Abstract
Background
Pulmonary rehabilitation training is of great significance for the prognosis of chronic obstructive pulmonary disease (COPD) patients. The purpose of this study was to investigate the therapeutic effect and pathway of a new sequential noninvasive positive pressure ventilation (NIPPV) + inspiratory muscle training (IMT) therapy.
Methods
A total of 100 COPD patients were enrolled and randomly divided into oxygen therapy (OT), NIPPV, IMT and sequential (NIPPV + IMT) group. Lung function, exercise endurance, quality of life, and dyspnea symptoms were examined and recorded. Then, reactive oxygen species (ROS), malonaldehyde (MDA), superoxide dismutase (SOD) and glutathione (GSH) levels were detected by enzyme-linked immunoassay, and suppressor of cytokine signaling 5 (SOCS5)/janus kinase 2 (JAK2)/signal transducer and activator of transcription 3 (STAT3) pathway expression changes were detected by quantitative real time-polymerase chain reaction (qRT-PCR) and western blot. A mouse model of COPD was then established to further verify the effects of SOCS5/JAK2/STAT3 pathways on lung function and oxidative stress.
Results
After 8 weeks of treatment, NIPPV, IMT or sequential (NIPPV + IMT) significantly improved exercise endurance, quality of life and dyspnea, reduced oxidative stress, promoted SOCS5 expression and inhibited the activation of JAK2/STAT3 pathway, and no significant effect was observed on lung function of COPD patients. Notably, sequential (NIPPV + IMT) showed better therapeutic outcomes than either IMT or NIPPV alone. Moreover, results at the animal level showed that overexpression of SOCS5 significantly reduced pulmonary inflammatory infiltration, pathological changes and oxidative stress levels in COPD mice, enhanced lung function, and inhibited the activation of JAK2/STAT3 pathway.
Conclusion
Our results elucidated that sequential (NIPPV + IMT) significantly relieved COPD development by regulating SOCS5/JAK2/STAT3 signaling-mediated oxidative stress.
Similar content being viewed by others
Introduction
Chronic obstructive pulmonary disease (COPD) is a common progressive respiratory disease characterized by persistent airflow restriction, and is a significant cause of disease death worldwide [1]. The latest epidemiological survey in China shows that the prevalence of COPD among people over 40 years old increased from 8.2% to 2008 to 13.7% in 2015, which is also related to the aging population [2]. Risk factors such as smoking, environmental pollution, occupational dust and chemical exposure, infection, and genetic factors can cause immune and inflammatory disorders, redox imbalances, and airway and vascular remodeling, leading to COPD occurrence and deterioration [3, 4]. However, the exactly pathogenesis is still not fully understood. Thus, it is of great significance to elucidate the pathogenesis of COPD for searching new effective therapeutic approaches.
Pulmonary rehabilitation is an individualized treatment strategy, which aims to improve the physical and psychological conditions of COPD patients and promote their long-term healthy behaviors through exercise training, self-control management and behavioral intervention [5]. Due to early airway obstruction, heavy respiratory load and low respiratory efficiency, respiratory work increases and respiratory muscle strength decreases, which leads to respiratory muscle dysfunction, resulting in dyspnea and decreased exercise tolerance in COPD patients [6, 7]. Although the current intervention of respiratory muscle function rehabilitation such as inspiratory muscle training (IMT) and calisthenics can partially improve the health status of COPD patients [8, 9]. However, training for specific respiratory muscle groups is rare. Noninvasive positive pressure ventilation (NIPPV) is a controversial form of therapy for COPD patients [10]. Clinical study showed that NIPPV could improve the survival in hypercapnia, stable COPD patients [11]. However, there was few COPD patients who receive acute or continuous NIPPV are at potential risk for serious complications such as pulmonary barotrauma and bleeding [12, 13]. On this basis, we proposed for the first time the rehabilitation strategy of sequential NIPPV + IMT that make the inspiratory muscles get a full rest after a certain intensity load exercise. However, the clinical efficacy of this strategy are still unclear.
The imbalance of oxidation/antioxidant system is one of driving mechanism in pathogenesis of COPD. For decades, there were a great number of reports confirmed that enhancing endogenous antioxidant stress is an effective strategy for treating COPD [14, 15]. Suppressor of cytokine signaling 5 (SOCS5) is a main member of SOCS family, which are known to be cytokine-inducible negative regulators of cytokine signaling, also called as signal transducer and activator of transcription (STAT)-induced STAT inhibitor (SSI) protein family. A recent finding revealed that the maintain of SOCS5 could alleviate lipopolysaccharide-induced oxidative stress and the release of pro-cytokine factors to attenuate myocardial injury [16]. More importantly, Diao et al’s study also confirmed that downregulation of SOCS5 targeted by miR-132 could aggravate the inflammatory responses in COPD pathophysiology [17]. Janus kinase 2 (JAK2)/STAT3 signaling as the main intracellular signaling chain that is a key downstream pathway of SOCS5, plays an important role in cell proliferation, apoptosis, oxidative stress, and immune response [18, 19]. For example, Piao et al’s report revealed that inhibition of IL-6/JAK2/STAT3 signaling could efficiency alleviate inflammatory responses and oxidative stress, thereby relieving pancreatitis-induced lung injury [20]. Therefore, it could be considered that SOCS5 might mediate JAK2/STAT3 pathway to regulate imbalance of oxidation/antioxidant processes in COPD.
