Abstract
Background
We analyzed the clinical characteristics of children with plastic bronchitis (PB) caused by Mycoplasma pneumoniae (MP) and explored its risk factors.
Methods
We prospectively analyzed clinical data of children with MP pneumonia (MPP) treated with fiberoptic bronchoscopy (FB). Patients were classified into a PB and non-PB group. General information, clinical manifestations, laboratory tests, results of computed tomography scan, and FB findings were compared between groups. We conducted statistical analysis of risk factors for developing PB.
Results
Of 1169 children who had MPP and were treated with FB, 133 and 1036 were in the PB and non-PB groups, respectively. There were no significant differences in sex, age, and incident season between groups (P > 0.05). The number of children in the PB group decreased during the COVID-19 pandemic. Compared with children in the non-PB group, those in the PB group had longer duration of hospitalization, increased levels of neutrophil (N), C-reactive protein (CRP), procalcitonin (PCT), D-dimer, lactate dehydrogenase (LDH), alanine transaminase (ALT) and aspartate transaminase (AST); lower levels of lymphocyte (L) and platelet (PLT); and higher incidence of lack of appetite, decreased breath sounds, single lobar infiltrate, pleural effusion, pericardial effusion, mucosal erosion and/or necrosis, and bronchial embolization. L levels and pleural effusion were identified as risk factors in multivariate logistic regression.
Conclusions
Children with PB caused by MPP had a strong and local inflammatory response. L levels and pleural effusion were independent risk factors of PB with MPP in children. Our findings will help clinicians identify potential PB in pediatric patients for early and effective intervention.
Similar content being viewed by others
Background
Mycoplasma pneumoniae (MP) is a common lower respiratory tract pathogen that causes MP pneumonia (MPP) in children. MPP is a major cause of community-acquired pneumonia (CAP), accounting for 10−40% of hospitalizations among children [1,2,3,4,5,6,7,8,9,10,11]. MPP is considered self-limiting and benign, but in recent years, several studies have shown that MPP can cause plastic bronchitis (PB) in children [12,13,14,15,16]. PB is an acute and critical pulmonary disease characterized by the formation of bronchial casts (BCs), which can partially or completely obstruct the tracheobronchial tree [17,18,19]. PB caused by infection in pediatric patients usually presents with productive cough, progressive dyspnea, repeated high fever, or pleuritic chest pain [17, 18]. Fiberoptic bronchoscopy (FB) and bronchoalveolar lavage (BAL) have high efficacy in the diagnosis and treatment of PB [12, 19]. In this prospective study, we analyzed the data of 1169 children with MPP who underwent FB. We aimed to explore the clinical features and risk factors of PB in children with MPP.
Methods
Study population
This study was approved by the Ethics Committee of the Affiliated Hospital of Jining Medical University (No. 2018C076). Children with MPP who were admitted to the department of pediatrics in the Affiliated Hospital of Jining Medical University from February 2019 to January 2020 and from August 2021 to July 2022 and were treated with FB were selected as study participants. The patients were divided into a PB group and a non-PB group, according to whether there was a plastic shape under FB. Inclusion criteria were: (1) hospitalized patients between 1 month and 14 years old; (2) symptoms and signs indicative of CAP, including fever, cough, abnormal lung auscultation, and new infiltration on chest radiograph; (3) positive laboratory results for MP, including an MP immunoglobulin M titer ≥ 1:160 or four-fold rising titer in acute and convalescent serum specimens; positive results for MP polymerase chain reaction tests in BAL fluid [20, 21]; (4) the patient’s condition met the diagnostic criteria of PB: plastic foreign body removed on FB; (5) informed consent was signed for FB and BAL; and (6) complete hospitalization data were available. The exclusion criteria were as follows: (1) previous recurrent respiratory tract infection, asthma, chronic lung disease, operation after cardiac disease, severe blood system disease, or immune deficiency disease; (2) foreign body inhalation; (3) patients currently recovering from MPP; (4) patients co-infected with other pathogens or tuberculosis; and (5) incomplete hospitalization data. Flowchart of the study population see Fig. 1.
Data collection
The clinical data of children with MPP were collected and mainly included the following: (1) general information: sex, age, etiological diagnostic methods and admission time (incident season); (2) clinical manifestations: time from illness onset to admission, duration of hospitalization, duration of fever and cough (time from the onset of fever and cough to the date of hospitalization), fatigue, dyspnea, wheezing, and other symptoms; decreased breath sounds, rales, rhonchi, hypoxia, and other signs; (3) laboratory tests: routine blood tests, inflammatory markers, and blood biochemistry; (4) computed tomography (CT) scan results; and (5) FB findings and histopathological examination.
