Abstract
Background
Some research found that elevated plasma cell-free DNA (cfDNA) concentrations and poor prognosis are associated in non-small cell lung cancer (NSCLC). However, more studies need to be carried out to verify this conclusion. Therefore, this study investigated the relationship between cfDNA concentration and treatment outcomes including prognosis in patients with advanced NSCLC.
Methods
We retrospectively collected medical records and cfDNA data from 160 patients with advanced NSCLC. Progression-free survival (PFS) were calculated using the Kaplan-Meier method and were compared between groups using the log rank test. Cox regression analysis was used for estimating the independent predictors of PFS. And we used logistic regression to evaluate the relationship between baseline biomarkers and efficacy. In our study, BT1 cfDNA, BT2 cfDNA, and BT3 cfDNA were defined as cfDNA concentration before the first treatment (baseline cfDNA concentration), cfDNA concentration before the second treatment, and cfDNA concentration before the third treatment, respectively.
Results
Patients with low cfDNA (BT1 cfDNA < 15 (ng/mL)) were reported a significantly prolonged median progression-free survival (mPFS) compared with patients with patients with high cfDNA (BT1 cfDNA ≥ 15(ng/mL)) (mPFS: 14.6 vs. 8.3 months, P = 0.002), as well as patients with neutrophil/lymphocyte ratio (NLR)<2.98 (mPFS: 13.1 vs. 7.9 months, P = 0.023). In addition, Cox proportional hazards regression analysis identified independent indicators associated with PFS including BT1 cfDNA ≥ 15 (ng/mL), NLR ≥ 2.98 and extrapulmonary metastasis. The best cut-off value for BT3 cfDNA for predicting disease progression is 41.46 (ng/mL) (Area Under the Curve (AUC): 0.652, 95%CI: 0.516–0.788), achieving 90.7% sensitivity and 37.5% specificity for the prediction of disease progression. BT3 cfDNA (OR = 6.08, 95% CI: 1.94–19.57, P = 0.002) was an independent factor for disease progression in patients with advanced NSCLC.
Conclusions
BT1 cfDNA may be a biomarker to assess the prognosis of advanced NSCLC. Patients with advanced NSCLC with lower cfDNA and NLR before treatment had a better prognosis. Increased BT3 cfDNA concentration was an independent factor of disease progression in advanced NSCLC patients. These findings may assist in identifying high-risk patients and guiding treatment strategies.
Similar content being viewed by others
Introduction
Lung cancer was the second most commonly diagnosed cancer and the leading cause of cancer-related death worldwide in 2020[1]. Non-small cell lung cancer (NSCLC) accounts for 80–85% of lung cancer, with a very high incidence and mortality. Based on histology, staging, genomics, and the patient’s condition, the current treatment options for NSCLC include surgery, radiotherapy, chemotherapy, immunotherapy, and molecular targeted therapy[2]. However, most patients are in the advanced stage when diagnosed, and palliative care is the only option for these patients. Therefore, the prognosis of patients with advanced NSCLC is often poor. Upon these, it is of great importance to actively explore novel prognostic biomarkers for advanced NSCLC patients to guide treatment decision-making to improve the survival of patients.
Over the past decade, traditional biomarkers have shown low sensitivity and specificity in predicting the diagnosis, efficacy and prognosis of NSCLC patients[3]. It is urgent to seek a non-invasive, minimally invasive, highly reproducible marker that can predict prognosis and dynamically monitor efficacy in NSCLC. Cell-free DNA (cfDNA) consists of short (≈ 160 (nt)) double-stranded DNA segments that are continuously cleared from bloodstrea (cfDNA has a half-life of 5-150 min)[4]. Thus, cfDNA is a “snapshot” of dying cells throughout the body and can be used to detect a wide and diverse range of biomarkers for a variety of diseases[5,6,7]. In particular, cfDNA has gained traction in cancer diagnosis over the past five year[8,9,10]. cfDNA is found mainly in blood, but also in other body fluids such as urine, pleural fluid, and cerebrospinal fluid[11]. The mechanisms of apoptosis, tumor cell necrosis, active release and lysis of circulating tumor cells have been proposed. Plasma cfDNA levels in cancer patients are often higher than those in healthy people. In addition, studies of NSCLC patients have found that higher cfDNA levels are significantly associated with poor prognosis in patients of NSCLC[12,13,14]. However, the predictive effect of cfDNA on prognosis and efficacy remains controversial and needs to be explored in more studies[15].
