Abstract
Background
The association of physical activity (PA) and lung function (LF) varies from no measurable effect to delayed LF decline. We assessed the association between accelerometery-assessed PA and LF in a sample of apparently healthy, community-dwelling subjects.
Methods
We included two cross-sectional studies using data from the PneumoLaus study (2014–17 and 2018–21), conducted in Lausanne, Switzerland. PA was assessed by accelerometry and categorised as inactivity, light, moderate or vigorous. Forced expiratory volume in 1 second (FEV1), forced volume capacity (FVC) and maximal mid-expiratory flow (MMEF) were measured by spirometry and expressed in percentage of predicted value (PV).
Results
Overall, 1′910 (54.7% women, 62.0 ± 9.7 years) and 1′174 (53.4% women, 65.8 ± 9.5 years) participants were included in the first and the second surveys, respectively. In both surveys, moderate and vigorous PA showed a weak but significant correlation with FEV1 in percentage (PV) (R = 0.106 and 0.132 for the first and 0.111 and 0.125 for the second surveys, p < 0.001). Similar correlations with FVC (p < 0.001) were found. Associations held irrespective of smoking status and remained after multivariable adjustment. Fewer associations were detected between LF and light PA or between MMEF and PA.
Conclusion
Moderate and vigorous intensity PA are associated with increased LF regardless of smoking status in apparently healthy community-dwelling European population. These associations are statistically but not clinically significant due to the small correlation coefficients (R < 0.30), corresponding to a weak association.
Similar content being viewed by others
Introduction
Physical activity (PA) has been shown to improve a wide range of physiological parameters, including lung function (LF) [1]. A prospective study over 25 years, published in 2003, reported a positive association between PA levels and forced expiratory volume in 1 second (FEV1) and forced vital capacity (FVC) [2]. The authors concluded that PA may delay the decline in LF that occurs in middle- and old-age, regardless of smoking status.
However, other cross-sectional and prospective studies only found this association among smokers [3, 4] and another found no association between PA and spirometric indices [5]. These divergent results may be due to the difficulty of PA quantification. Indeed, some studies used PA questionnaires [2,3,4, 6,7,8,9] which can be biased by subjective input. A study conducted in former and current smokers assessed PA via accelerometery and reported a positive relationship between moderate-to-vigorous PA (MVPA) and LF [10]. Consequently, there is conflicting or minor evidence on the association between objectively assessed PA and LF, and the degree of PA necessary to maintain adequate LF.
We aimed to repeat the associations between objectively assessed PA and LF in a sample of healthy, community-dwelling subjects in Switzerland, overall and according to smoking status, and to study this association at two different time points of our cohort. Our hypothesis was that these would show a good to strong correlation.
Methods
PneumoLaus study
The PneumoLaus study is part of the CoLaus|PsyCoLaus study (https://www.colaus-psycolaus.ch), an ongoing prospective study aiming to assess the determinants of cardiovascular and psychiatric diseases using a population-based sample drawn from the city of Lausanne, Switzerland [11]. In June 2014, all participants of the CoLaus|PsyCoLaus study were invited to take part in the PneumoLaus study. Baseline examinations were conducted between June 2014 and August 2017, and the follow-up survey was conducted between June 2018 and February 2021. All ethnic groups were included as the general population was randomly invited to participate in the CoLaus|PsyCoLaus study.
Ethical statement
The CoLaus|PsyCoLaus studiy were approved by the local Ethics Committee and participants provided written informed consent (https://www.cer-vd.ch; project number PB_2018–00038, reference 239/09).
Spirometry
PneumoLaus methodology was already described [12]. Briefly, LF was assessed using a MasterScreen-PFT spirometer (Carefusion, Hoechberg, Germany), with Sentry Suite software (Version 2.17). Each manoeuvre was automatically assessed by computer, based upon acceptability and reproducibility criteria according to the 2005 American Thoracic Society– European Respiratory Society standards [13].