Statistical analysis
All statistical analyses were performed using SAS software, version 9.4 (SAS Institute Inc., Cary, NC, USA). Enumeration data were expressed as percentage (%), measurement data with a normal distribution were described as mean ± standard deviation, and measurement data with a non-normal distribution were described as median (interquartile range). Fisher’s exact test was used for categorical data. The Kruskal–Wallis H test was used for continuous data. Logistic regression analysis was used to examine risk factors that were significant in the multivariate analysis. P < 0.05 was considered statistically significant.
Results
General information
A total of 1169 MPP children were eligible for inclusion. The PB group accounted for 133 (11.38%) cases and the non-PB group for 1036 (88.62%) cases. Male children comprised 53.38% (71 of 133) of the PB group and 54.15% (561 of 1036) of the non-PB group. The mean age of patients in the PB and non-PB group was 80.40 ± 29.85 months and 74.08 ± 30.68 months, respectively. Three methods were used to detect the pathogen, including single elevated IgM, seroconversion and PCR in BAL. The PCR in BAL was used to confirm MP infection. By using single elevated IgM as a diagnostic method, 119 children from the PB group (accounting for 93.23%, 119 of 133), and 862 children from the non-PB group (accounting for 83.20%, 862 of 1036) were found to have MP infection. The diagnostic method of seroconversion revealed that 120 children from the PB group (accounting for 90.23%, 120 of 133), and 859 children from the non-PB group (accounting for 82.92%, 859 of 1036) were infected with MP. The proportion of children hospitalized in autumn was significantly higher than that in other seasons. There were no significant differences in sex, age, etiological diagnostic methods and incident season between the two groups (P > 0.05). Compared with the period prior to the COVID-19 pandemic, the proportion of children in the PB group decreased (81 [60.90%] vs. 52 [39.10%]) whereas the proportion of children in the non-PB group increased (428 [41.31%] vs. 608 [58.69%]) during the pandemic (P < 0.0001) (Table 1).
Clinical manifestations
The median [IQR] duration of hospitalization was longer in the PB group than that in the non-PB group (9.00 [8.00, 10.00] days vs. 7.00 [6.00, 8.00] days; P < 0.0001). No differences were observed between groups for the time from illness onset to admission, duration of fever, and duration of cough. The incidence of lack of appetite was higher in the PB group than that in the non-PB group (98 [73.68%] vs. 616 [59.46%]; P = 0.0013). Compared with the non-PB group, patients in the PB group were more likely to have decreased breath sounds (93 [69.92%] vs. 607 [58.59%]; P = 0.0088). No remarkable differences in fatigue, dyspnea, wheezing, abdominal pain, diarrhea, chest pain, rales, rhonchi, tachypnea, hypoxia, and three depressions sign were observed between the two groups (Table 2).
Laboratory tests
There were significant differences in median [IQR] levels for neutrophil (N), lymphocyte (L), platelet (PLT), C-reactive protein (CRP), procalcitonin (PCT), D-dimer, lactate dehydrogenase (LDH), alanine transaminase (ALT), and aspartate transaminase (AST) between the PB and non-PB groups (P < 0.05). The levels of N (5.12 [4.01, 7.00] vs. 4.59 [3.36, 6.21] ×109/L; P = 0.0027), CRP (26.74 [13.29, 52.31] vs. 10.93 [3.97, 22.73] mg/dL; P < 0.0001), PCT (0.25 [0.13, 0.44] vs. 0.11 [0.07, 0.22] µg/L; P < 0.0001), D-dimer (1.68 [1.04, 2.68] vs. 0.58 [0.33, 1.09] mg/L; P < 0.0001), LDH (453.00 [349.00, 567.00] vs. 315.00 [267.00, 388.00] U/L; P < 0.0001), ALT (21.45 [14.80, 37.40] vs. 12.60 [9.80, 19.00] U/L; P < 0.0001), and AST (33.00 [26.00, 50.00] vs. 24.00 [20.00, 30.00] U/L; P < 0.0001) were significantly higher in the PB group than those in the non-PB group. Levels of L (1.70 [1.21, 2.40] vs. 2.47 [1.79, 3.37]×109/L; P < 0.0001) and PLT (288.50 [232.00, 336.00] vs. 316.00 [256.00, 393.00]×109/L; P < 0.0001) were significantly lower in the PB group than those in the non-PB group. There was no significant difference in the white blood cell count and erythrocyte sedimentation rate between groups (Table 2).