In this study, we aimed to clarify the relationship between cfDNA concentration and treatment outcomes including prognosis in patients with advanced NSCLC.
Materials and methods
Patient population
In this retrospective study, 160 stage IIIB-IV NSCLC patients treated at Jiangsu Cancer Hospital between January 2018 and January 2021 were included for analysis, and plasma cfDNA of the patients were analyzed.
Patients were treated according to the Declaration of Helsinki’s ethical principles for medical research involving human subjects. Patients were eligible if they were ≥ 18 years, histologically or cytologically confirmed NSCLC, stage IIIB-IV (according to version 8 of the AJCC), ECOG-PS 0–2 and not received clinical treatment or the interval of treatment for more than half a year, with baseline plasma cfDNA concentration, peripheral blood white blood cells, neutrophils, and lymphocyte counts available before treatment.
Patients were excluded if they were in acute infection, combined with severe impairment of organ function and diseases of the blood and autoimmune system, non-primary or combined with other malignancies, had incomplete baseline data, had less than two cycles of therapy, and did not have pre- and post-treatment imaging.
Sample collection and cfDNA extraction
Clinical data and routine blood tests which included blood cells, neutrophils and lymphocyte count were collected from patient charts and medical records at Jiangsu Cancer Hospital. neutrophil/lymphocyte ratio (NLR) was neutrophil/lymphocyte ratio.
Fasting venous blood was drawn from patients respectively one day before the first cycle of treatment, the second cycle of treatment and the third cycle of treatment. Each time approximately 2–4 (mL) of peripheral venous blood was collected using EDTA-K2 anticoagulation tubes. The peripheral venous blood was centrifuged at 3000 (g) for 10 min within 6 h. The plasma layer of about 1.5 (mL) was diluted with PBS buffer and the supernatant was stored by centrifugation at 1300 (g). The cfDNA was extracted from plasma using QuantiDNA Direct cfDNA Test (DiaCarta) according to manufacturer’s protocol. BT1 cfDNA, BT2 cfDNA, and BT3 cfDNA were defined as cfDNA concentration before the first treatment (baseline cfDNA concentration), cfDNA concentration before the second treatment, and cfDNA concentration before the third treatment, including immunotherapy, chemotherapy or targeted therapy, respectively. BT2-BT1 cfDNA、BT3-BT1 cfDNA was defined as the difference in cfDNA concentration before the second treatment and before the first treatment, and the difference in cfDNA concentration before the third treatment and before the first treatment.
Radiological evaluation of treatment efficacy by computed tomography (CT) scan was performed during treatment in our hospital, thereafter until disease progression and responses were evaluated by the response evaluation criteria in solid tumors (RECIST) version 1.1.
Statistical analysis
The Kolmogorov-Smirnov test was used for all continuous variables to test the hypothesis of normal distribution. We assessed correlations between BT1 cfDNA and continuous variables by using Spearman correlation analysis. Optimal cutoff values for both BT1 cfDNA and NLR were calculated by the survminer package surv cutpoint function. The Kaplan-Meier method was used to explore the prognostic effect of BT1 cfDNA and NLR on progression-free survival (PFS), and the log-rank test to count the difference in survival probability between groups. Cox Proportional Hazard Model were used to investigate independent prognostic factors for PFS (all preconditioning parameters with P < 0.05 found in univariate analyses were included as covariates in multivariate analyses). Multiple logistic regression models estimated the relationship between different efficacy groups (including progressive disease (PD), partial response (PR), stable disease (SD)) and BT1 cfDNA and NLR levels, with all statistics were performed in R. P < 0.05 was considered statistically significant. The factors affecting disease progression were analyzed by generalized linear regression model.
