Abstract
Objective
Using a system, which accuracy is equivalent to the gold standard Douglas Bag (DB) technique for measuring oxygen consumption (VO2), carbon dioxide generation (VCO2), and respiratory quotient (RQ), we aimed to continuously measure these metabolic indicators and compare the values between post-cardiothoracic surgery and critical care patients.
Methods
This was a prospective, observational study conducted at a suburban, quaternary care teaching hospital. Age 18 years or older patients who underwent mechanical ventilation were enrolled.
Results
We included 4 post-surgery and 6 critical care patients. Of those, 3 critical care patients died. The longest measurement reached to 12 h and 15 min and 50 cycles of repeat measurements were performed. VO2 of the post-surgery patients were 234 ± 14, 262 ± 27, 212 ± 16, and 192 ± 20 mL/min, and those of critical care patients were 122 ± 20, 189 ± 9, 191 ± 7, 191 ± 24, 212 ± 12, and 135 ± 21 mL/min, respectively. The value of VO2 was more variable in the post-surgery patients and the range of each patient was 44, 126, 71, and 67, respectively. SOFA scores were higher in non-survivors and there were negative correlations of RQ with SOFA.
Conclusions
We developed an accurate system that enables continuous and repeat measurements of VO2, VCO2, and RQ. Critical care patients may have less activity in metabolism represented by less variable values of VO2 and VCO2 over time as compared to those of post-cardiothoracic surgery patients. Additionally, an alteration of these values may mean a systemic distinction of the metabolism of critically ill patients.
Similar content being viewed by others
Introduction
Oxygen consumption (VO2), carbon dioxide generation (VCO2), and respiratory quotient (RQ), which is the ratio of VCO2 to VO2, are important measures of the metabolism in humans [1,2,3]. These measurements are widely used for patients with a variety of conditions, including post-surgery [1], shock [4], pulmonary and cardiac diseases [5], and critical illness requiring mechanical ventilation [2, 6, 7]. Indirect calorimetry is a non-invasive method, in which VO2 and VCO2 are measured from concentrations of oxygen and carbon dioxide of inhalation and exhalation [8,9,10]. Since it is non-invasive, indirect calorimetry has been widely used by clinicians [11, 12] as well as animal studies [13,14,15,16]. However, due to the lack of a gold standard, the accuracy has been questioned for nearly 100 years [6, 7, 17, 18].
One of the traditional but standard methods is the Douglas Bag (DB) collection technique, which was first introduced by a physiologist, Claude Gordon Douglas, in 1911 [19]. This technique is still the most reliable gold standard these days [18]. Central focus of the DB method is the accuracy of gas concentrations of inhalation and exhalation. Errors are propagated particularly when a high oxygen concentration gas is used [10]. The DB technique is a method using collection bags that equilibrate concentrations of gases inside a bag. The DB technique exerts higher accuracy if a gas concentration dynamically changes. The concentrations of oxygen and carbon dioxide in exhalation change dynamically during each breath. Therefore, the DB method enables reliable measurements when the accuracy of VO2, VCO2, and RQ is in need [18]. However, a complexity of the method and lengthy processing time limit the number of measurements that can be performed at bedside.
We developed an automation device that enables continuous and repeat measurements of VO2, VCO2, and RQ in patients undergoing mechanical ventilation [20]. The accuracy was validated, and it was equivalent to that of the DB technique. To the best of our knowledge, this is the first study that applied repeat measurements of VO2, VCO2, RQ by using a method equivalent to the DB technique and we will demonstrate the results of continuous metabolic measurements in post-cardiothoracic surgery and critical care patients.
Materials and methods
Study design
This was a prospective, observational study conducted at a suburban, quaternary care teaching hospital. Age 18 years or older patients who underwent mechanical ventilation were enrolled. The study protocol was approved by the Institutional Review Board (16–615-North Shore University Hospital). Written informed consent for participation was obtained from patients or next of kin prior to the procedures. If a patient did not hold a capacity for consent or did not have a legally authorized representative or next of kin, the patient was enrolled with waived consent. We excluded patients whose positive end-expiratory pressure (PEEP) setting was higher than 10 cm H2O due to an expected gas leak from the mechanical ventilation circuit. Our method and calculation algorithm enabled measurements of VO2, VCO2, and RQ at a variety range of fraction of inspired oxygen (FIO2) [21] and therefore, no upper limit was made on an FIO2 setting of mechanical ventilation.