In the present work, we aimed to explore the clinical therapeutic effect of sequential NIPPV + IMT in COPD patients and its association with SOCS5/JAK2/STAT3 signaling pathway, as well as the biological roles of SOCS5/JAK2/STAT3 signaling in oxidative stress damage in COPD mice model. Thus, we proposed a hypothesis that sequential NIPPV + IMT might improve exercise tolerance, quality of life, dyspnea and oxidative stress in COPD patients by regulating SOCS5/JAK2/STAT3 signaling pathway.
Methods
Clinical subject information and ethical statements
All clinical trials have been approved by The First Affiliated Hospital of Hunan Normal University (Hunan Provincial People’s Hospital, 2023 No. 180), and all subjects have signed the statement of interest before participating. In the present work, a total of 100 patients with COPD were included in this study, all of whom were diagnosed after medical history, physical examination, pulmonary function examination and X-ray examination according to the COPD diagnostic criteria specified by GOLD. All patients received conventional treatment and did not need to stop any of the conventional treatment drugs before examination.
The inclusion criteria were: (1) age range from 40 to 80 years old; (2) severe (30% pre ≤ FEV1% < 50% pre) or extremely severe (FEV1% < 30% pre or FEV1% < 50% pre with chronic respiratory failure) COPD patients; (3) difficulty breathing; (4) there was no acute exacerbation in the last 4 weeks. The exclusion criteria were as follows: (1) those who smoked more than 10 cigarettes a day; (2) BMI > 40 kg/m2; (3) patients with cardiac hemodynamic instability; (4) patients with other respiratory diseases, neuromuscular diseases and serious cerebrovascular accident sequelae.
Observation was terminated if the following conditions occurred: (1) the patient died; (2) refuse to participate in the IMT or NIPPV therapy or oxygen therapy (OT) in this study; (3) occur pneumothorax, the patient’s ability to clear secretions from oropharynx and upper respiratory tract is decreased, the patient can not normally use the nose mask, can no longer tolerate non-invasive ventilation, acute cardiovascular and cerebrovascular events, severe liver insufficiency, severe renal insufficiency, newly diagnosed malignant tumor or mental abnormality.
Experimental grouping and treatment
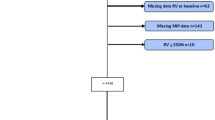
All subjects were randomly divided into 4 groups in equal proportions using a random number generator: (1) OT group (male 21, female 4), NIPPV group (male 23, female 2), IMT group (male 20, female 5) and NIPPV + IMT (male 21, female 4). Randomization was performed using opaque sealed envelopes that were opened during screening visits. All subjects received OT for at least 8 h per day with SPO2 > 90% and oxygen flow < 6 L/min. When NIPPV was treated, the initial EPAP was set at 4 cm H2O, and IPAP was gradually adjusted until the patient felt most comfortable and IPAP-EPAP was > 10 cm H2O. Oxygen absorption concentration (FiO2) to maintain blood oxygen saturation ≥ 90%, oxygen flow 4 ~ 6 L/min. The standby ventilation frequency is set to 14 times per min, and the suction rise time is set to 0.5-2 s. The duration of treatment was not less than 4 h in the daytime and continued at night. When IMT treatment, the patient was given a Power breath K5 inspiratory muscle trainer (Powerbreath Technologies Ltd, Birmingham, UK). The training intensity started at 40% PImax for 1 week, and then gradually increased by 10% until it reached 80% PImax. Exercise for 30 min twice a day. In NIPPT + IMT group, patients received IMT training for 30 min/session and followed by NIPPV for 2 h, twice a day. All patients were treated for 8 weeks. The flow chart was provided as Fig. 1.
Lung function examination
The lung function of the patients was measured using the Jaeger pulmonary function instrument Spi rostik CompTete (Jaeger, Germany), according to the guidelines formulated by the European Respiratory Society (ERS) and the American Thoracic Society (ATS) [21].
Exercise tolerance test
The exercise tolerance was measured by the 6-min walking test (6MWT) according to ATS guidelines for the Application of 6MWT [22]. In short, COPD patients walked from the starting point to the stopping point at the end of the flat indoor corridor with a reentry length of 30 m, the distance was measured (Supplementary Table 1). Recording the basic pulse, blood pressure, heart rate and pulse oxygen saturation at the before and after the test. Borg score was applied to assess basic dyspnea and fatigue index (Supplementary Table 2).
Score of health-related quality
The severe respiratory insufficiency (SRI) questionnaire developed and compiled by Professor Windisch’s team was used to evaluate the quality of life of the patients in this study [23]. The scale is completed by the subject independently, and the investigator is not allowed to make any suggestive reminders. The SRI scale consists of seven parts: physical functioning (SRI-PF), respiratory complaints (SRI-RC), attendant symptoms and sleep (SRI-AS), social relationships (SRI-SR), social functioning (SRI-SF), anxiety (SRI-AX) and psychological well-being (SRI-WB). After the survey, the score of each part and the total score of the whole questionnaire were calculated by the investigator through conversion and weighting. The score ranges from 0 to 100, and the score was positively correlated with the health status of patients.
Dyspnea score
Modified medical research council (MRC) scale was used to evaluate the degree of dyspnea of patients [24](Table 1), in which 0 was mild. A score of 1 is moderate; A score of 2–4 is considered severe.
Oxidative stress detection
By referencing the previous report, 5 mL blood samples were taken by fasting venipuncture into the EDTA vacuum collection vessel under sterile conditions [25]. After centrifugation, the serum sample was collected to examine the levels of reactive oxygen species (ROS, ab113851, Abcam, Cambridge, MA, USA), malondialdehyde (MDA, ab118970, Abcam) and activities of superoxide dismutase (SOD, ab80946, Abcam) and glutathione (GSH, ab239727, Abcam) according to the kit instructions.
