Abstract
Background
Standard high-flow nasal cannula (HFNC) is a respiratory support device widely used to manage post-extubation hypoxemic acute respiratory failure (hARF) due to greater comfort, oxygenation, alveolar recruitment, humidification, and reduction of dead space, as compared to conventional oxygen therapy. On the contrary, the effects of the new asymmetrical HFNC interface (Optiflow® Duet system (Fisher & Paykel, Healthcare, Auckland, New Zealand) is still under discussion. Our aim is investigating whether the use of asymmetrical HFNC interface presents any relevant difference, compared with the standard configuration, on lung aeration (as assessed by end-expiratory lung impedance (EELI) measured by electrical impedance tomography (EIT)), diaphragm ultrasound thickening fraction (TFdi) and excursion (DE), ventilatory efficiency (estimated by corrected minute ventilation (MV)), gas exchange, dyspnea, and comfort.
Methods
Pilot physiological crossover randomized controlled study enrolling 20 adults admitted to the Intensive Care unit, invasively ventilated for at least 24 h, and developing post-extubation hARF, i.e., PaO2/set FiO2 < 300 mmHg during Venturi mask (VM) within 120 min after extubation. Each HFNC configuration was applied in a randomized 60 min sequence at a flow rate of 60 L/min.
Results
Global EELI, TFdi, DE, ventilatory efficiency, gas exchange and dyspnea were not significantly different, while comfort was greater during asymmetrical HFNC support, as compared to standard interface (10 [7–10] and 8 [7–9], p-value 0.044).
Conclusions
In post-extubation hARF, the use of the asymmetrical HFNC, as compared to standard HFNC interface, slightly improved patient comfort without affecting lung aeration, diaphragm activity, ventilatory efficiency, dyspnea and gas exchange.
Clinical trial number
ClinicalTrial.gov. Registration number: NCT05838326 (01/05/2023).
New & noteworthy
The asymmetrical high-flow nasal cannula oxygen therapy (Optiflow® Duet system (Fisher & Paykel, Healthcare, Auckland, New Zealand) provides greater comfort as compared to standard interface; while their performance in term of lung aeration, diaphragm activity, ventilatory efficiency, dyspnea, and gas exchange is similar.
Similar content being viewed by others
Explore related subjects
Find the latest articles, discoveries, and news in related topics.Introduction
In recent years, high-flow nasal cannula (HFNC) oxygen therapy has become popular among intensivists to manage patients with hypoxemic acute respiratory failure (hARF) [1]. According to the last European Respiratory Society task force, HFNC is a valuable intervention for improving lung aeration, oxygenation and alveolar recruitment in different populations, such as post-operative patients and nonsurgical subjects at risk of extubation failure or pulmonary complications [1,2,3].
HFNC delivers up to 60 L/min of warmed humidified gas, with an inspired fraction of oxygen (FiO2) ranging from 21 to 100% [4, 5]. HFNC promotes naso-pharyngeal dead space washout, leading to a decrease of minute ventilation and diaphragm activity, and may increase to some extent the end-expiratory lung volume (EELV) consequent to a variable rise of end-expiratory airway pressure [2, 5, 6]. Finally, by delivering warm and well humidified gas, HFNC may facilitate the clearance of tracheobronchial secretions. Overall, HFNC has the potential to improve oxygenation and patient comfort, while increasing EELV and reducing inspiratory effort [5, 7, 8].
Indeed, they have been tested as first-line treatment for avoiding intubation in patients experiencing hARF or exacerbations of chronic obstructive pulmonary disease (COPD), for preventing re-intubation, especially in nonsurgical patients at low or moderate risk of extubation failure or in post-operative patients at low or high risk of pulmonary complications, and for preoxygenation during endotracheal intubation [5, 7,8,9,10,11,12,13,14,15,16].
Recently, a new HFNC interface using asymmetrical prongs was approved for clinical practice [17, 18]. Unlike standard nasal cannulas with equally sized prongs, the asymmetrical prongs deliver different flow rates between the two nostrils [17, 18]. Bench studies have demonstrated that the asymmetrical configuration resulted in higher positive end-expiratory pressure (PEEP) and accelerated clearance of the anatomical dead space [2, 17]. As compared to the conventional HFNC interface, Slobod and colleagues recently found the asymmetrical interface to be associated only with reduced minute ventilation and work of breathing in 10 non-intubated patients with mild-to-moderate hARF, likely attributable to the enhanced carbon dioxide (CO2) clearance from the upper airway [18].