Automation system
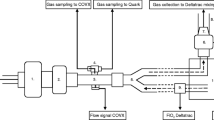
We previously reported the detail of our methods [20]. This is a unique technique, for which we have devoted most of our time to validate its accuracy and the results can be found in the other report [20]. In brief, ten minutes were given to all patients for acclimating to the apparatus before starting a measurement. For the measurements of VO2, VCO2, and RQ, inhalation and exhalation were independently analyzed. A commercially available gas analyzer (GF-210R Multi-Gas Module, Nihon Kohden Corporation, Irvine, CA, USA) was used. The gases were sampled from a mechanical ventilator (AVEA® ventilator, CareFusion, San Diego, CA, USA) and the gas concentrations of oxygen and carbon dioxide were measured by the gas analyzer. By using these values, we calculated fraction of expired oxygen (FEO2) and fraction of expired carbon dioxide (FECO2) from the concentrations measured at the exhaust port (FexhO2 and FexhCO2, respectively). The measurement was performed at bedside, which enabled a real-time and continuous collection of data. A dehumidification device (DHU-1000 Dehumidification Unit, Nihon Kohden Corporation, Tokyo, Japan) was set in conjunction with the gas analyzer: the dehumidification unit was intended for use in dehumidifying a sample gas. We have validated the accuracy of this automation system [20]. The validation included (i) a system response time; (ii) sensor accuracy of gas concentrations; (iii) the performance of mixing chamber; (iv) the calculation algorithm of our automation system; (v) and VO2, VCO2, and RQ in healthy volunteers compared between the device and the DB methods.
The gas concentrations of inhalation and exhalation were measured alternately, and the duty cycle was 15 min. The gas concentration in inhalation was measured at the first 6 min of the duty cycle. A valve switched the sampling port from inhalation to exhalation and 9 min were given for FEO2 and FECO2 measurements.
Calculations and analysis
FIO2, FEO2, FICO2 (fraction of inspired carbon dioxide), FECO2, in-circuit humidity and temperature in the dehumidification device, and ambient pressure and temperature around the mechanical ventilator circuit were measured. A minute ventilation volume of exhalation (VE), inhalation to exhalation (I:E) ratio, and bias flow setting were recorded from the mechanical ventilator. For the automation system, FEO2 and FECO2 were calculated from the gas concentrations measured at the ventilator exhaust port. The following equations are used in this study:
where VI is a minute ventilation volume of inhalation and VE is that of exhalation. FICO2 is zero since the inhalation gas does not contain CO2. RQ, VO2, and VCO2 are then transformed to equations as follows:
R is generally derived from the Haldane transformation with the assumption that nitrogen is neither produced nor retained by the body, and that no gases are present other than O2, CO2, and nitrogen [22]. Because the denominator includes FIO2 and it goes to zero as FIO2 increases to 1.0, R increases to infinite number when FIO2 is 1.0. Therefore, the Haldane transformation limits FIO2 generally up to 0.6. This is a significant limitation in critical care, in which patients normally require a high level FIO2. Therefore, we developed a method for measuring R and sought the number of R by using our rodent model [21]. Our results suggested that R was not 1.0 and so VI was not equal to VE. While our result is in line with the concept of the Haldane transformation that helps us interpret VI ≠ VE, the data from our rodent model also indicates that R may be constant as opposed to dependent on FIO2 as the calculation from the Haldane transformation outputs. We sought a value of human R calculated from the values obtained from previous human reports [22, 23] and determined it as 1.0097 in this study.
Statistical analysis
We reported data as mean and standard deviation and descriptive statistics were used. The values were reported as standard temperature and pressure and dry (STPD). Spearman’s rho and correlation coefficients were calculated, and Mann–Whitney U tests were used for comparison between two groups. Generalized linear mixed model, in which covariates include survival outcomes and number of measurements, was used to estimate the effects on continuous and repeat measurements. Prism for Mac version 9 (GraphPad Software, San Diego, CA) and SPSS version 27 (IBM, Armonk, NY) were used for statistical analysis, and P values less than 0.05 was considered statistically significant.