Western blot
The experimental procedure was performed by previous described [26]. The proteins were isolated with RIPA and the cut gels were transferred to the PVDF membrane (Millipore, MA, USA). Then, membranes were incubated overnight with antibodies against SOCS5 (PA5-21600, Thermo Fisher Scientific), JAK2 (ab108596, Abcam), p-JAK2 (ab32101, Abcam), STAT3 (ab119352, Abcam), p-STAT3 (ab32143, Abcam), and GAPDH (ab8245, Abcam). After washed with TBST, membranes were then incubated with secondary antibody (ab7090, Abcam) for 60 min. The membranes were visualized and imaged by GEL imaging system (Bio-Rad, CA, USA). The bands were quantified by Image J (National Institutes of Health, MD, USA).
Establishment of COPD mouse model
All animal experiments were performed in accordance with the animal ethics approval of The First Affiliated Hospital of Hunan Normal University (Hunan Provincial People’s Hospital, 2021 No. 43). C57BL/6 male mice (6–8 weeks) were purchased from SLAC Laboratory (Shanghai, China) and kept in an animal facility at 21 ~ 24 °C for 12/12 h with light and dark cycles and free access to water and food. Mice were randomly divided into Air + pc-NC group, Cigarette smoke (CS) + pc-NC group and CS + pc-SOCS5 group (n = 6/per group). In brief, mice were exposed to room air or CS of five cigarettes (Furongwang, HUNAN Tobacco Industrial Co., Ltd. China) four times each day for 30 min/time, 5 days/weeks for up to 24 weeks. The lentivirus-packed SOCS5 overexpressing vector (pc-SOCS5) and empty vector (pc-NC) assembled by GenePharma (Shanghai, China) were intranasally injected into the mice once every 2 weeks (2 × 107 TU in 50 µL). The lung function of mice in each group were examined as the previous described [26]. After last CS/air exposure, the mice were euthanized by intraperitoneal injection of an overdose of pentobarbital sodium (800 mg/kg) on another day and pathological analysis was performed.
H&E staining
As previous described [26], H&E staining was conducted to assess pathologic changes. The lung tissues of each group were collected and fixed with 4% buffer formalin solution and embedded in paraffin and then cut into 4 μm thick slices. Then, the slices were subjected to H&E staining by using H&E staining kit (Beyotime, China) according to instruction protocol. Finally, the slices were photographed under an optical microscope (Olympus, China).
Statistical analysis
Statistical data was analyzed by SPSS 19.0 (IBM, NY, USA) and expressed as means ± SD. The differences between-group differences and multi-group comparisons were determined using Student’s t test and one-way ANOVA followed by Tukey’s post hoc tset, respectively. The p values less than 0.05 were considered significant.
Results
Effect of sequential NIPPV + IMT intervention on pulmonary function in patients with COPD
As shown in Table 2, patients in each group had no significant differences in age, BMI, lung function, respiratory muscle strength, blood gas analysis, exercise tolerance, dyspnea and quality of life scores. After 8 weeks of treatment, lung function was measured in each group. Compared with before intervention, IMT, NIPPV and sequential NIPPV + IMT had no significant effect on various pulmonary function indexes FVC, FEV1 and FEV1/FVC (Fig. 2A-C). These data indicated that sequential NIPPV + IMT intervention had no significant effect on pulmonary function in COPD patients.
Effect of sequential NIPPV + IMT intervention on pulmonary function in patients with COPD. A total of 100 COPD patients were enrolled and randomly divided into OT group, NIPPV group, IMT group and NIPPV + IMT group. After 8 weeks of treatment, the lung function related indexes including FVC(A), FEV1(B) and FEV1/FVC(C) were measured by Jaeger pulmonary function instrument. All indices were determined at least three times
Sequential NIPPV + IMT intervention enhanced the exercise tolerance of COPD patients
After 8 weeks of treatment, exercise tolerance was measured using the 6MWT method. As shown in Fig. 3, compared with before intervention, 6-min walking distance (6MWD) in NIPPV, IMT and sequential NIPPV + IMT groups was significantly increased. Moreover, sequential NIPPV + IMT treatment was significantly higher than that of IMT or NIPPV treatment (Fig. 3).
Sequential NIPPV + IMT intervention enhanced the exercise tolerance of COPD patients. A total of 100 COPD patients were enrolled and randomly divided into OT group, NIPPV group, IMT group and NIPPV + IMT group. After 8 weeks of treatment, 6MWT method was used to examine the exercise tolerance. All indices were determined at least three times. *P < 0.05
Sequential NIPPV + IMT intervention significantly improved the quality of life in COPD patients
Compared to OT group, IMT, NIPPV and sequential NIPPV + IMT treatment remarkably enhanced the score of health-related quality (Fig. 4). In addition, compared to IMT and NIPPV groups, COPD patients in sequential NIPPV + IMT group had significantly better quality of life (Fig. 4).
Sequential NIPPV + IMT intervention significantly improved the quality of life in COPD patients. A total of 100 COPD patients were enrolled and randomly divided into OT group, NIPPV group, IMT group and NIPPV + IMT group. After 8 weeks of treatment, SRI questionnaire was carried out to assess the quality of life of COPD patients. *P < 0.05, **P < 0.01
Sequential NIPPV + IMT treatment markedly alleviated dyspnea COPD patients
The efficacy of sequential NIPPV + IMT treatment for dyspnea in patients with COPD were evaluated by MRC scale. After 8 weeks of treatment, the MRC score was strikingly reduced in patients in NIPPV, IMT and sequential NIPPV + IMT groups compared that in OT group (Fig. 5). Importantly, the MRC score in patients of sequential NIPPV + IMT group were also lower than that in patients of NIPPV and IMT groups (Fig. 5).