However, no study has, in so far, focused on patients developing hARF after extubation and on assessing the effect of the asymmetrical HFNC interface to prevent extubation failure.
We designed this pilot study for investigating whether in patients developing hARF early after extubation, the use of asymmetrical HFNC interface presents any relevant difference, compared with the standard configuration, on lung aeration - as assessed by end-expiratory lung impedance (EELI) measured by electrical impedance tomography (EIT) -, on diaphragm ultrasound thickening fraction (TFdi) and excursion (DE) -, ventilatory efficiency - estimated by corrected minute ventilation (MV) -, gas exchange, dyspnea, and comfort.
Materials and methods
This pilot physiological crossover randomized controlled study included all consecutive adult patients, admitted to the intensive care unit (ICU) of the University Hospital of Padua (Italy) between May 8th and June 10th 2023, undergoing invasive mechanical ventilation for at least 24 h and experiencing post-extubation hARF, defined as arterial partial pressure of oxygen (PaO2) to set inspiratory fraction of oxygen (FiO2) ratio < 300 mmHg during VenturiMask (VM) support [8], within 120 min after extubation. Exclusion criteria were: (i) long-term oxygen therapy, (ii) need for rescue noninvasive ventilation after extubation (based on predefined criteria [19]), (iii) chronic pulmonary disease, (iv) moderate-severe cardiac failure, (v) severe acute respiratory syndrome coronavirus-2 infection, (vi) pregnancy, (vii) presence of tracheostomy, (viii) contraindications to EIT [20] or HFNC interface [4, 21], and (ix) requiring nasogastric tubes for mandatory clinical reasons, i.e., delayed gastric emptying, upper abdominal surgery.
Attending ICU physicians identified patients as ready to undergo the first spontaneous breathing trial when they met the following predefined criteria in a daily screening, as previously described [22,23,24]: (1) PaO2/FiO2 ≥ 200 mmHg with PEEP ≤ 8 cmH2O and FiO2 ≤ 0.4; (2) respiratory rate (RR) ≤ 30/min (during pressure support ventilation); (3) a cooperative cognitive state (Richmond agitation-sedation scale between 0 and − 1); and (4) hemodynamic stability (heart rate < 140 beats min−1 and mean arterial pressure > 60 mmHg with norepinephrine < 0.1 mcg/kg/min or dobutamine < 5 mcg/kg/min and without epinephrine). After a 30-minute spontaneous breathing trial, the patient was extubated only in the absence of any of these criteria: (1) signs of acute respiratory distress (RR ≥ 35/min, agitation, recruitment of accessory muscles, and peripheral oxygen saturation < 90%); (2) life-threatening cardiac arrhythmias; (3) copious secretions [22,23,24].
The study was approved by the Institutional Ethics Committee of Padua University Hospital (reference number: AOP2949) and registered on ClinicalTrial.gov (registration number NCT05838326, 01/05/2023). The study was carried out according to the Declaration of Helsinki and written informed consent was obtained from all patients. The asymmetrical HFNC interfaces were kindly provided by Fisher & Paykel Healthcare (New Zealand) only for research purposes, without any economic interests. The industry was not involved in any phase of the study.
Randomization
Randomization was performed within 120 min after extubation, immediately after validation of the oxygenation criteria, defined as PaO2/set FiO2 < 300 mmHg during VM support. According to a web-based blocked random sequence, all patients received HFNC therapy through the asymmetrical interface Optiflow® Duet system (Fisher & Paykel, Healthcare, Auckland, New Zealand) and through the conventional interface.
Either with conventional HFNC or asymmetrical device, the set FiO2 was titrated to maintain a peripheral oxygen saturation between 92% and 98%, the gas flow rate was set at 50–60 L/min (AIRVO 2, Fisher&Paykel Healthcare, New Zealand), based on the size of the nostril, and the temperature of the heated humidifier (Fisher&Paykel Healthcare, New Zealand) was set at 37° C (absolute humidity delivered 44 mgH2O/L) for the entire study period. Each step was 60 min long and a 10-min ‘wash-out’ phase with VM support was required before each step. The standard and asymmetrical interfaces were identically sized, i.e., small, medium, or large, according to the distance between the patient’s nostrils, as recommended by the manufacturers [21].