Reference values were applied according to the Global Lung Function Initiative (GLI) 2012, adjusting for the following ethnic origins: Caucasian, African, Northeast Asian, Southeast Asian and other [14]. If FEV1/FVC or FVC was found to be below the lower limits of normal (LLN), spirometry was repeated 10–15 minutes after administration of 4 × 100 μg of salbutamol via a metered-dose inhaler and a spacer. Normal spirometry was defined by baseline FEV1/FVC ratio and FVC above LLN, representing the lower 5th percentile (corresponding to a z-score of − 1.645) based on age, sex, height and ethnicity [15]. The maximal mid expiratory flow (MMEF) was defined by the mean forced expiratory flow between 25 and 75% of the FVC. An experienced respiratory technician and a consultant pulmonologist evaluated the quality of spirometry manoeuvres. Spirometry was included using recognised acceptability and reproducibility criteria [16].
Physical activity assessment
PA was assessed using a wrist-worn triaxial accelerometer (GENEActiv, Activinsights Ltd., United Kingdom, https://www.activinsights.com). The accelerometer has been validated against caliometry demonstrating excellent correlations against METs (Metabolic Equivalent of Task). The area under the Receiver Operating Characteristic (ROC) curves to discriminate sedentary activity ranged from 0.844–0.896, and for moderate to vigorous activity from 0.991–0.993 [17].
The devices were pre-programmed with a 50 Hz sampling frequency and subsequently attached to the participants’ right wrist. Participants were requested to wear the device continuously for 14 days in their free-living conditions. A valid day was defined as ≥10 hours of diurnal wear-time on weekdays and ≥ 8 hours on week-end days. At least 5 week days and 2 week-end days of valid data were required [18, 19].
PA was categorised into inactivity (< 85 m-g), light (85–180 m-g), moderate (181–437 m-g) and vigorous (> 437 m-g) PA, based on the thresholds defined by White et al. [20] and the average daily time spent within each category was utilised for analysis. MVPA was obtained by summing time spent in moderate and vigorous PA.
Other covariates
Educational level was categorised into high (university), middle (high school), and low (apprenticeship or mandatory) [21]. Nationality was categorised as born in Switzerland or not. Marital status was categorised as living with or without a partner. Smoking status was self-reported and categorised as never, former, or current smoker [22].
Body weight and height were measured with participants barefoot and in light indoor clothes. Body weight was measured in kilograms to the nearest 100 g using a Seca® scale (Hamburg, Germany). Height was measured to the nearest 5 mm using a Seca® (Hamburg, Germany) height gauge. Body mass index (BMI) was categorised as normal (< 25 kg/m2), overweight (≥25 and < 30 kg/m2) and obese (≥30 kg/m2) [23].
Inclusion and exclusion criteria
Participants from the PneumoLaus study (2014–17 and 2018–21) were considered as eligible. Those with possible restrictive ventilatory impairment defined as FVC < LLN before and after bronchodilation or obstructive ventilatory impairment defined as FEV1/FVC < LLN or missing any information concerning PA and smoking status were excluded.
Data analysis
Data was analysed employing Stata version 17.0 for Windows (Stata Corp, College Station, Texas, USA). Descriptive statistics were presented as number of participants (percentage) for categorical variables or as average ± standard deviation for continuous variables. Between-group comparisons were performed using chi-square or Fisher’s exact test for categorical variables and student’s t-test or analysis of variance for continuous variables.
Within each PA level, the associations between LF (in absolute volumes and in percentage of predicted value) and time spent in the corresponding PA level were assessed by Spearman rank correlation and the coefficients of determination (R-squared or R2), defined as the square value of the correlation coefficient or the percentage of variance explained by the multivariable linear regression model were computed. The R2 coefficient was considered “high” when it was > 0.70, “good” between 0.50–0.70, “fair” between 0.30–0.50 and “weak or no association” if it was < 0.30 [24]. Multivariable analysis was conducted using linear regression, with LF as the dependent variable and time spent in a PA level as independent variable. Adjustments were performed for age (continuous), BMI (continuous) and smoking category (never, former, current) and results were expressed as standardised beta coefficients. Statistical significance was considered for a two-sided test with p < 0.05. A mixed method analysis was then performed, grouping the data from both follow-ups, thus including some participants with data in each.