Sequential NIPPV + IMT treatment markedly alleviated dyspnea COPD patients. A total of 100 COPD patients were enrolled and randomly divided into OT group, NIPPV group, IMT group and NIPPV + IMT group. After 8 weeks of treatment, dyspnea in patients with COPD were evaluated by MRC scale. *P < 0.05, **P < 0.01, ***P < 0.001
Sequential NIPPV + IMT treatment relived the oxidative stress of COPD patients
By ELISA assay, we could observe that the ROS and MDA levels in patients of NIPPV, IMT and sequential NIPPV + IMT groups were obviously reduced, but the activities of SOD and GSH were upregulated, compared those in patients in OT group (Fig. 6A-D), suggesting NIPPV, IMT and sequential NIPPV + IMT treatment could alleviate the oxidative stress of COPD patients. Notably, the oxidation inhibition effect of sequential NIPPV + IMT treatment was significantly stronger than that of IMT or NIPPV treatment alone (Fig. 6A-D).
Sequential NIPPV + IMT treatment relived the oxidative stress of COPD patients. The serum samples were obtained from COPD patients in each groups, then the levels of ROS (A) and MDA (B) as well as the activities of SOD (C) and GSH (D) were tested by ELISA assay. All indices were determined at least three times. *P < 0.05, **P < 0.01, ***P < 0.001
Effects of sequential NIPPV + IMT treatment on SOCS5/JAK2/STAT3 signaling pathway
The results from western blot assay showed that the protein level of SOCS5 was upregulated, but the phosphorylation of JAK2 and STAT3 were reduced after NIPPV, IMT or sequential NIPPV + IMT treatment (Fig. 7A-D). Moreover, sequential NIPPV + IMT treatment showed a better intervention effect on the SOCS5/JAK2/STAT3 pathway than NIPPV or IMT treatment (Fig. 7A-D).
Effects of sequential NIPPV + IMT treatment on SOCS5/JAK2/STAT3 signaling pathway. (A-D) The protein levels of SOCS5, p-JAK2/JAK2, p-STAT3/STAT3 in serum samples of COPD patients were determined by western blot assay (The result shows the cut gel). All examinations were determined at least three times. *P < 0.05, **P < 0.01, ***P < 0.001
SOCS5 suppressed COPD development by inactivating JAK2/STAT3 signaling
To further confirm the biological roles of SOCS5/JAK2/STAT3 signaling in COPD, we established COPD mouse model. H&E staining showed that the pulmonary parenchymal inflammatory cell infiltration, small airway thickening and alveolar cavity collapse were increased in the CS + pc-NC group compared to Air + pc-NC group, while this effect was alleviated by SOCS5 overexpression (Fig. 8A). Lung function measurement results showed that compared with the Air + pc-NC group, CS exposure markedly destroyed lung function, which was represented by increased functional residual capacity, total lung capacity and resistance index, and decreased FEV0.1/FVC. However, these effects were partially reversed after overexpression of SOCS5 (Fig. 8B-E). Next, the oxidative stress of mice were further examined. As shown in Fig. 8F-I, CS exposure significantly increased ROS and MDA level as well as downregualted the activities of SOD and GSH, and all of these effects were dramatically diminished by SOCS5 overexpression. Western blot analysis also presented that compared to Air + pc-NC group, the protein level of SOCS5 was reduced and the phosphorylation of JAK2 and STAT3 were enhanced in lung tissues of mice in CS + pc-NC group, and these protein levels were remarkably reversed by SOCS5 overexpression (Fig. 8J). Therefore, these results showed that SOCS5 improved COPD development by inactivating JAK2/STAT3 signaling pathway.
SOCS5 suppressed COPD development by inactivating JAK2/STAT3 signaling. C57BL/6 male mice were randomly divided into Air + pc-NC group, CS + pc-NC group and CS + pc-SOCS5 group (n = 6/per group). (A) H&E staining was carried out to assess the pathological changes. (B-E) Lung function index including Function residual capacity (B), Total lung capacity (C), Resistance index (D) and FEV0.1/FVC (E) were examined. (F-I) The levels of ROS (F), MDA (G), SOD (H) and GSH (I) were tested by ELISA assays. (J) The protein levels of SOCS5, p-JAK2/JAK2, p-STAT3/STAT3 were determined by western blot assay (The result shows the cut gel). *P < 0.05, **P < 0.01, ***P < 0.001
Discussion
COPD is the most common chronic respiratory disease, with a high prevalence rate, high disability rate, high mortality characteristics, a serious threat to human health. According to a statistical report, the number of COPD patients worldwide reached 544.9 million in 2017, an increase of 39.8% since 1990 [27]. To data, there is still no effective treatment for COPD in clinic. With the continuous deterioration of COPD patients, it often involves multiple systems, resulting in respiratory muscle, limb muscle dysfunction and malnutrition, so that the emergence of year by year increased dyspnea, exercise tolerance and quality of life decreased, causing a great burden to society [28, 29]. Therefore, it is important to seek effective strategies to treat COPD and elucidate its pathways of action.