Baseline demographic and clinical data were collected from electronic health records. During the last 10 min of each phase (i.e., MV, standard HFNC, and asymmetrical HFNC) the following variables were collected: respiratory rate, pH, arterial oxygen saturation (SaO2), PaO2/set FiO2, arterial pressure of carbon dioxide (PaCO2) and FiO2, systolic blood pressure, diastolic blood pressure, comfort, dyspnea, and EIT and ultrasound variables. Comfort and dyspnea were evaluated using a numerical scale (NRS) (ranging between 0 and 10) and the Borg scale, respectively [7, 25]. All patients were blinded to the novelty of the asymmetrical interface.
EIT
After meeting the inclusion criteria, a 16-electrode EIT belt was placed around the chest, as previously described [20, 26]. The following EIT parameters were recorded during the last 10 min of each step and before ultrasound assessment: (i) the average global tidal volume (VT) and the percentage of VT distributed to non-dependent and dependent lung regions (VTglob, VTnon-dep, and VTdep, respectively); (ii) the MV and the corrected MV, calculated as [(VTglob x PaCO2)/40 mmHg]* respiratory rate per minute− 1, where 40 mmHg is the ideal value of PaCO2 [27, 28]; (iii) the global and regional changes in EELI (estimating EELV) during the VM and in each HFNC phase (ΔEELIglob, ΔEELInon-dep, and ΔEELIdep, respectively); (iv) the global inhomogeneity index (GI) and the regional ventilation delay (RVD) [29, 30].
Diaphragm ultrasound
Diaphragm ultrasound evaluation was performed at the bedside, during quiet breathing, with the patient in a semi-recumbent position, by two trained intensivists (AB and TP) [19], using a 4–12 MHz linear array transducer (Mindray M9, North America, NJ, USA), placed perpendicular to the right chest wall between the 9th and 10th intercostal spaces (at the level of apposition) after the EIT evaluation, as previously published [19, 31].
The diaphragm thickness was measured at both end-expiration and inspiration, and TFdi was calculated as the average of three respiratory cycles, according to the formula: TFdi (%) = (inspiratory thickness-expiratory thickness)/expiratory thickness*100 [32]. Diaphragm ultrasound assessment was performed only on the right side due to the lower interobserver reproducibility on the left side [19, 33, 34]. The intra- and inter-observer agreement between the two observers was previously published [19].
DE was measured with a low frequency curved array probe (2–5 MHz) positioned just below the costal arch at the midclavicular line and by angling the ultrasound beam as much as possible cranially and perpendicular to the diaphragmatic dome. The diaphragm is identified as a bright line that covers the liver and the spleen. During inspiration, the diaphragm should move toward the probe [32, 35]. Excursion is quantified in M-mode, with the M-line placed perpendicularly to the direction of motion, and as the mean of three quiet breathings [32, 36].
Statistical analysis
Continuous data are presented as median and interquartile range [IQR]. Being a pilot study, a sample size was not calculated a priori. Comparisons between different interfaces were analyzed using the Wilcoxon signed rank test for paired data and all p-values were adjusted by Benjamini and Hochberg method. Missing data was omitted from the analysis. Subset analyses were performed according to the improvement on lung aeration during the asymmetrical support. All statistical tests were two-tailed and statistical significance was defined by p < 0.05. Analyses were conducted using Prism (version 5.0; GraphPad Software, Inc, La Jolla, CA, USA) and R (version 4.0.3, R Foundation for Statistical Computing, Vienna, Austria).
Results
As shown in Fig. 1, we evaluated for enrollment 35 patients and excluded 14 patients not meeting inclusion criteria. One patient was dropped out because of inadequate EIT recordings, leaving 20 patients eligible for analysis.
CONSORT flow diagram for crossover trials. *Patients not meeting inclusion criteria: rescue noninvasive ventilation after extubation N = 5, chronic pulmonary disease N = 2, tracheostomy N = 2, mandatory nasogastric tube N = 5. Abbreviations: HFNC high flow nasal cannula, N number, EIT electrical impedance tomography
Patients’ characteristics are reported in Table 1. Median age was 65 [55–76] years and seven (35%) were female. The most frequent etiology for admission to the ICU was elective abdominal surgery (35%), followed by septic shock (15%) and trauma (15%) (Table 1). Extubation occurred after the first spontaneous breathing trial in 17 out of 20 (85%) patients, and after the second attempt in 3 (15%) patients. During VM support, PaO2/FiO2 was 195 [177–259] mmHg. The total duration of invasive mechanical ventilation was 30 [27–84] hours, and ICU stay was 2 [2–5] days. One patient (5%) died during the ICU stay (Table 1).