A final analysis categorised the duration of MVPA in tertiles and compared LF parameters between them.
Results
Selected participants
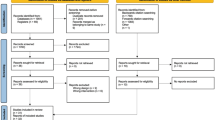
Of the initial 4881 participants of the baseline survey, 1910 (39.1% participation rate, 54.7% women, 62.0 ± 9.7 years) were included in the analysis. Of the initial 3751 participants of the follow-up survey, 1174 (participation rate 31.3, 53.4% women, 65.8 ± 9.5 years) were included. The reasons for exclusion and the characteristics of the included and excluded participants are summarised in Fig. 1 and Supplementary Table 1, respectively. In the baseline survey, included participants were younger, more frequently professionally active, of higher educational level and less frequently smokers or hypertensive than excluded participants. In the follow-up survey, included participants were older, less professionally active, more frequently of middle educational level, and less frequently smokers than excluded participants.
Association between PA and spirometry metrics as % of predicted values
The results of bivariate and multivariable analyses for percentage of PV are summarised respectively in Tables 1 and 2, and the R2 for bivariate associations are provided in Supplementary Table 2. In both studies and analyses, vigorous PA was significantly and positively associated with FEV1 and FVC except in current smokers of the follow-up study. R2 were low, ranging between 0 and 4.2%.
In the multivariable analysis of both studies, moderate PA was significantly and positively associated with FEV1 and FVC except in current smokers of the follow-up. No significant association was found between any PA levels and MMEF, except for a positive association for moderate and vigorous PA in current smokers of the baseline study. Significant associations were found between light PA and both FVC and FEV1 as % of PV, except for current smokers of the follow-up.
Table 3 shows the mixed method analysis, merging both follow-ups, and including repeated measures of participants who performed LF in both follow-ups. The results confirm those obtained separately for each follow-up. For example, an increase of 15 minutes in inactivity leads to a decrease in FEV1 of 0.12%.
Similar findings were found upon LF in absolute values (Supplementary Tables 3, 4 and 5). In fact, only association between vigorous PA and both FEV1 and FVC were significantly positive when adjusting for BMI and age. A visual depiction of the bivariate associations between spirometry values and vigorous PA, respectively inactivity, is provided in Supplementary Figs. 1 and 2.
Discussion
For most PA parameters, we observed small correlations with LF parameters, with R2 below 10%. This suggests that LF has limited importance in explaining PA in a large, healthy, community-dwelling population, despite statistically significant associations even after adjusting for age and BMI.
The significantly positive correlation seen between vigorous PA and absolute volume persisted for FEV1 and FVC as % of PV, except for current smokers in the follow-up study, also due to a lack of power. There was no association between MMEF in % of PV and PA. Nevertheless, when analysed univariately with spirometry values as % of PV, the correlation with moderate PA remained.
The association between PA and lung volumes could be partly due to the influence of different lifestyles on lung volumes. Just as PA, certain diets, such as the Mediterranean one, have a positive association with LF as they reduce inflammation in tissues, including in lungs [25, 26]. A positive association has been demonstrated both in a population without lung disease [25] and in case of cystic disease or chronic obstructive pulmonary disease [27,28,29].