We calculated the sample size of the multivariable Cox regression model for PFS using the previously reported method. Given the widely accepted rule of thumb of 20 events per variable and given there were 3 variables in the final Cox model, the total number of events expected was 60. Taking into account an approximate 64% 1-year event rate and a 20% lost-to-review rate among the participants, we required a total sample size of at least 117 patients.
Results
Baseline characteristics
Patient characteristics are summarized in Table 1. A total of 160 patients were enrolled, and the median age was 61.58 years old. The screening process and results are shown in Supplementary Figure S1.The pathological type of most patients was adenocarcinoma (78.1%). 57 patients received previous chemotherapy alone (35.6%). 63 patients (39.4%) received chemotherapy and Bevacizumab, 33 patients (20.6%) received immunotherapy, and 7 patients (4.4%) received oral targeted agents.
Information including absolute values of white blood cells (WBC), neutrophils (NEU), lymphocytes (LYM), NLR, BT1, BT2, and BT3 cfDNA concentrations in routine blood tests is shown in Table 2, mPFS of patients: 10.37 months (95% CI: 8.37–13.2), median follow-up time:17.5 months (95% CI: 13.6–25.4).
Correlation between BT1 cfDNA concentration and clinical characteristics
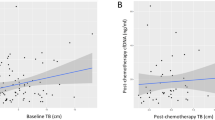
In plasma samples respectively collected from 160 NSCLC patients, cfDNA concentrations ranged from 3.24 to 277.90 (ng/mL). Spearman analysis showed that BT2 and BT3 were significantly correlated with BT1. (Fig. 1a, b). When comparing the relationship between BT1 cfDNA and clinicopathological features, we found higher cfDNA concentrations in the subgroup with tumor size ≥ 5 (cm) (median 22.37 vs. 16.48 (ng/mL), P = 0.004) and age ≥ 65 (median 26.45 vs. 16.97 (ng/mL), P = 0.009). (Fig. 2, Supplementary Table S1)
Prognostic of BT1 cfDNA and NLR
BT1 cfDNA and NLR analyses were performed in 160 patients at baseline. The optimal cutoff values for BT1 cfDNA and NLR is 15 (ng/mL) and 2.98.(Supplementary Figure S2a, c). The mPFS of patients with high BT1 cfDNA (BT1 cfDNA ≥ 15(ng/mL)) was 8.3 months and 14.6 months in patients with low BT1 cfDNA (BT1 cfDNA < 15(ng/mL)) (Log-rank P = 0.002, HR = 1.97 95Cl: 1.29–3.01). The mPFS of patients with high NLR at baseline (NLR ≥ 2.98) was 7.9 months and 13.1 months in patients with low NLR (NLR<2.98) (Log-rank P = 0.023, HR = 1.57 95Cl: 1.06–2.32). (Supplementary Figure S2b, d)
Analyses of prognostic biomarkers in advanced NSCLC
In the univariate Cox regression model analysis, we found that the extrapulmonary metastases, tumor size, BT1 cfDNA and NLR were associated with PFS (P<0.05). Then, Multivariable analysis identified three significant factors associated with independent PFS: cfDNA ≥ 15 (ng/mL), (HR: 1.98, 95%CI: 1.26–3.11, P = 0.003), NLR ≥ 2.98 (HR:1.86, 95% CI: 1.22–2.84, P = 0.004) and extrapulmonary metastases (HR: 1.68, 95%CI: 1.10–2.55, P = 0.016) (Table 3). Upon this, we combined BT1 cfDNA and NLR into the following four risk subgroups: 1: “Low grade” (BT1 cfDNA < 15 (ng/mL) and NLR < 2.98); 2: “Low-intermediate” (BT1 cfDNA < 15 (ng/mL) and NLR ≥ 2.98); 3: " High Intermediate” (BT1 cfDNA ≥ 15 (ng/mL) and NLR < 2.98); 4: “High Grade” (BT1 cfDNA ≥ 15 (ng/mL) and NLR ≥ 2.98). Supplementary Figure S3 contains the results of univariate Log-rank tests and mPFS for each variable group. Analysis of the four risk subgroups revealed that patients in the high-risk subgroup had a significantly worse prognosis compared to those in the low-risk subgroup, with nearly threefold the risk of progression compared to the low-risk subgroup (HR = 2.96, 95Cl: 1.64–5.35, P < 0.001) (Supplementary Table S2, Fig. 3). The mPFS of the four subgroups were 17.17 m, 14.57 m, 11.23 m, and 6.73 m, respectively.