Results
Data from 10 patients were analyzed in this study. Table 1 shows baseline characteristics of the study subjects. Out of 10 subjects, 4 were patients post cardiothoracic surgery, 5 were patients in the intensive care units (ICU), and 1 was in the emergency room (ER). Sequential organ failure assessment (SOFA) score, blood lactate level (mmol/L), and P/F ratio (arterial partial oxygen pressure to FIO2) were obtained at ICU admission. Female patients had significantly lower weights and heights than male (p < 0.05 and p < 0.05, respectively). None of the post-cardiothoracic surgery patients had major adverse events nor died. This study included 6 critical care patients, whose metabolic measurements were performed in the ER or ICU, and 3 were died.
Initial metabolic values
Table 2 demonstrates values of initial metabolic measurements. SOFA score negatively correlated with RQ (- 0.657, p < 0.05). Initial blood lactate levels negatively correlated with VCO2 (-0.671, p < 0.05) but adjusting it by body weight rejected the significance. FIO2 negatively correlated with RQ (-0.696, p < 0.05) (Supplement).
Continuous and repeat metabolic measurements
We performed continuous and repeat metabolic measurements by the automation device and Fig. 1 and Table 2 show the results. The minimum number of measurements were 5 cycles for 75 min occurred in ER and the longest measurement lasted for 12 h and 15 min and 50 cycles of repeat measurements were performed. Figure 1 A shows the trend of FIO2 over time. There was a downward trend of FIO2 in the post-surgery patients because all patients were weaned off from mechanical ventilation during the post-day 1 of surgery. In contrast, there was no consistent pattern of FIO2 in the critical care patients.
Continuous and Repeat Metabolic Measurements. The measurements were started from the admission to the cardiothoracic intensive care unit for post-surgical patients. For critical care patients, the measurements were started from the earliest possible timing after enrollment. A Fraction of inspired oxygen (FIO2). B VO2 at standard temperature and pressure and dry (STPD). C VCO2 at STPD. D RQ. n = 4 for post-surgery, n = 6 for critical care patients. Subjects IDs are corresponding to those in Table 1
The mean VO2 in the 4 post-surgery patients were 234 ± 14, 262 ± 27, 212 ± 16, and 192 ± 20 mL/min, respectively. Those in the critical care patients were 122 ± 20, 189 ± 9, 191 ± 7, 191 ± 24, 212 ± 12, and 135 ± 21 mL/min, respectively. The value of VO2 was variable in the post-surgery patients and the range was 44, 126, 71, and 67, respectively. These values seemed more variable than those (22, 42, 20, 60, 38, 69) of critical care patients. The range of non-survivors (22, 42, 20) appeared to be less than those (60, 38, 69) of survivors.
The mean VCO2 in the 4 post-surgery patients were 160 ± 9, 184 ± 20, 149 ± 14, and 131 ± 6 mL/min, respectively. Those in critical care patients were 58 ± 9, 133 ± 5, 107 ± 5, 129 ± 3, 155 ± 7, and 103 ± 16 mL/min, respectively. The value of VCO2 was variable in 4 post-surgery patients and the range was 31, 103, 59, and 24, respectively. These values seemed more variable than those (23, 21, 18, 8, 21, 48) of critical care patients. Figure 2 shows the result of detailed analysis on VCO2. According to Eq. (5), VCO2 is derived from VE and FECO2. The higher VO2 found in post-surgery patients seemed associated with both variables. Non-survivors had lower FECO2 than survivors and the same trend was found by the plots in fraction of CO2 at exhaust port of the mechanical ventilator (FexhCO2). Generalized linear mixed model identified the effects of outcome on VO2 (p < 0.05), VCO2 (p < 0.01), and VCO2 per weight (p < 0.01), but not on VO2 per weight.
Ventilation and Carbon Dioxide Levels. A Minute ventilation volume (SPTD) measured at the exhaust port. B Fraction of expired carbon dioxide (FECO2). C Fraction of expired carbon dioxide measured at the exhaust port. n = 4 for post-surgery, n = 6 for critical care patients. Subjects IDs are corresponding to those in Table 1
The mean RQ in the 4 post-surgery patients were 0.69 ± 0.05, 0.70 ± 0.02, 0.70 ± 0.02, 0.69 ± 0.06 respectively. Those in critical care patients were 0.47 ± 0.04, 0.71 ± 0.03, 0.56 ± 0.01, 0.68 ± 0.06, 0.73 ± 0.06, 0.76 ± 0.01, respectively. The range of RQ of the post-surgery patients were 0.17, 0.10, 0.08, 0.17 and those of the critical care patients were 0.12, 0.14, 0.04, 0.16, 0.22, and 0.05, respectively.