Currently, glucocorticoids, as the most common used anti-inflammatory drugs, are widely used in the treatment of acute COPD exacerbation and stable stage [30]. Although glucocorticoid therapy can improve lung function and reduce acute exacerbations, long-term use of glucocorticoid therapy does not prevent the decline of lung function, seriously affecting the quality of life of patients [31, 32]. In recent years, studies have shown that patients with different degrees of COPD can benefit from pulmonary Rehabilitation and exercise Training, including improved activity tolerance, reduced dyspnea and improved quality of life [33, 34]. For the majority of COPD patients, IMT is an effective stand-alone intervention that can be transitioned to aerobic and resistance training as part of a multidimensional respiratory rehabilitation training program for patients with dyspnea and exercise intolerance, as well as for patients who avoid activity due to fear of motor dyspnea [35]. Studies have shown that IMT therapy can increase type I and type II fibers of intercostal external muscles, induce remodeling of training muscles, and change the adaptive structure of respiratory muscles [8]. Beaumont et al. also uncovered that IMT effectively improves the strength and endurance of the inspiratory muscles, reduces breathing difficulties in activities of daily living, and improves walking distance and quality of life [33]. In addition, NIPPV was confirmed to significantly reduce the rate of endotracheal intubation, shorten the length of hospital stay, and avoid complications of invasive ventilation such as airway injury and ventilator-associated pneumonia [36]. Tang et al. highlighted that long term NIPPV treatment improved arterial blood gas, enhanced exercise endurance and quality of life, and reduced mortality in patients with COPD [37]. Similar results also found in Wiles et al’s work [10]. However, there is still some controversy about NIPPV’s role in COPD [38]. The failure of NIPPV treatment leads to the delay of endotracheal intubation time and treatment time, which increases the mortality of patients, which might relate to high CRP concentration, serum albumin, combined renal insufficiency, vomit aspiration [39,40,41,42]. Based on the above research status, we proposed for the first time a novel therapy with sequential NIPPV + IMT. Compared with IMT and NIPPV alone, sequential NIPPV + IMT treatment increased exercise endurance, improved quality of life and dyspnea symptoms in patients, and this process was associated with reduced levels of oxidative stress.
Oxidative stress directly damages airway and lung tissues, leads to protease/antiprotease imbalance, promotes inflammation, and plays an extremely important role in the occurrence and development of COPD [15]. Enhancing the antioxidant capacity of COPD patients and reducing oxidative stress damage are also considered to be the key to improve the progression of COPD [43]. Wang et al. revealed that aerobic exercise efficiency relieved emphysema and pulmonary fibrosis of COPD mice by suppressing oxidative stress and inflammation [44]. A pulmonary rehabilitation study showed that patients with COPD significantly reduced the oxidative stress capacity of exercise after 8 weeks of pulmonary rehabilitation training [45]. In our study, we demonstrated for the first time that both IMT and NIPPV treatment significantly reduced the levels of MDA and ROS in the serum of COPD patients, enhanced the activities of SOD and GSH, and significantly enhanced the antioxidant capacity of COPD patients. Importantly, sequential NIPPV + IMT treatment showed better therapeutic efficacy, suggesting that sequential NIPPV + IMT may be a novel way to improve COPD progression by intervening in oxidative stress.
Additionally, we also observed increased SOCS5 expression and inactivation of the JAK2/STAT3 pathway after IMT, NIPPV and sequential NIPPV + IMT therapy, suggesting that the SOCS5/JAK2/STAT3 signaling pathway might be involved in its mediated changes in oxidative stress levels and disease progression in COPD patients. SOCS family proteins are a class of negative regulators of JAK2-STAT3 signaling pathway, consisting of SOCS1-7, both containing Src-homology domian and a conserved C-termial domain [46]. Among them, SOCS5 is expressed in a variety of adult tissues, playing anti-inflammatory, anti-tumor, anti-oxidative stress and other functions [16, 47, 48]. Xi et al. uncovered that salidroside could promote SOCS5 transcription to reduce airway inflammation and airway remodeling in asthmatic mice [48]. Tsai et al. also proved that SOCS5 was highly expressed in high glucose-induced renal mesangial cells, which led to the inactivation of JAK2/STAT3 pathway [49]. Wang el al also reported that inhibition of SOCS5 led to the apoptosis and inflammation in lung epithelial cells by activation of JAK2/STAT3 pathway [50]. It is well known that the JAK2/STAT3 pathway, as a key intracellular signaling pathway, has also been proved to be involved in the progression of COPD disease through inflammation, oxidative stress and apoptosis [51, 52]. A recent study showed that inhibiting SOCS5 significantly promoted cigarette extract-induced inflammatory responses, exacerbating COPD progression [17]. It also shown that maintaining SOCS5 expression could reduce LPS-induced oxidative stress and inflammation and improve myocardial injury [16]. Here, we established a COPD mouse model, whose results further verified that overexpression of SOCS5 inhibited JAK2/STAT3 pathway activation, improved lung function and reduced oxidative stress in COPD mice. The above reports further confirm our conjecture that SOCS5/JAK2/STAT3 signaling may be involved in the occurrence and development of COPD by influencing oxidative stress levels. Besides, the alteration of SOCS5 in diseases was confirmed to be related various regulation such as miRNAs sponge, DNA methylation, m6A modification and transcription regulation [46, 48, 53], and these regulatory pathways were acted an important role in COPD pathogenesis [54,55,56]. Thus, it was implied that the exact mechanism of abnormally expressed SOCS5 in COPD was worthy of research.