Comparison with other studies
The present study is consistent with the positive association found in three other cross-sectional studies which also used accelerometer-assessed PA [10, 30, 31]. In contrast to our study, the association was described as stronger for former and current smokers compared to non-smokers [10, 30] but this difference might only be due to lack of power of our small smoker group. On the contrary, Barboza et al. [32], who also used accelerometer-assessed PA, found no association between PA and LF, nor did Smith et al. [5]. Our distinct results may be attributed to the difference in definition of PA intensity levels, as several studies assessed PA based on questionnaires and others on accelerometry but without forcibly applying the same thresholds as we did. Similarly, there were differences between the studied populations. Ours was older: mean 62.0 ± 9.7 years at baseline and 65.8 ± 9.5 years at follow-up versus 47 ± 14 years (males) and 53 ± 8 years (females) in Barboza et al. [32] and 15.2 ± 0.25 years in Smith et al. [5]. Our population was also less overweight: mean BMI 26.3 ± 4.6 kg/m2 at baseline and 26.2 ± 4.3 at follow-up versus 27.4 ± 4.5 kg/m2 (males) and 29.8 ± 7.07 kg/m2 (females) in Barboza et al. [32]. In fact, Barboza et al. [32] used both questionnaire and accelerometery-assessed PA but only show accelerometer-derived inactivity data. Also, the device was worn on the wrist in our study, versus the hip in the others [5, 32]. The waist location has indeed been shown to have better accuracy, followed by the left and then the right wrist, which was used in our study [33]. As inflammation increases with age, one hypothesis to explain our findings could be that PA counterbalances excessive inflammation, which is not present in youth [26]. Indeed, Garcia-Aymerich et al. found an association between PA and LF only in older people (> 40 years) [3], already starting with moderate PA. Our study seems to confirm the age-related association but not the association between moderate PA and spirometric indices. Moreover, as shown in Rose et al.s systematic review and metanalysis, the intensity of exercise did not influence chronic inflammatory response in general, even though sub-analyses suggest that higher training intensity may have more effect in middle-aged adults [34]. For ease of understanding, Table 4 summarises the results of the cited studies.
Strengths and limitations
The strength of this study is the objectively assessed PA by accelerometery and LF by spirometry. As this study was conducted in a sample of apparently healthy, community-dwelling people, our results indicate that maintaining an adequate level of PA is associated with a better LF. Finally, our sample size is larger than most other studies conducted in healthy people (n = 62 for Barboza et al. [32], n = 322 for Luzak et al. [30], n = 895 for Smith et al. [5]), thus allowing a bigger statistical power.
Our study also has limitations. First, it was conducted in a single city with mainly subjects of European ancestry, hence, results might not be generalisable in other settings or other ethnicities, although we believe that such associations should hold in most cases. Second, the cross-sectional design does not allow to establish the cause-effect relationship between PA and LF or to assess whether a long-term relationship between PA and LF exists. A dedicated prospective analysis would be necessary to answer this. Third, we did not determine the correlation between PA and static lung volumes such as total lung capacity, functional residual capacity, and residual volume. Fourth, due to low R2, clinical significance is not guaranteed despite statistically significative results, as shown in other studies. A meta-analysis would allow to draw stronger conclusions. Nevertheless, this small fraction of influence on spirometry metrics is similar or even higher than that obtained in polygenic risk scores for asthma, where 95% of R2 values are below 6% [35] or in similar scores in psychiatry, where R2 reach 2% for major depressive disorders, 5% for bipolar disorders or 6% for attention deficit hyperactivity disorders [36,37,38,39]. Fifth, the accelerometer was positioned in the dominant (right) wrist, contrary to other studies that used the nondominant side. This was done to increase participation, as wearing the accelerometer on the left wrist would interfere with the wristwatch that most participants wore. A study [33] suggested that results obtained using the right wrist might be less reliable than using the left one, but the study was conducted during a very limited period of time (1 hour) and in a laboratory setting. Hence, the findings might not be applicable to free-living people. Nevertheless, it would be important that other studies be conducted using the accelerometer on the nondominant hand and with a sample size like ours.
Conclusion
The present study shows a weak correlation between vigorous PA and MVPA, with better LF, irrespective of smoking status, in an apparently healthy community-dwelling population. Lesser degrees of PA have no significant impact on LF.
Availability of data and materials
The data of CoLaus|PsyCoLaus and PneumoLaus studies used in this article cannot be fully shared as they contain potentially sensitive personal information on participants. According to the Ethics Committee for Research of the Canton of Vaud, sharing these data would be a violation of the Swiss legislation with respect to privacy protection. However, coded individual-level data that do not allow researchers to identify participants are available upon request to researchers who meet the criteria for data sharing of the CoLaus|PsyCoLaus Datacenter (CHUV, Lausanne, Switzerland). Any researcher affiliated to a public or private research institution who complies with the CoLaus|PsyCoLaus standards can submit a research application to or research.psycolaus@chuv.ch. Proposals requiring baseline data only, will be evaluated by the baseline (local) Scientific Committee (SC) of the CoLaus and PsyCoLaus studies. Proposals requiring follow-up data will be evaluated by the follow-up (multicentric) SC of the CoLaus|PsyCoLaus cohort study. Detailed instructions for gaining access to the CoLaus|PsyCoLaus data used in this study are available at https://www.colaus-psycolaus.ch/professionals/how-to-collaborate/.