Lung aeration and diaphragm activity
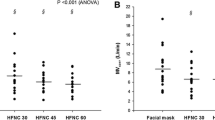
As shown in Fig. 2 and S1, no differences were found in the percent change of global EELI (p = 0.159) and its distribution in dependent (p = 0.364) and not-dependent (p = 0.836) lung regions when passing from VM and asymmetrical or standard HFNC. Also, MV and corrected MV were similar between asymmetrical and standard HFNC, as well as global VT, GI and RVD (Table 2). Furthermore, TFdi (p = 0.910) and DE (p = 0.891) were not different in the two HFNC phases (Fig. 3 and S2).
Electrical impedance tomography during standard and asymmetrical HFNC oxygen therapy. Variables are expressed as median, with an interquartile range [IQR]. Additional data are reported in Fig. S1. A: global lung aeration; B: lung aeration in dependent area; C: lung aeration in non-dependent area. Abbreviations: ns not significant, HFNC high-flow nasal cannula, dep dependent, non-dep non-dependent, EELI end-expiratory lung impedance (measured as percent change from VM)
Diaphragm ultrasound evaluation during standard and asymmetrical HFNC oxygen therapy. Variables are expressed as median, with an interquartile range [IQR]. Additional data are reported in Fig. S2. A: TFdi; B: diaphragm excursion. Abbreviations: HFNC high-flow nasal cannula, TFdi diaphragm thickening fraction
Gas exchange, dyspnea, and comfort
As shown in Table 3, respiratory rate, SaO2, PaO2/set FiO2, gas exchange and hemodynamic parameters were not different between asymmetrical and standard HFNC. Comfort, but not dyspnea, was higher during asymmetrical HFNC, compared to the conventional interface (p = 0.044 and p = 0.763, respectively) (Table 3).
Asymmetrical HFNC vs. venturi mask
Asymmetrical HFNC oxygen therapy decreased corrected MV and respiratory rate, as compared to VM (p = 0.050 and p = 0.007, respectively); while SaO2 and PaO2/set FiO2 were higher during asymmetrical HFNC, as compared to VM (p = 0.006 and p = 0.001, respectively) (Table 2). Finally, both comfort and dyspnea were not different as compared to VM (p = 0.104) (Table 3). Additional data on asymmetrical HFNC oxygen therapy, as compared to VM, are reported in the Supplementary materials (Table S3).
Subset analysis
Additional analyses were performed considering only patients (14, 70%) improving lung aeration using standard HFNCs, as shown in the Supplementary materials (Tables S4 and S5). Once again, the asymmetrical interface, as compared to the standard configuration, shows higher patient comfort (p = 0.016).
Discussion
In this pilot physiological study, randomizing 20 ICU patients with post-extubation hARF, the use of the asymmetrical HFNC, despite showing similar performances in terms of lung aeration, TFdi, DE, ventilatory efficiency, and gas exchange, was associated with improved patient comfort, compared to standard HFNC interface.
While conventional HFNC has been shown to generate a ‘PEEP effect’, promoting alveolar recruitment and improving oxygenation, with a flow-dependent increase in global EELI, a valid surrogate of alveolar recruitment, clinical evidences on the potential benefits of the use of asymmetrical prongs on lung aeration are still lacking [2, 5,6,7,8, 18].
Recent bench studies, collecting data from anatomically ‘correct’ three-dimensional upper airway models, showed that an increase in asymmetrical nare occlusion led to a significant improvement of the ‘PEEP effect’ [17, 37, 38]. On the contrary, in 10 ICU patients affected by mild-to-moderate hARF, Slobod et al. showed that the asymmetrical HFNC interface did not affect alveolar recruitment, dorsal fraction of ventilation and end-expiratory lung impedance, thus suggesting no major effect on alveolar aeration [18]. Our results are in keeping with those findings despite uneven populations. Indeed, in our cohort of adults experiencing post-extubation hARF, EELI was similar between asymmetrical and standard configuration, without any difference between ventral and dorsal aeration. However, our results on lung aeration may be limited because, first, we did not measure EELV directly with computed tomography [39], but only through a derived EIT parameter (i.e., EELI) and, second, because we cannot exclude that some patients breathe with their mouths open, which may decrease the ‘PEEP effect’ associated with HFNC oxygen therapy [11, 40]. However, all above mentioned bench studies, describing an increased ‘PEEP effect’ during the asymmetrical HFNC, suffer an important limitation worthy of discussion, such as collecting data from ‘normal’ upper airway models, and not accounting for anatomical abnormalities that may affect nasal flow distribution and ‘PEEP effect’ [17, 37, 38].