Conclusions
In conclusion, our results suggest that sequential NIPPV + IMT treatment alleviated dyspnea and improved exercise tolerance and quality of life in COPD patients, which might be related to reduced levels of oxidative stress mediated by SOCS5/JAK2/STAT3 signaling pathway. Our proposed a new and more effective therapeutic strategy for COPD patients and elucidated its potential pathways of action, providing a direction for the clinical treatment of patients with COPD.
Data Availability
All data generated or analyzed during this study are included in this published article.
Abbreviations
- COPD:
-
Chronic obstructive pulmonary disease
- NIPPV:
-
Noninvasive positive pressure ventilation
- IMT:
-
Inspiratory muscle training
- OT:
-
Oxygen therapy
- SOCS5:
-
Suppressor of cytokine signaling 5
- STAT:
-
Signal transducer and activator of transcription
- SSI:
-
STAT inhibitor
- JAK2:
-
Janus kinase 2
- 6MWT:
-
6-minute walking test
- ATS:
-
American Thoracic Society
- SRI:
-
Severe respiratory insufficiency
- ROS:
-
Reactive oxygen species
- MDA:
-
Malondialdehyde
- SOD:
-
Superoxide dismutase
- GSH:
-
Glutathione
- qRT-PCR:
-
Quantitative real-time polymerase chain reaction
References
Vestbo J, Hurd SS, Agusti AG, Jones PW, Vogelmeier C, Anzueto A, Barnes PJ, Fabbri LM, Martinez FJ, Nishimura M, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med. 2013;187(4):347–65.
Wang C, Xu J, Yang L, Xu Y, Zhang X, Bai C, Kang J, Ran P, Shen H, Wen F, et al. Prevalence and risk factors of chronic obstructive pulmonary disease in China (the China Pulmonary Health [CPH] study): a national cross-sectional study. Lancet. 2018;391(10131):1706–17.
Huang X, Mu X, Deng L, Fu A, Pu E, Tang T, Kong X. The etiologic origins for chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis. 2019;14:1139–58.
Ritchie AI, Wedzicha JA. Definition, causes, Pathogenesis, and Consequences of Chronic Obstructive Pulmonary Disease Exacerbations. Clin Chest Med. 2020;41(3):421–38.
Spruit MA, Singh SJ, Garvey C, ZuWallack R, Nici L, Rochester C, Hill K, Holland AE, Lareau SC, Man WD, et al. An official american thoracic Society/European respiratory society statement: key concepts and advances in pulmonary rehabilitation. Am J Respir Crit Care Med. 2013;188(8):e13–64.
Geltser BI, Kurpatov IG, Dej AA, Kozhanov AG. Respiratory muscles dysfunction and respiratory diseases. Ter Arkh. 2019;91(3):93–100.
Barreiro E, Gea J. Respiratory and Limb Muscle Dysfunction in COPD. COPD 2015, 12(4):413–426.
Figueiredo RIN, Azambuja AM, Cureau FV, Sbruzzi G. Inspiratory muscle training in COPD. Respir Care. 2020;65(8):1189–201.
Basso-Vanelli RP, Di Lorenzo VA, Labadessa IG, Regueiro EM, Jamami M, Gomes EL, Costa D. Effects of Inspiratory muscle training and calisthenics-and-breathing exercises in COPD with and without respiratory muscle weakness. Respir Care. 2016;61(1):50–60.
Wiles SP, Aboussouan LS, Mireles-Cabodevila E. Noninvasive positive pressure ventilation in stable patients with COPD. Curr Opin Pulm Med. 2020;26(2):175–85.
Kohnlein T, Windisch W, Kohler D, Drabik A, Geiseler J, Hartl S, Karg O, Laier-Groeneveld G, Nava S, Schonhofer B, et al. Non-invasive positive pressure ventilation for the treatment of severe stable chronic obstructive pulmonary disease: a prospective, multicentre, randomised, controlled clinical trial. Lancet Respir Med. 2014;2(9):698–705.
Raghavan R, Ellis AK, Wobeser W, Sutherland KB, O’Donnell DE. Hemopneumothorax in a COPD patient treated with noninvasive positive pressure ventilation: the risk of attendant anticoagulation. Can Respir J. 2004;11(2):159–62.
Yamanoglu A, Bilgin S, Celebi Yamanoglu NG, Topal FE. A rare complication of continuous positive airway pressure treatment - rectus sheath hematoma: a case report. Braz J Anesthesiol. 2021;71(4):461–3.
Barnes PJ. Oxidative stress-based therapeutics in COPD. Redox Biol. 2020;33:101544.
Wiegman CH, Li F, Ryffel B, Togbe D, Chung KF. Oxidative stress in Ozone-Induced chronic lung inflammation and Emphysema: a facet of Chronic Obstructive Pulmonary Disease. Front Immunol. 2020;11:1957.
Yu M, Xie D, Hu CY, Cui Y. LncRNA small nucleolar RNA host gene 16 reduces sepsis-induced myocardial damage by regulating miR-421/suppressor of cytokine signaling 5 axis. Kaohsiung J Med Sci. 2022;38(6):517–29.
Diao X, Zhou J, Wang S, Ma X. Upregulation of miR-132 contributes to the pathophysiology of COPD via targeting SOCS5. Exp Mol Pathol. 2018;105(3):285–92.
Fu Y, Xu Y, Chen S, Ouyang Y, Sun G. MiR-151a-3p promotes postmenopausal osteoporosis by targeting SOCS5 and activating JAK2/STAT3 signaling. Rejuvenation Res. 2020;23(4):313–23.
Sun Y, Cheng M, Liang X, Chen S, Wang M, Zhang X. JAK2/STAT3 involves oxidative stress-induced cell injury in N2a cells and a rat MCAO model. Int J Neurosci. 2020;130(11):1142–50.