Abbreviations
- PA:
-
Physical activity
- LF:
-
Lung functions
- FEV1 :
-
Forced expiratory volume in 1 second
- FVC:
-
Forced vital capacity
- MET:
-
Metabolic equivalent of task
- MMEF:
-
Maximum mid-expiratory flow
- MVPA:
-
Moderate and vigorous physical activity
- GLI:
-
Global lung initiative
- ROC:
-
Receiver operating characteristic
References
Garcia-Aymerich J, Lange P, Benet M, Schnohr P, Antó JM. Regular physical activity reduces hospital admission and mortality in chronic obstructive pulmonary disease: a population based cohort study. Thorax. 2006;61(9):772–8.
Pelkonen M, Notkola IL, Lakka T, Tukiainen HO, Kivinen P, Nissinen A. Delaying decline in pulmonary function with physical activity: a 25-year follow-up. Am J Respir Crit Care Med. 2003;168(4):494–9.
Garcia-Aymerich J, Lange P, Benet M, Schnohr P, Antó JM. Regular physical activity modifies smoking-related lung function decline and reduces risk of chronic obstructive pulmonary disease: a population-based cohort study. Am J Respir Crit Care Med. 2007;175(5):458–63.
Fuertes E, Carsin AE, Antó JM, Bono R, Corsico AG, Demoly P, et al. Leisure-time vigorous physical activity is associated with better lung function: the prospective ECRHS study. Thorax. 2018;73(4):376–84.
Smith MP, von Berg A, Berdel D, Bauer CP, Hoffmann B, Koletzko S, et al. Physical activity is not associated with spirometric indices in lung-healthy German youth. Eur Respir J. 2016;48(2):428–40.
Bédard A, Carsin AE, Fuertes E, Accordini S, Dharmage SC, Garcia-Larsen V, et al. Physical activity and lung function—cause or consequence? Szczesniak RD, editor. PLoS One. 2020;15(8):e0237769.
Svartengren M, Cai GH, Malinovschi A, Theorell-Haglöw J, Janson C, Elmståhl S, et al. The impact of body mass index, central obesity and physical activity on lung function: results of the EpiHealth study. ERJ Open Res. 2020;6(4):00214–2020.
Luzak A, Karrasch S, Wacker M, Thorand B, Nowak D, Peters A, et al. Association of generic health-related quality of life (EQ-5D dimensions) and inactivity with lung function in lung-healthy German adults: results from the KORA studies F4L and age. Qual Life Res. 2018;27(3):735–45.
Nystad W, Samuelsen SO, Nafstad P, Langhammer A. Association between level of physical activity and lung function among Norwegian men and women: the HUNT study. Int J Tuberc Lung Dis Off J Int Union Tuberc Lung Dis. 2006;10(12):1399–405.
Benadjaoud MA, Menai M, van Hees VT, Zipunnikov V, Regnaux JP, Kivimäki M, et al. The association between accelerometer-assessed physical activity and respiratory function in older adults differs between smokers and non-smokers. Sci Rep. 2019;9(1):10270.
Firmann M, Mayor V, Vidal PM, Bochud M, Pécoud A, Hayoz D, et al. The CoLaus study: a population-based study to investigate the epidemiology and genetic determinants of cardiovascular risk factors and metabolic syndrome. BMC Cardiovasc Disord. 2008;8(1):6.
Lenoir A, Fitting JW, Marques-Vidal PM, Vollenweider P, Nicod LP. GLI 2012 equations define few spirometric anomalies in the general population: the PneumoLaus study. Respir Res. 2018;19(1):250.