CT scan results
The incidences of single lobar infiltrate (66 [49.62%] vs. 397 [38.32%]; P = 0.0187), pleural effusion (51 [38.35%] vs. 159 [15.35%]; P < 0.0001), and pericardial effusion (7 [5.26%] vs. 22 [2.12%]; P = 0.0391) were higher in the PB group than those in the non-PB group. The incidence of multilobar infiltrates (unilateral) (26 [19.55%] vs. 304 [29.34%]; P = 0.0252) was lower in the PB group than that in the non-PB group (P < 0.05). There were no significant differences in the incidence of consolidation and multilobar infiltrates (bilateral) between the groups (Table 2).
FB findings and histopathological examination
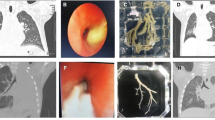
The proportions of mucosal erosion and/or necrosis (101 [75.94%] vs. 357 [34.46%]; P < 0.0001) and bronchial embolization (82 [61.65%] vs. 130 [12.55%]; P < 0.0001) were higher in the PB group than those in the non-PB group, and the proportion of mucosal hyperemia and/or edema (32 [24.06%] vs. 679 [65.54%]; P < 0.0001) was lower in the PB group than that in the non-PB group. No remarkable differences in bronchial obstruction and bronchiectasis were observed between the two groups (Table 2). BC removed from one of patients in PB group is shown in Fig. 2A. Hematoxylin and eosin staining demonstrated that the BC contained numerous inflammatory necrosis and neutrophils (Fig. 2B).
Multivariate regression analysis of PB in MPP
We performed multifactorial analysis with possible risk factors as the independent variables and development of PB as the dependent variable to determine the independent risk factors of PB in the context of MPP. The results showed that L levels (odds ratio [OR] = 1.591, 95% confidence interval [CI]: 1.236 ~ 2.096) and pleural effusion (OR = 2.466, 95% CI: 1.619 ~ 3.724) were independent factors influencing the development of PB with MPP (Table 3).
Discussion
PB is a relatively rare respiratory disease that can result in severe respiratory complications, such as respiratory failure and death [19, 22,23,24]. Its etiology is still poorly understood, but PB is associated with several cardiac and pulmonary conditions, including cyanotic congenital heart disease, asthma, cystic fibrosis, respiratory infections, lymphatic abnormalities, sickle cell anemia, neoplasms, and lung transplantation [25,26,27,28]. Respiratory infections are reported to be the main cause of PB in Asia, and MP is the primary pathogenic bacterium causing PB [12, 13, 29, 30]. In the present study, we analyzed clinical characteristics among children who had MPP with and without PB.
The underlying mechanisms of BC formation in patients with MPP are unclear at present. However, previous studies have shown that MP infection not only directly causes necrosis of the airway epithelium through adhesion damage and membrane fusion damage but also induces cilia removal dysfunction to promote the formation of mucus plug owing to excessive inflammation [12, 14, 31,32,33]. In this study, we found that the proportion of children with PB decreased dramatically during the COVID-19 pandemic. This may be owing to restrictive public health safety measures in place during the pandemic, such as wearing masks in public, sanitizing hands regularly, taking classes online, limiting public gatherings, health monitoring, travel restrictions, and border closures. These measures also helped to successfully control MP transmission [34,35,36].
We found that the duration of hospitalization in the PB group was longer than that in the non-PB group. This was consistent with reports by Zhong et al. [33] and Hua [37] and may be attributed to the following two aspects. On the one hand, the clinical manifestations of PB caused by MP infection are not specific, and MPP is considered self-limiting. PB caused by MPP cannot be treated in a timely manner during the early stage [38]. On the other hand, the mechanism of PB development after MP infection may be closely associated with MP drug resistance, increasing the difficulty of treatment [39].
The clinical manifestation of PB depends on the size and location of BCs and the primary disease. Our results showed that the incidence of lack of appetite was higher in the PB group than in the non-PB group. At present, there are no reports on the association between lack of appetite and PB. The main signs of MPP combined with PB are tachypnea, three depressions sign, and decreased breath sounds. Our study showed that patients in the PB group were more prone to decreased breath sounds, which is considered to be related to the positions and degrees of airway obstruction owing to BCs [13].