Likewise, data on the role of asymmetrical nostrils in reducing the patients’ work of breathing are still conflicting. Interestingly, Slobod et al. found that the inspiratory esophageal pressure-time product was slightly reduced with the asymmetrical HFNC, in comparison with the standard interface [18]. Since we cannot exclude that the presence of the esophageal catheter, useful for measuring the esophageal pressure-time product or the diaphragm electrical activity, may affect either the dead space clearance or the ‘PEEP effect’, we decided to remove any nasogastric tube before protocol initiation [37, 41]. As an alternative, we decided to explore the patient inspiratory effort by ultrasound assessment, a less invasive technique, with easy learning and high reproducibility [11, 40, 42]. Based on our findings, both TFdi and DE were similar between different interfaces.
Furthermore, standard HFNC support has been shown to reduce dead space and to improve CO2-washout in mixed populations (i.e., hypoxemic ICU patients, hypercapnic COPD subjects ect) [1]. In keeping with those previous findings, Tatkov et al. showed an increased CO2-washout, and Slobod et al. observed an increased ventilatory efficiency during asymmetrical HFNC support, as compared to the standard interface [17, 18]. According to our findings, the asymmetrical HFNC performed similarly to the standard interface, probably due to an important heterogeneity in patients’ baseline characteristics that may affect the comparison with the above-mentioned study [18]. Indeed, in our cohort only 4 out of 20 (20%) patients were intubated for ‘primary’ ARF or pneumonia (i.e., patient n. 1, 3, 4, 17), while Slobod et al. enrolled 6 out of 10 (60%) patients with ‘primary’ ARF [18].
Finally, despite the absence of relevant differences between the standard and asymmetrical interface, our results suggest greater comfort during the asymmetrical HFNC interface, favoring their application routinely. The reasons why our patients reported greater comfort during the asymmetrical interface are not entirely clear. However, our results seem to be promising for realizing further studies investigating the impact of asymmetrical cannulas on lung aeration and diaphragm activity in different clinical settings, with different patient selection.
Our study has some limitations. First, during our trial, it was not possible to control the potential impact of spontaneous patient movements on EIT recordings [26], although we marked the initial EIT belt position, as previously described [26]. Second, we cannot exclude that the absence of a ‘PEEP effect’ may be due to the potential impact of mouth breathing during HFNC, as previously described [11, 40]. Third, we enrolled patients with a median invasive mechanical ventilation of 30 h. So doing, the effect on alveolar recruitment and CO2 clearance in case of longer endotracheal intubation remains unclear and further studies are required to clarify this issue. Finally, due to the explorative nature of our investigation we cannot exclude that our study could be underpowered to assess any difference between the asymmetrical and standard interface. In fact, our sample size could not be enough to measure a possible effect on lung aeration and diaphragm activity. In addition, further studies are necessary to explore the impact of asymmetrical HFNCs in different clinical settings, with different patient selection.
In conclusion, in acute post-extubation hARF, the use of the asymmetrical HFNC, as compared to standard HFNC, improved patient comfort slightly, despite similar performances in terms of lung aeration, TFdi, DE, ventilatory efficiency, dyspnea and gas exchange.
Data availability
The datasets used and/or analysed during the current study are available for the corresponding author on reasonable request.
Abbreviations
- BMI:
-
body mass index
- CO2 :
-
carbon dioxide
- COPD:
-
chronic obstructive pulmonary disease
- DBP:
-
diastolic blood pressure
- DE:
-
diaphragm excursion
- dep:
-
dependent
- EELI:
-
end-expiratory lung impedance
- EIT:
-
electrical impedance tomography
- EELV:
-
end-expiratory lung volume
- GI:
-
global inhomogeneity index
- HFNC:
-
high-flow nasal cannula
- hARF:
-
hypoxemic acute respiratory failure
- ICU:
-
intensive care unit
- IMV:
-
invasive mechanical ventilation
- IQR:
-
interquartile range
- LOS:
-
length of stay
- MV:
-
minute ventilation
- N:
-
number
- non-dep:
-
non-dependent
- NRS:
-
numerical scale
- PaO2 :
-
arterial partial pressure of oxygen
- PaCO2 :
-
arterial pressure of carbon dioxide
- PEEP:
-
positive end-expiratory pressure
- RVD:
-
regional ventilation delay
- RR:
-
respiratory rate
- SAPS II:
-
simplified acute physiology score II PaO2
- SaO2 :
-
arterial oxygen saturation
- SBP:
-
systolic blood pressure
- Tot:
-
total
- VT :
-
tidal volume
- TFdi:
-
diaphragm ultrasound thickening fraction
- VM:
-
Venturi mask
References
Oczkowski S, Ergan B, Bos L, Chatwin M, Ferrer M, Gregoretti C, et al. ERS clinical practice guidelines: high-flow nasal cannula in acute Respiratory Failure. Eur Respir J. 2022;59(4):2101574.