Miller MR, Hankinson J, Brusasco V, Burgos F, Casaburi R, Coates A, et al. Standardisation of spirometry. Eur Respir J. 2005;26(2):319–38.
Quanjer PH, Stanojevic S, Cole TJ, Baur X, Hall GL, Culver BH, et al. Multi-ethnic reference values for spirometry for the 3-95-yr age range: the global lung function 2012 equations. Eur Respir J. 2012;40(6):1324–43.
Stanojevic S, Kaminsky DA, Miller MR, Thompson B, Aliverti A, Barjaktarevic I, et al. ERS/ATS technical standard on interpretive strategies for routine lung function tests. Eur Respir J. 2022;60(1):2101499.
Hankinson JL, Eschenbacher B, Townsend M, Stocks J, Quanjer PH. Use of forced vital capacity and forced expiratory volume in 1 second quality criteria for determining a valid test. Eur Respir J. 2015;45(5):1283–92.
McDevitt B, Connolly J, Duddy D, Doherty R, Condell J. Preliminary investigations of the validity and Interinstrument reliability for classification of accelerometer physical activity cut-points against indirect Caliometry in healthy adults. In: 2022 33rd Irish signals and systems conference (ISSC) [internet]. Cork, Ireland: IEEE. p. 1–9. 2022 [cited 2023 Nov 15]. . Available from: https://ieeexplore.ieee.org/document/9826199/.
Gubelmann C, Vollenweider P, Marques-Vidal P. Of weekend warriors and couch potatoes: socio-economic determinants of physical activity in Swiss middle-aged adults. Prev Med. 2017;105:350–5.
Rovero M, Preisig M, Marques-Vidal P, Strippoli MPF, Vollenweider P, Vaucher J, et al. Subtypes of major depressive disorders and objectively measured physical activity and sedentary behaviors in the community. Compr Psychiatry. 2024;129:152442.
White T, Westgate K, Wareham NJ, Brage S. Estimation of physical activity energy expenditure during free-living from wrist Accelerometry in UK adults. PLoS One. 2016;11(12):e0167472.
https://www.bfs.admin.ch/bfs/fr/home/statistiques/catalogues-banques-donnees.assetdetail.223674.html. Date of last visit : 12.03.2024.
https://www.who.int/data/gho/indicator-metadata-registry/imr-details/358. Date of last visit : 12.03.2024.
Weir CB, Jan A. BMI Classification Percentile And Cut Off Points. In: StatPearls [internet]. Treasure Island (FL): StatPearls Publishing; 2023. [cited 2024 Jan 22]. Available from: http://www.ncbi.nlm.nih.gov/books/NBK541070/.
Hinkle D, Wiersma W, Jurs S. Applied statistics for the behavioral sciences, vol. 663. 5th ed. Houghton Mifflin; 2003.
Gutiérrez-Carrasquilla L, Sánchez E, Hernández M, Polanco D, Salas-Salvadó J, Betriu À, et al. Effects of Mediterranean diet and physical activity on pulmonary function: a cross-sectional analysis in the ILERVAS project. Nutrients. 2019;11(2):329.
Gleeson M, Bishop NC, Stensel DJ, Lindley MR, Mastana SS, Nimmo MA. The anti-inflammatory effects of exercise: mechanisms and implications for the prevention and treatment of disease. Nat Rev Immunol. 2011;11(9):607–15.
De Castro MF, Ducharme-Smith K, Mora-Garcia G, Alqahtani SA, Ruiz-Diaz MS, Moreira A, et al. Household food insecurity, lung function, and COPD in US adults. Nutrients. 2021;13(6):2098.
Xu Z, Xue Y, Wen H, Chen C. Association of oxidative balance score and lung health from the National Health and nutrition examination survey 2007–2012. Front Nutr. 2023;9(9):961950.
Shaheen SO, Jameson KA, Syddall HE, Aihie Sayer A, Dennison EM, Cooper C, et al. The relationship of dietary patterns with adult lung function and COPD. Eur Respir J. 2010;36(2):277–84.