Discussion
We developed a system for measuring FIO2, FEO2, and FECO2 in humans undergoing mechanical ventilation. These gas concentrations are critical components when seeking accurate VO2, VCO2, and RQ. The accuracy of our system was equivalent to the gold standard DB collection technique [20]. Our automation system allows for continuous and repeat measurements, to which the DB technique has a significant limitation due to the complexity of its procedure. By using our automation system, we successfully collected continuous values of VO2, VCO2, and RQ in post-surgical and critical care patients.
The volume ratio of inhalation to exhalation defined as R in this study is an essential component of calculating VO2. However, there is a significant technical difficulty of measuring the small differences between VI and VE. Therefore, VI is commonly calculated by using the Haldane transformation, which limits the use of FIO2 up to 0.6. Assuming VI equals VE and ignoring this small difference eliminate the concern. However, failure to account for this small difference can erroneously decrease VO2 by 17% [10], if VI is actually not equal to VE, and the error propagates at higher FIO2 levels. The adequacy of the Haldane transformation [22] supports that VI is not equal to VE.
We have developed a method for measuring the small differences between VI and VE [21]. Our results from the rodent model showed that R ranged from 1.0081 ± 0.0017 to 1.0092 ± 0.0029 at FIO2 0.3 and 1.0, respectively, indicating that VI is not equal to VE (R ≠ 1.0000). While our results were in line with the concept of the Haldane transformation supporting the idea of VI ≠ VE, our data revealed that R is an independent value from FIO2 as opposed to that calculated by the Haldane transformation, which includes FIO2 in its equation. We sought a value of human R calculated from the values obtained from previous human reports [22, 23] and determined it as 1.0097 in this study. These data justify the use of R = 1.0097 to all calculations that include various FIO2 levels that depend on patient’s condition.
Our automation system allows for continuous and repeat measurements of VO2, VCO2, and RQ. The data of 10 patients were evaluated in this study. Post-surgical patients had higher VO2 and VCO2 and appeared to have more variable numbers as compared to those of critical care patients. It is inferred that the post-surgical patients had more active metabolism than critical care patients, which might be attributed to purposefully minimized sedatives to wean off the patients from mechanical ventilation. The greater variability of metabolic values argues the unmet need of continuous measurements. In other words, one point measurement such as the traditional DB technique, even though it is accurate and reliable, may not be sufficient to identify an important signal that varies over time. We also found that non-survivors appeared to have low FECO2. This is inferred that patients who are critically ill may have less activity in metabolism.
Differences in study values were observed between survivors and non-survivors at the beginning of the measurements. This suggests that significant systemic distinctions occurred at the starting period of our measurements and those could induce changes in the values. Indeed, SOFA scores were higher in non-survivors and there was a negative correlation of VCO2 with the initial value of blood lactate, while there was no statistically significant correlation between SOFA and VCO2. Both SOFA scores and blood lactate are known to indicate the severity of diseases but the difference of the correlation patters of VCO2 with these values may be led by a mechanistic response. Moreover, our study subjects include female patients, whose weights and heights were significantly lower than male. Adjusting VCO2 by weight rejected the correlation of VCO2 with blood lactate, meaning it might be confounded by the body size. On the other hand, RQ is not affected by the body size as its calculation cancels the effect of body weight. Indeed, there were negative correlations of RQ with SOFA and FIO2. We found that high FIO2 leads to lower RQ due to the increase in VO2 [20]. Therefore, this finding might mean that the lowered RQ could be led by the distinctions of the patients or high FIO2 or both. However, due to the small number of our study population, we could not draw a conclusion. Further studies may warrant the investigation of these mechanisms.
This study is subject to several limitations. The number of samples, first and foremost, is limited in this study owing to the nature of explanatory design of our current work. Second, the study lacks a mechanistic information. More detailed information such as the dose of inotropes, vasoactive inotropic score, and nutrition would help with identifying specific characteristics or reasons that alter VO2, VCO2, or RQ in the ICU settings. Particularly because nutrition [3] and/or disease conditions [24, 25] may alter RQ, more focused information on these variables needs to be investigated. The automation system aids clinicians to collect more information on metabolism of patients. Moreover, our method enables the gas concentration measurements that are equivalent to the gold standard DB method. Therefore, we expect to see more metabolic data from patients in the future.
