri M, Miskoff J. Anti-synthetase syndrome: a rare and challenging diagnosis for bilateral ground-glass opacities—a case report with literature review. BMC Pulm Med. 2021;21(1):11.
Wu W, Guo L, Fu Y, Wang K, Zhang D, Xu W, Chen Z, Ye S. Interstitial Lung Disease in Anti-MDA5 Positive Dermatomyositis. Clin Rev Allergy Immunol. 2021;60(2):293–304.
Li C. Liang Me, Jiang H, Zhao J, Wu C, Wang Q, Zhang L, Zhao Y: The long-term prognosis of pneumomediastinum associated with dermatomyositis: a two-centre retrospective cohort study. Rheumatology. 2021;60(5):2288–95.
Le Goff B, Chérin P, Cantagrel A, Gayraud M, Hachulla E, Laborde F, Papo T, Sibilia J, Zabraniecki L, Ravaud P, et al. Pneumomediastinum in interstitial lung disease associated with dermatomyositis and polymyositis. Arthritis Care Res. 2008;61(1):108–18.
Papadopoulou C, Chew C, Wilkinson MGL, McCann L, Wedderburn LR. Juvenile idiopathic inflammatory myositis: an update on pathophysiology and clinical care. Nat Rev Rheumatol. 2023;19(6):343–62.
Jhajj AS, Shun Yeung JH, To F, Tsay GJ. Spontaneous Pneumomediastinum due to Anti-Melanoma Differentiation-Associated Protein 5 Requiring a Bilateral Lung Transplant. Case Reports in Rheumatology. 2021;2021:1–4.
Saint-Georges F, Mulliez P, Lebas D, Modiano P, Bisch D. Pneumomediastinum complicating the anti-synthetase syndrome. Rev Mal Respir. 2005;22(6 Pt 1):1031–34.
Vinicki JP, Pellet SC, Raimondi A, Dubinsky D, Nasswetter G. Antisynthetase syndrome with subcutaneous emphysema and pneumomediastinum. J Clin Rheumatol. 2014;20(7):401–2.
Adams RA, Fernandes-Cerqueira C, Notarnicola A, Mertsching E, Xu Z, Lo W-S, Ogilvie K, Chiang KP, Ampudia J, Rosengren S, et al. Serum-circulating His-tRNA synthetase inhibits organ-targeted immune responses. Cell Mol Immunol. 2019;18(6):1463–75.
Joosse BA, Jackson JH, Cisneros A, Santhin AB, Smith SA, Moore DJ, Crofford LJ, Wilfong EM, Bonami RH. High-Throughput Detection of Autoantigen-Specific B Cells Among Distinct Functional Subsets in Autoimmune Donors. Front Immunol. 2021;12:685718.
Galindo-Feria AS, Albrecht I, Fernandes-Cerqueira C, Notarnicola A, James EA, Herrath J, Dastmalchi M, Sandalova T, Rönnblom L, Jakobsson PJ, et al. Proinflammatory Histidyl-Transfer RNA Synthetase-Specific CD4+ T Cells in the Blood and Lungs of Patients With Idiopathic Inflammatory Myopathies. Arthritis & Rheumatology. 2019;72(1):179–91.
Gonçalves TA, Parente DB, Barreto MM. Pneumomediastinum and pneumorrhachis as complications of dermatomyositis. J Bras Pneumol. 2021;47(6):e20210352.
Abu-Rumeileh S, Marrani E, Maniscalco V, Maccora I, Pagnini I, Mastrolia MV, Rouster-Stevens K, Simonini G. Lung involvement in juvenile idiopathic inflammatory myopathy: A systematic review. Autoimmun Rev. 2023;22(10):103416.
Zhang D, Wang H, Zhou X, Yang J, Liu Y, Wang W, Jiang P, Fan B. Clinical characteristics and prognostic analysis of idiopathic inflammatory myopathy with positive anti‐aminoacyl‐tRNA synthetase antibodies: A single center experience. Immun Inflamm Dis. 2023;11(11):e1085.
Sawal N, Mukhopadhyay S, Rayancha S, Moore A, Garcha P, Kumar A, Kaul V. A narrative review of interstitial lung disease in anti-synthetase syndrome: a clinical approach. J Thorac Dis. 2021;13(9):5556–71.
Sodsri T, Petnak T, Ngamjanyaporn P. Clinical Characteristics of Anti-Synthetase Syndrome and Variables Associated with Interstitial Lung Disease and Mortality: A Retrospective Cohort Study. J Clin Med. 2023;12(21):6849.
Pinal-Fernandez I, Casal-Dominguez M, Huapaya JA, Albayda J, Paik JJ, Johnson C, Silhan L, Christopher-Stine L, Mammen AL, Danoff SK. A longitudinal cohort study of the anti-synthetase syndrome: increased severity of interstitial lung disease in black patients and patients with anti-PL7 and anti-PL12 autoantibodies. Rheumatology. 2017;56(6):999–1007.
Tang HS, Tang IYK, Ho RTC, Young JKY, Lai BTL, Chung JYK, Yung AKM, Cheung CCL, Lee PML, So H: Clinical heterogeneity and prognostic factors of anti-synthetase syndrome: a multi-centred retrospective cohort study. Rheumatology 2023:kead671.
Funding
This work was supported by the Sciences and Technology Project of Shenzhen (Grant No. JCYJ20220530155805012), the Sanming Project of Medicine in Shenzhen (Grant No. SZSM202011005), Guangdong High-level Hospital Construction Fund (No. ynkt2022-zz38).
Author information
Authors and Affiliations
Contributions
Jieqiong Lin collected the clinical data and wrote the original draft. Yaowen Li, Longwei Sun collected the clinical data and reviewed the manuscript. Weisheng Sun prepared the chest CT figures. Qimeng Fan, Xin Zhao prepared the clinical data. Hongwu Zeng reviewed the manuscript. All authors revised and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
No applicable. Ethical approval to report this case was not required due to its retrospective nature.
Consent for publication
Written informed consent was obtained from the patient for publication of this case report and any accompanying images.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Lin, J., Li, Y., Fan, Q. et al. Anti-synthetase syndrome in a child with pneumomediastinum: a case report and literature review. BMC Pulm Med 24, 158 (2024). https://doi.org/10.1186/s12890-024-02984-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12890-024-02984-0