Pinkham M, Tatkov S. Effect of flow and cannula size on generated pressure during nasal high flow. Crit Care Lond Engl. 2020;24(1):248.
Goligher EC, Dres M, Patel BK, Sahetya SK, Beitler JR, Telias I, et al. Lung- and diaphragm-protective ventilation. Am J Respir Crit Care Med. 2020;202(7):950–61.
Roca O, Hernández G, Díaz-Lobato S, Carratalá JM, Gutiérrez RM, Masclans JR, et al. Current evidence for the effectiveness of heated and humidified high flow nasal cannula supportive therapy in adult patients with Respiratory Failure. Crit Care Lond Engl. 2016;20(1):109.
Mauri T, Alban L, Turrini C, Cambiaghi B, Carlesso E, Taccone P, et al. Optimum support by high-flow nasal cannula in acute hypoxemic Respiratory Failure: effects of increasing flow rates. Intensive Care Med. 2017;43(10):1453–63.
Lee JH, Rehder KJ, Williford L, Cheifetz IM, Turner DA. Use of high flow nasal cannula in critically ill infants, children, and adults: a critical review of the literature. Intensive Care Med. 2013;39(2):247–57.
Maggiore SM, Idone FA, Vaschetto R, Festa R, Cataldo A, Antonicelli F, et al. Nasal high-flow versus Venturi mask oxygen therapy after extubation. Effects on oxygenation, comfort, and clinical outcome. Am J Respir Crit Care Med. 2014;190(3):282–8.
Maggiore SM, Jaber S, Grieco DL, Mancebo J, Zakynthinos S, Demoule A, et al. High-Flow Versus VenturiMask Oxygen Therapy to Prevent Re-intubation in Hypoxemic patients after Extubation: a Multicenter, Randomized Clinical Trial. Am J Respir Crit Care Med. 2022;206(12):1452–62.
Arman PD, Varn MN, Povian S, Davis A, Uchakin P, Bhar A, WEANING, AND EXTUBATION [Internet]. Effects of Direct Extubation to High-Flow Nasal Cannula Compared to Standard Nasal Cannula in Patients in the Intensive Care Unit. In: A53 CRITICAL CARE: PROBLEMS RELATED TO INTUBATION,. American Thoracic Society; 2017 [cited 2022 Aug 26]. p. A1887–A1887. (American Thoracic Society International Conference Abstracts). Available from: https://www.atsjournals.org/doi/abs/https://doi.org/10.1164/ajrccm-conference.2017.195.1_MeetingAbstracts.A1887.
Cho JY, Kim HS, Kang H, Kim SH, Choe KH, Lee KM, et al. Comparison of Postextubation Outcomes Associated with High-Flow Nasal Cannula vs. Conventional Oxygen Therapy in patients at high risk of Reintubation: a Randomized Clinical Trial. J Korean Med Sci. 2020;35(25):e194.
Longhini F, Pisani L, Lungu R, Comellini V, Bruni A, Garofalo E, et al. High-Flow Oxygen Therapy after Noninvasive Ventilation interruption in patients recovering from Hypercapnic Acute Respiratory Failure: a physiological crossover trial. Crit Care Med. 2019;47(6):e506–11.
Ferrer M, Sellarés J, Valencia M, Carrillo A, Gonzalez G, Badia JR, et al. Non-invasive ventilation after extubation in hypercapnic patients with chronic respiratory disorders: randomised controlled trial. Lancet Lond Engl. 2009;374(9695):1082–8.
Fernandez R, Subira C, Frutos-Vivar F, Rialp G, Laborda C, Masclans JR, et al. High-flow nasal cannula to prevent postextubation Respiratory Failure in high-risk non-hypercapnic patients: a randomized multicenter trial. Ann Intensive Care. 2017;7(1):47.
Ranieri VM, Tonetti T, Navalesi P, Nava S, Antonelli M, Pesenti A, et al. High-Flow nasal oxygen for severe hypoxemia: oxygenation response and outcome in patients with COVID-19. Am J Respir Crit Care Med. 2022;205(4):431–9.