Conclusions
We developed an automation system that enables repeat measurements of VO2, VCO2, and RQ. Critical care patients may have less variability of VO2 and VCO2 as compared to the post-cardiothoracic surgery patients.
Availability of data and materials
The datasets generated and/or analyzed during the current study are not publicly available due the current sponsorship made with Nihon Kohden Corporation but are available from the corresponding author on reasonable request.
References
Gump FE, Kinney JM, Price JB Jr. Energy metabolism in surgical patients: oxygen consumption and blood flow. J Surg Res. 1970;10:613–27.
Manthous CA, Hall JB, Olson D, Singh M, Chatila W, Pohlman A, Kushner R, Schmidt GA, Wood LD. Effect of cooling on oxygen consumption in febrile critically ill patients. Am J Respir Crit Care Med. 1995;151:10–4.
McClave SA, Lowen CC, Kleber MJ, McConnell JW, Jung LY, Goldsmith LJ. Clinical use of the respiratory quotient obtained from indirect calorimetry. JPEN J Parenter Enteral Nutr. 2003;27:21–6.
Siegel JH, Fabian M, Smith JA, Kingston EP, Steele KA, Wells MR, Kaplan LJ. Oxygen debt criteria quantify the effectiveness of early partial resuscitation after hypovolemic hemorrhagic shock. J Trauma. 2003;54:862–80 (discussion 880).
Guazzi M, Adams V, Conraads V, Halle M, Mezzani A, Vanhees L, Arena R, Fletcher GF, Forman DE, Kitzman DW, Lavie CJ, Myers J. EACPR/AHA scientific statement. clinical recommendations for cardiopulmonary exercise testing data assessment in specific patient populations. Circulation. 2012;126:2261–74.
Sundstrom M, Tjader I, Rooyackers O, Wernerman J. Indirect calorimetry in mechanically ventilated patients. A systematic comparison of three instruments. Clin Nutr. 2013;32:118–21.
Graf S, Karsegard VL, Viatte V, Heidegger CP, Fleury Y, Pichard C, Genton L. Evaluation of three indirect calorimetry devices in mechanically ventilated patients: which device compares best with the Deltatrac II((R))? A prospective observational study. Clin Nutr. 2015;34:60–5.
Matarese LE. Indirect calorimetry: technical aspects. J Am Diet Assoc. 1997;97:S154-160.
da Rocha EE, Alves VG, da Fonseca RB. Indirect calorimetry: methodology, instruments and clinical application. Curr Opin Clin Nutr Metab Care. 2006;9:247–56.
Branson RD, Johannigman JA. The measurement of energy expenditure. Nutr Clin Pract. 2004;19:622–36.
Soussi S, Vallee F, Roquet F, Bevilacqua V, Benyamina M, Ferry A, Cupaciu A, Chaussard M, De Tymowski C, Boccara D, Mimoun M, Chaouat M, Anstey J, Mebazaa A, Legrand M. Measurement of oxygen consumption variations in critically ill burns patients: are the fick method and indirect calorimetry interchangeable? Shock. 2017;48:532–8.
Hensel M, Kox WJ. Increased intrapulmonary oxygen consumption in mechanically ventilated patients with pneumonia. Am J Respir Crit Care Med. 1999;160:137–43.
Musch TI, Bruno A, Bradford GE, Vayonis A, Moore RL. Measurements of metabolic rate in rats: a comparison of techniques. J Appl Physiol. 1985;1988(65):964–70.
van Klinken JB, van den Berg SA, van Dijk KW. Practical aspects of estimating energy components in rodents. Front Physiol. 2013;4:94.
Yo K, Yu YM, Zhao G, Bonab AA, Aikawa N, Tompkins RG, Fischman AJ. Brown adipose tissue and its modulation by a mitochondria-targeted peptide in rat burn injury-induced hypermetabolism. Am J Physiol Endocrinol Metab. 2013;304:E331-341.
Even PC, Nadkarni NA. Indirect calorimetry in laboratory mice and rats: principles, practical considerations, interpretation and perspectives. Am J Physiol Regul Integr Comp Physiol. 2012;303:R459-476.
Hagedorn HC. An apparatus for the graphic recording of oxygen consumption and carbon dioxide output, especially adapted for clinical work. Biochem J. 1924;18:1301–7.