Piao X, Zou Y, Sui X, Liu B, Meng F, Li S, Zhang Q, Ma C, Wu T. Hydrostatin-SN10 ameliorates Pancreatitis-Induced Lung Injury by affecting IL-6-Induced JAK2/STAT3-Associated inflammation and oxidative stress. Oxid Med Cell Longev. 2019;2019:9659757.
Stanojevic S, Kaminsky DA, Miller MR, Thompson B, Aliverti A, Barjaktarevic I, Cooper BG, Culver B, Derom E, Hall GL et al. ERS/ATS technical standard on interpretive strategies for routine lung function tests. Eur Respir J 2022, 60(1).
Laboratories, ATSCoPSfCPF. ATS statement: guidelines for the six-minute walk test. Am J Respir Crit Care Med. 2002;166(1):111–7.
Kotanen P, Kainu A, Brander P, Bergman P, Lehtomaki A, Kreivi HR. Validation of the finnish severe respiratory insufficiency questionnaire. Clin Respir J. 2020;14(7):659–66.
Pisi R, Aiello M, Calzetta L, Frizzelli A, Tzani P, Bertorelli G, Chetta A. The COPD assessment test and the modified Medical Research Council scale are not equivalent when related to the maximal exercise capacity in COPD patients. Pulmonology. 2023;29(3):194–9.
Ismail M, Hossain MF, Tanu AR, Shekhar HU. Effect of spirulina intervention on oxidative stress, antioxidant status, and lipid profile in chronic obstructive pulmonary disease patients. Biomed Res Int. 2015;2015:486120.
Wu Y, Guan S, Ge Y, Yang Y, Cao Y, Zhou J. Cigarette smoke promotes chronic obstructive pulmonary disease (COPD) through the miR-130a/Wnt1 axis. Toxicol in Vitro. 2020;65:104770.
Collaborators GBDCRD. Prevalence and attributable health burden of chronic respiratory diseases, 1990–2017: a systematic analysis for the global burden of Disease Study 2017. Lancet Respir Med. 2020;8(6):585–96.
Laaban JP, Kouchakji B, Dore MF, Orvoen-Frija E, David P, Rochemaure J. Nutritional status of patients with chronic obstructive pulmonary disease and acute respiratory failure. Chest. 1993;103(5):1362–8.
Belfer MH, Reardon JZ. Improving exercise tolerance and quality of life in patients with chronic obstructive pulmonary disease. J Am Osteopath Assoc. 2009;109(5):268–78. quiz 280 – 261.
Hasan SS, Capstick T, Zaidi STR, Kow CS, Merchant HA. Use of corticosteroids in asthma and COPD patients with or without COVID-19. Respir Med. 2020;170:106045.
Calverley PM, Anderson JA, Celli B, Ferguson GT, Jenkins C, Jones PW, Yates JC, Vestbo J, investigators T. Salmeterol and fluticasone propionate and survival in chronic obstructive pulmonary disease. N Engl J Med. 2007;356(8):775–89.
Magnussen H, Disse B, Rodriguez-Roisin R, Kirsten A, Watz H, Tetzlaff K, Towse L, Finnigan H, Dahl R, Decramer M, et al. Withdrawal of inhaled glucocorticoids and exacerbations of COPD. N Engl J Med. 2014;371(14):1285–94.
Beaumont M, Forget P, Couturaud F, Reychler G. Effects of inspiratory muscle training in COPD patients: a systematic review and meta-analysis. Clin Respir J. 2018;12(7):2178–88.
Cornelison SD, Pascual RM. Pulmonary Rehabilitation in the management of chronic lung disease. Med Clin North Am. 2019;103(3):577–84.
Beaumont M, Le Tallec F, Villiot-Danger E. [Inspiratory muscle training during pulmonary rehabilitation]. Rev Mal Respir. 2021;38(7):754–67.
Osadnik CR, Tee VS, Carson-Chahhoud KV, Picot J, Wedzicha JA, Smith BJ. Non-invasive ventilation for the management of acute hypercapnic respiratory failure due to exacerbation of chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2017;7:CD004104.
Tang Q, Qin G. [Therapeutic effect of long-term noninvasive positive pressure ventilation on stable chronic obstructive pulmonary disease: a systematic review]. Zhong Nan Da Xue Xue Bao Yi Xue Ban. 2016;41(3):319–27.
Altintas N. Update: non-invasive positive pressure ventilation in chronic respiratory failure due to COPD. COPD. 2016;13(1):110–21.
Fan L, Zhao Q, Liu Y, Zhou L, Duan J. Semiquantitative cough strength score and associated outcomes in noninvasive positive pressure ventilation patients with acute exacerbation of chronic obstructive pulmonary disease. Respir Med. 2014;108(12):1801–7.
Pacilli AM, Valentini I, Carbonara P, Marchetti A, Nava S. Determinants of noninvasive ventilation outcomes during an episode of acute hypercapnic respiratory failure in chronic obstructive pulmonary disease: the effects of comorbidities and causes of respiratory failure. Biomed Res Int. 2014;2014:976783.
Miniati M, Monti S, Bottai M, Cocci F, Fornai E, Lubrano V. Prognostic value of C-reactive protein in chronic obstructive pulmonary disease. Intern Emerg Med. 2011;6(5):423–30.
Ciledag A, Kaya A, Ercen Diken O, Onen ZP, Sen E, Demir N. The risk factors for late failure of non-invasive mechanical ventilation in acute hypercapnic respiratory failure. Tuberk Toraks. 2014;62(3):177–82.