Fernando SM, Tran A, Sadeghirad B, Burns KEA, Fan E, Brodie D, et al. Noninvasive respiratory support following extubation in critically ill adults: a systematic review and network meta-analysis. Intensive Care Med. 2022;48(2):137–47.
Thille AW, Monseau G, Coudroy R, Nay MA, Gacouin A, Decavèle M, et al. Non-invasive ventilation versus high-flow nasal oxygen for postextubation Respiratory Failure in ICU: a post-hoc analysis of a randomized clinical trial. Crit Care Lond Engl. 2021;25(1):221.
Taktov S, Rees M, Gulley A, van den Lgt H, Nilius GN. Asymmetrical nasal high flow ventilation improves clearance of CO2 from the anatomical dead space and increases positive airway pressure. J Appl Physiol (1985). 2023;134(2):365–77.
Slobod D, Spinelli E, Crotti S, Lissoni A, Galazzi A, Grasselli G, et al. Effects of an asymmetrical high flow nasal cannula interface in hypoxemic patients. Crit Care Lond Engl. 2023;27(1):145.
Boscolo A, Sella N, Pettenuzzo T, Pistollato E, Calabrese F, Gregori D, et al. Diaphragm dysfunction predicts weaning outcome after bilateral lung transplant. Anesthesiology. 2023. https://doi.org/10.1097/ALN.000000000004729.
Sella N, Pettenuzzo T, Zarantonello F, Andreatta G, De Cassai A, Schiavolin C, et al. Electrical impedance tomography: a compass for the safe route to optimal PEEP. Respir Med. 2021;187:106555.
Park S. High-flow nasal cannula for Respiratory Failure in adult patients. Acute Crit Care. 2021;36(4):275–85.
Boles JM, Bion J, Connors A, Herridge M, Marsh B, Melot C, et al. Weaning from mechanical ventilation. Eur Respir J. 2007;29(5):1033–56.
Dres M, Dubé BP, Mayaux J, Delemazure J, Reuter D, Brochard L, et al. Coexistence and impact of Limb muscle and diaphragm weakness at Time of Liberation from Mechanical Ventilation in Medical Intensive Care Unit patients. Am J Respir Crit Care Med. 2017;195(1):57–66.
Dubé BP, Dres M, Mayaux J, Demiri S, Similowski T, Demoule A. Ultrasound evaluation of diaphragm function in mechanically ventilated patients: comparison to phrenic stimulation and prognostic implications. Thorax. 2017;72(9):811–8.
Vitacca M, Bianchi L, Zanotti E, Vianello A, Barbano L, Porta R, et al. Assessment of physiologic variables and subjective comfort under different levels of pressure support ventilation. Chest. 2004;126(3):851–9.
Zarantonello F, Sella N, Pettenuzzo T, Andreatta G, Calore A, Dotto D, et al. Early physiologic effects of Prone Positioning in COVID-19 Acute Respiratory Distress Syndrome. Anesthesiology. 2022;137(3):327–39.
Fusina F, Albani F, Bertelli M, Cavallo E, Crisci S, Caserta R, et al. Corrected Minute Ventilation is Associated with Mortality in ARDS caused by COVID-19. Respir Care. 2021;66(4):619–25.
Zheng M. Dead space ventilation-related indices: bedside tools to evaluate the ventilation and perfusion relationship in patients with acute respiratory distress syndrome. Crit Care Lond Engl. 2023;27(1):46.
Zhao Z, Möller K, Steinmann D, Frerichs I, Guttmann J. Evaluation of an electrical impedance tomography-based Global Inhomogeneity Index for pulmonary ventilation distribution. Intensive Care Med. 2009;35(11):1900–6.
Hsu YL, Tien AJ, Chang MY, Chang HT, Möller K, Frerichs I, et al. Regional ventilation redistribution measured by electrical impedance tomography during spontaneous breathing trial with automatic tube compensation. Physiol Meas. 2017;38(6):1193–203.
Bernardinello N, Cocconcelli E, Boscolo A, Castelli G, Sella N, Giraudo C, et al. Prevalence of diaphragm dysfunction in patients with interstitial lung Disease (ILD): the role of diaphragmatic ultrasound. Respir Med. 2023;216:107293.
Haaksma ME, Smit JM, Boussuges A, Demoule A, Dres M, Ferrari G, et al. EXpert consensus on Diaphragm UltraSonography in the critically ill (EXODUS): a Delphi consensus statement on the measurement of diaphragm ultrasound-derived parameters in a critical care setting. Crit Care Lond Engl. 2022;26(1):99.