Black C, Grocott MP, Singer M. Metabolic monitoring in the intensive care unit: a comparison of the Medgraphics Ultima, Deltatrac II, and Douglas bag collection methods. Br J Anaesth. 2015;114:261–8.
Shephard RJ. A critical examination of the Douglas bag technique. J Physiol. 1955;127:515–24.
Shinozaki K, Yu PJ, Zhou Q, Cassiere HA, Stanley J, Rolston DM, Garg N, Li T, Johnson J, Saeki K, Goto T, Okuma Y, Miyara SJ, Hayashida K, Aoki T, Wong V, Molmenti EP, Lampe JW, Becker LB. An automation system equivalent to the Douglas bag technique enables continuous and repeat metabolic measurements in patients undergoing mechanical ventilation. Clin Ther. 2022;44:1471–9.
Shinozaki K, Okuma Y, Saeki K, Miyara SJ, Aoki T, Molmenti EP, Yin T, Kim J, Lampe JW, Becker LB. A method for measuring the molecular ratio of inhalation to exhalation and effect of inspired oxygen levels on oxygen consumption. Sci Rep. 2021;11:12815.
Wilmore JH, Costill DL. Adequacy of the Haldane transformation in the computation of exercise VO2 in man. J Appl Physiol. 1973;35:85–9.
Herron JM, Saltzman HA, Hills BA, Kylstra JA. Differences between inspired and expired minute volumes of nitrogen in man. J Appl Physiol. 1973;35:546–51.
Shinozaki K, Becker LB, Saeki K, Kim J, Yin T, Da T, Lampe JW. Dissociated oxygen consumption and carbon dioxide production in the post-cardiac arrest rat: a novel metabolic phenotype. J Am Heart Assoc. 2018;7:e007721.
Uber A, Grossestreuer AV, Ross CE, Patel PV, Trehan A, Donnino MW, Berg KM. Preliminary observations in systemic oxygen consumption during targeted temperature management after cardiac arrest. Resuscitation. 2018;127:89–94.
Acknowledgements
None.
Funding
This research was supported by the research grant of Nihon Kohden Corporation.
Author information
Authors and Affiliations
Contributions
K. Shinozaki has full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis; K. Shinozaki, K. Saeki, J. W. Lampe , L. B. Becker designed the conception of the study; K. Shinozaki, P. J. Yu, Q. Zhou, D. M. Rolston, J. Johnson, and Y. Okuma performed acquisition of data and K. Shinozaki analyzed data; all authors made interpretations of data; all authors added intellectual content of revisions to the paper and gave full approval of the version to be published.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The Northwell Health Human Research Protection Program is guided by the ethical principles as set forth in the 1979 report of the National Commission for the Protection of Human Subjects of Biomedical and Behavioral Research (entitled: Ethical Principles and Guidelines for the Protection of Human Subjects of Research, the “Belmont Report”), following the Declaration of Helsinki, which has been revised several times since 1964.
The study protocol was approved by the Institutional Review Board (16–615-North Shore University Hospital). Written informed consent for participation was obtained from patients or next of kin prior to the procedures.
Consent for publication
Not applicable.
Competing interests
Saeki belongs to Nihon Kohden Innovation Center, INC and Goto to Nihon Kohden Corporation as employee. This does not invade the authors’ adherence to all the Journal's policies. Shinozaki and Becker own intellectual property of metabolic measurement in critically ill patients. Shinozaki has grant/research supported by Grants-in-Aid for Scientific Research (KAKEN, 23K19643) and Nihon Kohden Corp. Becker has grant/research supported by Philips Healthcare, the National Institutes of Health, Nihon Kohden Corp., BeneChill Inc., Zoll Medical Corp, Medtronic Foundation, and patents in the areas of hypothermia induction and perfusion therapies. All the other authors have no disclosures.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1:
Table S1. Differences of values between post-surgery and critical care patients. Table S2. Differences of values between survivors and non-survivors. Table S3. Differences of values between male and female patients. Table S4. Correlation between study values. Table S5. Mechanical ventilator settings.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Shinozaki, K., Yu, PJ., Zhou, Q. et al. Continuous and repeat metabolic measurements compared between post-cardiothoracic surgery and critical care patients. BMC Pulm Med 23, 390 (2023). https://doi.org/10.1186/s12890-023-02657-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12890-023-02657-4