Laboratory tests are a convenient and practical method to assess the severity of PB. Levels of N, CRP, PCT, D-dimer, LDH, ALT, and AST were significantly higher in the PB group, and levels of L and PLT were significantly lower. These results were consistent with recent research [33, 40, 41]. The increase in N is related to the activation of neutrophils through toll-like receptor identifying MP lipid-associated membrane proteins after MP infection [42]. As sensitive indicators in the acute phase of inflammation, CRP and PCT are helpful in identifying the formation of PB caused by MPP [13]. LDH, a cytoplasmic enzyme that exists in various important organs, is a non-specific inflammatory biomarker of lysing in lung tissue or cell membrane damage. LDH serves as an important indicator to monitor infection severity and inflammatory disease [43]. The higher levels of N, CRP, PCT, and LDH in the PB group reflect an excessive inflammatory response, which can promote the formation of BCs. Systemic inflammation caused by MP infection leads to an imbalance between the blood coagulation and anticoagulation systems, causing a hypercoagulable state and higher D-dimer levels [14]. These lead to reduced air exchange capacity of the lung tissue and changes in the microcirculation, causing retention of inflammatory factors in the lungs, increased oozing of mucus, and the formation of BCs [13]. In addition to intrapulmonary damage, MP infection can cause extrapulmonary damage, such as damage to the liver [44]. Levels of ATL and AST reflect the degree of liver damage. The more severe the disease in children with MPP, the higher the levels of ALT and AST. In our study, the decreased level of PLT in the PB group was notable. As reported by Zhao et al. [41], the main reason for this is that MP infection and the formation of BCs could increase PLT damage and depletion.
Imaging findings in this study indicated that the incidence of single lobar infiltrate was higher and the incidence of multilobar infiltrates (unilateral) was lower in the PB group as compared with the non-PB group, suggesting that plastic casts in the PB group were more localized. Zhang et al. [42] found that PB caused by MP infection was likely to cause fragmented partial BCs with different pathogens. The incidences of pleural effusion and pericardial effusion were higher in the PB group than those in the non-PB group, which was consistent with data from studies in China and other countries suggesting that the local immune response was stronger in MP-induced PB cases than in non-PB cases [13, 37, 40,41,42, 45, 46].
In our study, FB and BAL were performed to make a definite diagnosis of PB and to clear airway obstructions. Under the bronchoscopy, the proportions of mucosal erosion and/or necrosis and bronchial embolization was higher in the PB group than those in the non-PB group, suggesting a strong inflammatory reaction; this was in line with the research findings of Zhang et al [42]. No remarkable difference in bronchial obstruction was observed between the two groups, we speculated might have been due to (1) BCs formation in segmental or subsegmental bronchus and (2) the timely intervention of bronchoscopy, preventing the spread of BCs throughout the airways [15].
Unlike previous single-factor analyses of risk factors for PB [33], in this study, we conducted multifactor analysis of the risk factors for developing PB in MPP. In line with published reports [37, 40,41,42], our results showed that L levels and pleural effusion were independent risk factors of PB with MPP. Our results will help clinicians to identify potential cases of PB for early intervention, especially FB, which is an invasive procedure.
Despite these strengths, several limitations remain in our study. First, we did not conduct patient follow-up. Second, this was a single-center study; further multicenter and large-sample studies are needed to reduce bias. Third, further exploration of the specific mechanism of BC formation caused by MPP is needed.
Conclusions
In conclusion, children with PB caused by MPP had a strong and local inflammatory response. L levels and pleural effusion were independent risk factors of PB with MPP in children. Our findings will help clinicians to identify potential PB in pediatric patients for early and effective intervention.
Data Availability
All data generated or analysed during this study are included in this published article.
Change history
09 December 2023
This article has been corrected since original publication; please see the linked erratum for further details.