Xu Y, Liu H, Song L. Novel drug delivery systems targeting oxidative stress in chronic obstructive pulmonary disease: a review. J Nanobiotechnol. 2020;18(1):145.
Wang X, Wang Z, Tang D. Aerobic Exercise alleviates inflammation, oxidative stress, and apoptosis in mice with chronic obstructive Pulmonary Disease. Int J Chron Obstruct Pulmon Dis. 2021;16:1369–79.
Mercken EM, Hageman GJ, Schols AM, Akkermans MA, Bast A, Wouters EF. Rehabilitation decreases exercise-induced oxidative stress in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2005;172(8):994–1001.
Sharma ND, Nickl CK, Kang H, Ornatowski W, Brown R, Ness SA, Loh ML, Mullighan CG, Winter SS, Hunger SP, et al. Epigenetic silencing of SOCS5 potentiates JAK-STAT signaling and progression of T-cell acute lymphoblastic leukemia. Cancer Sci. 2019;110(6):1931–46.
Wei YQ, Jiao XL, Zhang SY, Xu Y, Li S, Kong BH. MiR-9-5p could promote angiogenesis and radiosensitivity in cervical cancer by targeting SOCS5. Eur Rev Med Pharmacol Sci. 2019;23(17):7314–26.
Ming X, Yu X, Li J, Wang J, Zheng J, Xiong L. Salidroside attenuates Airway inflammation and remodeling via the miR-323-3p/SOCS5 Axis in Asthmatic mice. Int Arch Allergy Immunol. 2022;183(4):424–34.
Tsai YC, Kuo PL, Hung WW, Wu LY, Wu PH, Chang WA, Kuo MC, Hsu YL. Angpt2 induces Mesangial Cell apoptosis through the MicroRNA-33-5p-SOCS5 Loop in Diabetic Nephropathy. Mol Ther Nucleic Acids. 2018;13:543–55.
Wang L, Yuan N, Li Y, Ma Q, Zhou Y, Qiao Z, Li S, Liu C, Zhang L, Yuan M, et al. Stellate ganglion block relieves acute lung injury induced by severe acute pancreatitis via the miR-155-5p/SOCS5/JAK2/STAT3 axis. Eur J Med Res. 2022;27(1):231.
Milara J, Ballester B, de Diego A, Calbet M, Ramis I, Miralpeix M, Cortijo J. The pan-JAK inhibitor LAS194046 reduces neutrophil activation from severe asthma and COPD patients in vitro. Sci Rep. 2022;12(1):5132.
Liu CW, Lee TL, Chen YC, Liang CJ, Wang SH, Lue JH, Tsai JS, Lee SW, Chen SH, Yang YF, et al. PM2.5-induced oxidative stress increases intercellular adhesion molecule-1 expression in lung epithelial cells through the IL-6/AKT/STAT3/NF-kappaB-dependent pathway. Part Fibre Toxicol. 2018;15(1):4.
Song RH, Du P, Gao CQ, Liu XR, Zhang JA. METTL3 is involved in the Development of Graves’ disease by inducing SOCS mRNA m6A modification. Front Endocrinol (Lausanne). 2021;12:666393.
Huang X, Lv D, Yang X, Li M, Zhang H. m6A RNA methylation regulators could contribute to the occurrence of chronic obstructive pulmonary disease. J Cell Mol Med. 2020;24(21):12706–15.
Huo X, Jin S, Wang Y, Ma L. DNA methylation in chronic obstructive pulmonary disease. Epigenomics. 2021;13(14):1145–55.
Li CX, Gao J, Skold CM, Wheelock AM. miRNA-mRNA-protein dysregulated network in COPD in women. Front Genet. 2022;13:1010048.
Acknowledgements
Not applicable.
Funding
This study was supported by the grants from Changsha Science and Technology Plan Project (No. kq2004114) and Scientific Research Project of Education Department of Hunan Province (No. 18C0019).
Author information
Authors and Affiliations
Contributions
Yirou Lei: Study Concepts, Study Design, Literature Research, Clinical Studies, Experimental Studies, Data Acquisition, Data Analysis, Statistical Analysis, Writing-Original draft, Writing-Reviewing. Jiaying He: Clinical Studies, Experimental Studies, Data Acquisition. Fang Hu: Clinical Studies. Hao Zhu: Clinical Studies. Jing Gu: Clinical Studies. Lijuan Tang: Clinical Studies.Man Luo: Definition of Intellectual Content, Literature Research, Clinical Studies, Experimental Studies, Data Acquisition, Manuscript Review.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
All clinical trials have been approved by The First Affiliated Hospital of Hunan Normal University (Hunan Provincial People’s Hospital, 2023 No. 180), and all methods were carried out in accordance with relevant guidelines and regulations. Informed consent has been obtained from all subjects and/or their legal guardian(s). All animal experiments were carried out in accordance with relevant guidelines and regulations, and reported in accordance with ARRIVE guidelines. All experimental protocols were approved by ethics committee/IRB of the First Affiliated Hospital of Hunan Normal University (Hunan Provincial People’s Hospital, 2021 No. 43).
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Lei, Y., He, J., Hu, F. et al. Sequential inspiratory muscle exercise-noninvasive positive pressure ventilation alleviates oxidative stress in COPD by mediating SOCS5/JAK2/STAT3 pathway. BMC Pulm Med 23, 385 (2023). https://doi.org/10.1186/s12890-023-02656-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12890-023-02656-5