Cammarota G, Rossi E, Vitali L, Simonte R, Sannipoli T, Anniciello F, et al. Effect of awake prone position on diaphragmatic thickening fraction in patients assisted by noninvasive ventilation for hypoxemic acute Respiratory Failure related to novel coronavirus Disease. Crit Care Lond Engl. 2021;25(1):305.
Cammarota G, Simonte R, Longhini F, Spadaro S, Vetrugno L, De Robertis E. Advanced Point-of-care Bedside Monitoring for Acute Respiratory Failure. Anesthesiology. 2023;138(3):317–34.
Tuinman PR, Jonkman AH, Dres M, Shi ZH, Goligher EC, Goffi A, et al. Respiratory muscle ultrasonography: methodology, basic and advanced principles and clinical applications in ICU and ED patients-a narrative review. Intensive Care Med. 2020;46(4):594–605.
Santana PV, Cardenas LZ, de Albuquerque ALP, de Carvalho CRR, Caruso P. Diaphragmatic ultrasound: a review of its methodological aspects and clinical uses. J Bras Pneumol Publicacao of Soc Bras Pneumol E Tisilogia. 2020;46(6):e20200064.
Brüning J, Hildebrandt T, Heppt W, Schmidt N, Lamecker H, Szengel A, et al. Characterization of the airflow within an average geometry of the Healthy Human Nasal Cavity. Sci Rep. 2020;10(1):3755.
Narang I, Carberry JC, Butler JE, Gandevia SC, Chiang AKI, Eckert DJ. Physiological responses and perceived comfort to high-flow nasal cannula therapy in awake adults: effects of flow magnitude and temperature. J Appl Physiol. 2021;131(6):1772–82.
Gattinoni L, Caironi P, Valenza F, Carlesso E. The role of CT-scan studies for the diagnosis and therapy of acute respiratory distress syndrome. Clin Chest Med. 2006;27(4):559–70.
Garofalo E, Bruni A, Pelaia C, Cammarota G, Murabito P, Biamonte E, et al. Evaluation of a New Interface Combining High-Flow Nasal Cannula and CPAP. Respir Care. 2019;64(10):1231–9.
Parke RL, McGuinness SP. Pressures delivered by nasal high flow oxygen during all phases of the respiratory cycle. Respir Care. 2013;58(10):1621–4.
Cammarota G, Sguazzotti I, Zanoni M, Messina A, Colombo D, Vignazia GL, et al. Diaphragmatic Ultrasound Assessment in subjects with Acute Hypercapnic Respiratory Failure admitted to the Emergency Department. Respir Care. 2019;64(12):1469–77.
Acknowledgements
We are indebted to all ICU colleagues who made this work possible (Eugenio Serra MD, Arianna Peralta MD, Luisa Muraro MD, Paolo Persona MD, Enrico Petranzan MD). Furthermore, the authors thank Kirstin Elisabeth Rose, MD, for proofreading the article and editing English.
Funding
No funding was received for the present study. The asymmetrical HFNC interfaces were kindly provided by Fisher & Paykel Healthcare (New Zealand) only for research purposes, without any economic interests. The industry was not involved in any phase of the study.
Author information
Authors and Affiliations
Contributions
AB, TP, FZ, NS and PN substantially contributed to the study design, data interpretation and the writing of the manuscript. GL, EP, ADC, SC, FT, IP, GB, SC and GM contributed to data collection, interpretation, and the writing of the manuscript. GL, ADC and DG conceived, performed and guaranteed the accuracy of data analysis. AB, ZF, NS, DG and PT critically revised the manuscript for important intellectual content. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study was approved by the Institutional Ethics Committee of Padua University Hospital (reference number: AOP2949) and written informed consent was obtained from all patients.
Consent for publication
Not applicable.
Competing interests
PN research lab received grants/research equipment by Draeger, Mindray, Intersurgical SPA and Gilead. PN receives royalties from Intersurgical SPA for Helmet Next invention. He also received speaking fees from Getinge, Intersurgical SPA, Gilead, MSD, Draeger. NS and FZ received speaking fees from Getinge. The other authors have no other competing interests to declare.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Boscolo, A., Pettenuzzo, T., Zarantonello, F. et al. Asymmetrical high-flow nasal cannula performs similarly to standard interface in patients with acute hypoxemic post-extubation respiratory failure: a pilot study. BMC Pulm Med 24, 21 (2024). https://doi.org/10.1186/s12890-023-02820-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12890-023-02820-x