11 December 2023
A Correction to this paper has been published: https://doi.org/10.1186/s12890-023-02800-1
Abbreviations
- MP:
-
Mycoplasma pneumoniae
- MPP:
-
MP pneumonia
- CAP:
-
Community-acquired pneumonia
- PB:
-
Plastic bronchitis
- BCs:
-
Bronchial casts
- FB:
-
Fiberoptic bronchoscopy
- BAL:
-
Bronchoalveolar lavage
- CT:
-
Computed tomography
- N:
-
Neutrophil
- L:
-
Lymphocyte
- PLT:
-
Platelet
- CRP:
-
C-reactive protein
- PCT:
-
Procalcitonin
- LDH:
-
Lactate dehydrogenase
- ALT:
-
Alanine transaminase
- AST:
-
Aspartate transaminase
References
Su M, Wang Q, Li D, Wang LL, Wang CY, Wang JL, et al. Prevalence and clinical characteristics of hospitalized children with community-acquired Mycoplasma pneumoniae Pneumonia during 2017/2018, Chengde, China. Med (Baltim). 2021;100(5):e23786.
Guo Q, Li L, Wang C, Huang Y, Ma F, Cong S, et al. Comprehensive virome analysis of the viral spectrum in paediatric patients diagnosed with Mycoplasma pneumoniae Pneumonia. Virol J. 2022;19(1):181.
Han Z, Zhang Y, Liao S, Zhou N. The clinical characteristics of Mycoplasma pneumoniae Pneumonia and its relationship between hypokalemia in West China. Transl Pediatr. 2021;10(2):406–14.
Gao LW, Yin J, Hu YH, Liu XY, Feng XL, He JX, et al. The epidemiology of paediatric Mycoplasma pneumoniae Pneumonia in North China: 2006 to 2016. Epidemiol Infect. 2019;147:e192.
Zhang Y, Zheng W, Ning H, Liu J, Li F, Ju X. Interleukin-6 in blood and bronchoalveolar lavage fluid of hospitalized children with community-acquired Pneumonia. Front Pediatr. 2022;10:922143.
Chi H, Huang YC, Liu CC, Chang KY, Huang YC, Lin HC, et al. Characteristics and etiology of hospitalized pediatric community-acquired Pneumonia in Taiwan. J Formos Med Assoc. 2020;119(10):1490–9.
Roh EJ, Lee MH, Lee JY, Kim HB, Ahn YM, Kim JK, et al. Analysis of national surveillance of respiratory pathogens for community-acquired Pneumonia in children and adolescents. Bmc Infect Dis. 2022;22(1):330.
Yun KW, Wallihan R, Desai A, Alter S, Ambroggio L, Cohen DM, et al. Clinical characteristics and etiology of community-acquired Pneumonia in US children, 2015–2018. Pediatr Infect Dis J. 2022;41(5):381–7.
Wrotek A, Robakiewicz J, Pawlik K, Rudzinski P, Pilarska I, Jaron A, et al. The etiology of Community-Acquired Pneumonia correlates with serum inflammatory markers in children. J Clin Med. 2022;11:19.
Cannesson A, Elenga N. Community-Acquired Pneumonia requiring hospitalization among French Guianese Children. Int J Pediatr. 2021;2021:4358818.
Merida-Vieyra J, Aquino-Andrade A, Palacios-Reyes D, Murata C, Ribas-Aparicio RM, De Colsa RA. Detection of Mycoplasma pneumoniae in Mexican children with community-acquired Pneumonia: experience in a tertiary care hospital. Infect Drug Resist. 2019;12:925–35.
Wang L, Wang W, Sun JM, Ni SW, Ding JL, Zhu YL, et al. Efficacy of fiberoptic bronchoscopy and bronchoalveolar lavage in childhood-onset, complicated plastic Bronchitis. Pediatr Pulmonol. 2020;55(11):3088–95.
Huang JJ, Yang XQ, Zhuo ZQ, Yuan L. Clinical characteristics of plastic Bronchitis in children: a retrospective analysis of 43 cases. Respir Res. 2022;23(1):51.
Shen F, Dong C, Zhang T, Yu C, Jiang K, Xu Y, et al. Development of a Nomogram for Predicting Refractory Mycoplasma pneumoniae Pneumonia in Children. Front Pediatr. 2022;10:813614.
Lu S, Liu J, Cai Z, Shuai J, Huang K, Cao L. Bronchial casts associated with Mycoplasma pneumoniae Pneumonia in children. J Int Med Res. 2020;48(4):1220710815.
Zhang T, Han C, Guo W, Ning J, Cai C, Xu Y. Case Report: clinical analysis of Fulminant Mycoplasma pneumoniae Pneumonia in Children. Front Pediatr. 2021;9:741663.
Li Y, Williams RJ, Dombrowski ND, Watters K, Daly KP, Irace AL, et al. Current evaluation and management of plastic Bronchitis in the pediatric population. Int J Pediatr Otorhinolaryngol. 2020;130:109799.