Luzak A, Karrasch S, Thorand B, Nowak D, Holle R, Peters A, et al. Association of physical activity with lung function in lung-healthy German adults: results from the KORA FF4 study. BMC Pulm Med. 2017;17(1):215.
Schweitzer L, Geisler C, Johannsen M, Glüer CC, Müller MJ. Associations between body composition, physical capabilities and pulmonary function in healthy older adults. Eur J Clin Nutr. 2017;71(3):389–94.
Barboza ML, Barbosa ACB, Spina GD, Sperandio EF, Arantes RL, ARDT G, et al. Association between physical activity in daily life and pulmonary function in adult smokers. J Bras Pneumol Publicacao Of Soc Bras Pneumol E Tisilogia. 2016;42(2):130–5.
Esliger DW, Rowlands AV, Hurst TL, Catt M, Murray P, Eston RG. Validation of the GENEA accelerometer. Med Sci Sports Exerc. 2011;43(6):1085–93.
Rose GL, Skinner TL, Mielke GI, Schaumberg MA. The effect of exercise intensity on chronic inflammation: a systematic review and meta-analysis. J Sci Med Sport. 2021;24(4):345–51.
Richardson TG, Harrison S, Hemani G, Davey SG. An atlas of polygenic risk score associations to highlight putative causal relationships across the human phenome. eLife. 2019;5(8):e43657.
Demontis D, Walters RK, Martin J, Mattheisen M, Als TD, Agerbo E, et al. Discovery of the first genome-wide significant risk loci for attention deficit/hyperactivity disorder. Nat Genet. 2019;51(1):63–75.
Wray NR, Ripke S, Mattheisen M, Trzaskowski M, Byrne EM, Abdellaoui A, et al. Genome-wide association analyses identify 44 risk variants and refine the genetic architecture of major depression. Nat Genet. 2018;50(5):668–81.
Mullins N, Forstner AJ, O’Connell KS, Coombes B, Coleman JRI, Qiao Z, et al. Genome-wide association study of more than 40,000 bipolar disorder cases provides new insights into the underlying biology. Nat Genet. 2021;53(6):817–29.
Plomin R, Von Stumm S. Polygenic scores: prediction versus explanation. Mol Psychiatry. 2022;27(1):49–52.
Acknowledgements
The authors thank the participants of the study as well as Ms. Antigone Askitoglu, Division of Pulmonology, Department of Medicine, Lausanne University Hospital (CHUV) and University of Lausanne (UNIL), Lausanne, Switzerland, lung function technician, who performed all spirometries and oversaw the completion of all questionnaires and Ms. Céline Maglieri, Innovation and Clinical Research Directorate, Lausanne University Hospital (CHUV), for rereading all the results.
Funding
Open access funding provided by University of Lausanne The CoLaus|PsyCoLaus study was supported by unrestricted research grants from GlaxoSmithKline, the Faculty of Biology and Medicine of Lausanne, the Swiss National Science Foundation (grants 3200B0–105993, 3200B0–118308, 33CSCO-122661, 33CS30–139468, 33CS30–148401, 33CS30_177535 and 3247730_204523) and the Swiss Personalised Health Network (grant 2018DRI01).
Author information
Authors and Affiliations
Contributions
S.C: investigation, visualization, writing – original draft. B.T: resources, investigation, writing - review & editing. C.vG: resources, writing - review & editing. P.M-V: resources, investigation, data curation, formal analysis, writing - review & editing. V.K: conceptualization, supervision, writing - review & editing.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The institutional Ethics Committee of the University of Lausanne, which afterwards became the Ethics Commission of Canton Vaud (https://www.cer-vd.ch) approved the CoLaus|PsyCoLaus study (project number PB_2018–00038, reference 239/09). All participants provided their informed consent before entering the study.
Consent for publication
Non applicable (NA) as this manuscript does not contain information or images that could lead to identification of a study participant.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Collaud, S., Touilloux, B., von Garnier, C. et al. Physical activity and lung function association in a healthy community-dwelling European population. BMC Pulm Med 24, 169 (2024). https://doi.org/10.1186/s12890-024-02979-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12890-024-02979-x