Patel N, Patel M, Inja R, Krvavac A, Lechner AJ. Plastic Bronchitis in Adult and Pediatric patients: a review of its presentation, diagnosis, and treatment. Mo Med. 2021;118(4):363–73.
Soyer T, Yalcin S, Emiralioglu N, Yilmaz EA, Soyer O, Orhan D, et al. Use of serial rigid bronchoscopy in the treatment of plastic Bronchitis in children. J Pediatr Surg. 2016;51(10):1640–3.
Expert Committee on Rational Use of Medicines for Children Pharmaceutical Group N. (2020). Expert consensus on laboratory diagnostics and clinical practice of Mycoplasma pneumoniae infection in children in China (2019). Chin J Pediatr. 2020;58(5):366 – 73.
Meyer SP, Unger W, van Rossum A, Berger C. The art and science of diagnosing Mycoplasma pneumoniae Infection. Pediatr Infect Dis J. 2018;37(11):1192–5.
Kunder R, Kunder C, Sun HY, Berry G, Messner A, Frankovich J, et al. Pediatric plastic Bronchitis: case report and retrospective comparative analysis of epidemiology and pathology. Case Rep Pulmonol. 2013;2013:649365.
Yuan L, Huang JJ, Zhu QG, Li MZ, Zhuo ZQ. Plastic Bronchitis associated with adenovirus serotype 7 in children. Bmc Pediatr. 2020;20(1):268.
Ding XF, Zhong LL, Zhang B, Lin L, Huang H, Liang M. Clinical features and pathogens of plastic Bronchitis in children: an analysis of 9 cases. Chin J Contemp Pediatr. 2014;16(7):729–33.
Perez RE, Lopez CM, Caro AP, Perez FJ. Management and treatment of Pediatric Plastic Bronchitis. Arch Bronconeumol. 2017;53(8):467–8.
Raghuram N, Pettignano R, Gal AA, Harsch A, Adamkiewicz TV. Plastic Bronchitis: an unusual complication associated with sickle cell Disease and the acute chest syndrome. Pediatrics. 1997;100(1):139–42.
Kuperman T, Wexler ID, Shoseyov D, Weintraub M, Revel-Vilk S, Kerem E. Plastic Bronchitis caused by neoplastic infiltrates in a child. Pediatr Pulmonol. 2006;41(9):893–6.
Eberlein M, Parekh K, Hansdottir S, Keech J, Klesney-Tait J. Plastic Bronchitis complicating primary graft dysfunction after lung transplantation. Ann Thorac Surg. 2014;98(5):1849.
Zhang FZ, Qin L, Yuan JX, Tang LF. Plastic Bronchitis due to adenoviral Infection: a case report. Bmc Pediatr. 2020;20(1):61.
Zeng L, Wei J, Tang Y, Liu E, Li Q, Zang N. Clinical characteristics of human adenovirus Plastic Bronchitis in 10 Pediatric cases: a retrospective study of seven years. Virol Sin. 2021;36(3):550–4.
Eberlein MH, Drummond MB, Haponik EF. Plastic Bronchitis: a management challenge. Am J Med Sci. 2008;335(2):163–9.
Liang H, Jiang W, Han Q, Liu F, Zhao D. Ciliary ultrastructural abnormalities in Mycoplasma pneumoniae Pneumonia in 22 pediatric patients. Eur J Pediatr. 2012;171(3):559–63.
Zhong H, Yin R, Zhao R, Jiang K, Sun C, Dong X. Analysis of clinical characteristics and risk factors of Plastic Bronchitis in Children with Mycoplasma pneumoniae Pneumonia. Front Pediatr. 2021;9:735093.
Cheng Y, Cheng Y, Dai S, Hou D, Ge M, Zhang Y, et al. The prevalence of Mycoplasma Pneumoniae among Children in Beijing before and during the COVID-19 pandemic. Front Cell Infect Microbiol. 2022;12:854505.
Zhang LN, Cao L, Meng LH. Pathogenic changes of community-acquired Pneumonia in a children’s hospital in Beijing, China before and after COVID-19 onset: a retrospective study. World J Pediatr. 2022;18(11):746–52.
Li L, Guo P, Ma J, Sun H, Mei S. Impact of COVID-19 on the Epidemiological Features of Mycoplasma pneumoniae Infection in children with community-acquired Pneumonia in Henan, China. Microbiol Spectr. 2023;11(1):e491122.
Hua J. Analysis of risk factors for plastic Bronchitis in children with refractory Mycoplasma pneumoniae Pneumonia. Chin J Appl Clin Pediatr. 2019;34(16):1219–22.
Zhang Y, Zhou Y, Li S, Yang D, Wu X, Chen Z. The clinical characteristics and predictors of Refractory Mycoplasma pneumoniae Pneumonia in Children. PLoS ONE. 2016;11(5):e156465.
Choi YJ, Chung EH, Lee E, Kim CH, Lee YJ, Kim HB et al. Clinical characteristics of Macrolide-Refractory Mycoplasma pneumoniae Pneumonia in Korean Children: a Multicenter Retrospective Study. J Clin Med. 2022;11(2).
Zhang H, Yang J, Zhao W, Zhou J, He S, Shang Y, et al. Clinical features and risk factors of plastic Bronchitis caused by refractory Mycoplasma pneumoniae Pneumonia in children: a practical nomogram prediction model. Eur J Pediatr. 2023;182(3):1239–49.
Zhao L, Zhang T, Cui X, Zhao L, Zheng J, Ning J, et al. Development and validation of a nomogram to predict plastic Bronchitis in children with refractory Mycoplasma pneumoniae Pneumonia. Bmc Pulm Med. 2022;22(1):253.
Zhang R, Wang T, Dai G, Wang MJ, Yan YD, Zhou YW, et al. Analysis of clinical characteristics and risk factors of plastic Bronchitis caused by Mycoplasma pneumoniae Infection. Chin J Appl Clin Pediatr. 2021;36(11):811–6.
Lu A, Wang C, Zhang X, Wang L, Qian L. Lactate Dehydrogenase as a Biomarker for Prediction of Refractory Mycoplasma pneumoniae Pneumonia in Children. Respir Care. 2015;60(10):1469–75.
Waites KB, Xiao L, Liu Y, Balish MF, Atkinson TP. Mycoplasma pneumoniae from the respiratory tract and Beyond. Clin Microbiol Rev. 2017;30(3):747–809.
Shu LL, Zhong L, Qiu L, Li Y, Zhang R, Liu HM. Clinical analysis of 86 cases of children with plastic Bronchitis. J SCU Med Sci Edi. 2021;52(5):855–8.
Ma Y, Gu Y, Zhang X, Gu W, Wang T, Sun H et al. High Expression of MUC5AC, MUC5B, and Layilin Plays an Essential Role in Prediction in the Development of Plastic Bronchitis Caused by MPP. Front Microbiol. 2022;13:911228.
Acknowledgements
The authors would like to thank the clinical staff, specifically the medical doctors and nurses, for their help with collecting clinical specimens, and all of the children and their parents for their participation and cooperation.
Funding
This work was supported by the Postdoctoral program of Affiliated Hospital of Jining Medical University (< grant number JYFY303579> [to < Lei Yang>]); Health Commission of Shandong Province (< grant number 202006010928> [to < Ruihan Liu>]); Key research and development plan in Jining City (< grant number 2021YXNS068> [to < Yuyan Zhang>]); Jining Medical University (< grant number JYP2019KJ32> [to < Jun Ning>]); and the Affiliated Hospital of Jining Medical University (< grant number 2018-BS-004> [to < Ruihan Liu>]).
Author information
Authors and Affiliations
Contributions
LY: drafting the manuscript. LY, RL and JN: conception of the work, participation in data analysis and interpretation, critical revision of the manuscript, final approval of the version to be published. YZ, CS and JN: operation of bronchoscopy. ZL: collection of experimental specimens. TH: collection of patients’ information. LY, RL, YZ and JN: funding acquisition. FN and YW: supervision. All the authors: conception of the work, critical revision of the manuscript, final approval of the version to be published.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
The study was conducted according to the guidelines of the Declaration of Helsinki, and approved by the Ethics Committee of the Affiliated Hospital of Jining Medical University (protocol code: 2018C076). All subjects’ legal guardians provided written informed consent.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Yang, L., Zhang, Y., Shen, C. et al. Clinical features and risk factors of plastic bronchitis caused by Mycoplasma pneumoniae pneumonia in children. BMC Pulm Med 23, 468 (2023). https://doi.org/10.1186/s12890-023-02766-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12890-023-02766-0