Relationship between dynamics of cfDNA levels and treatment efficacy
The disease control rate (DCR) of the 160 patients enrolled was 85% (54 PR and 82 SD), and the remaining 24 patients were PD. Median cfDNA concentrations (BT1, BT2, BT2-BT1, BT3-BT1) did not differ between the three groups (PD, PR and SD). In addition, there was simply no statistical difference in BT3 among the three groups (P = 0.048), though patients in the PD group had higher BT3 cfDNA concentrations than those in the PR and SD groups (Supplementary Table S3, Supplementary Figure S4). When patients were divided into disease control (PR + SD) and PD groups,BT3 cfDNA concentrations in the (PR + SD) (median 19.10 (ng/mL)) and PD (median 24.96 (ng/mL)) groups were found to be statistically different (P = 0.020) (Supplementary Table S4 and S5). The diagnostic efficacy of BT3 cfDNA concentration and BT3-BT1 cfDNA concentration in predicting disease progression was shown in Fig. 4. BT3 cfDNA concentration was more effective in identifying PD patients with 90.7% sensitivity and 37.5% specificity(AUC: 0.652, 95%CI: 0.516–0.788, cut-off value 41.46 (ng/mL)). The results demonstrated that BT3 cfDNA concentration could be predictive of disease progression in patients with advanced NSCLC.
Factors associated with disease progression in patients with advanced NSCLC
We used logistic regression models to explore the factors associated with disease progression in patients with advanced NSCLC. We found that BT3 cfDNA and BT3-BT1 cfDNA concentrations were associated with disease progression (P < 0.05).We further found that BT3 cfDNA (OR: 6.08, 95% CI: 1.94–19.57, P = 0.002) was an independent factor affecting disease progression in patients with advanced NSCLC (Table 4).
Discussion
In this retrospective study, we found that BT1 cfDNA levels was an independent prognostic indicator for advanced NSCLC patients. In addition, we revealed that patients with advanced NSCLC with increased levels of both BT1 cfDNA and NLR had a worse prognosis. We also revealed that BT3 cfDNA could identify patients with advanced NSCLC who may have disease progression and could be used as a simple and convenient monitoring indicator.
The search for mechanisms and prognostic markers of tumors has been ongoing[16,17,18,19]. The predictive role of cfDNA in prognosis has been demonstrated in many studies in recent years. These studies have important clinical implications that serum samples were often obtained as part of routine blood tests, potentially eliminating the need for additional samples for cfDNA analysis. This study demonstrated that baseline cfDNA levels could be used as an independent prognostic indicator for advanced non-small cell lung cancer, which is consistent with some previous findings that elevated plasma cfDNA concentrations and poor prognosis are associated [20,21,22,23,24]. Hyun et al[25] found that high cfDNA concentrations may be an independent predictor of PFS and OS in NSCLC. A recent study revealed that the lack of increase in ctDNA levels was linked to a considerably longer PFS (median: 0.7 months versus 12.0 months)[26]. Our present study focused on the inclusion of stage IIIB-IV NSCLC patients, and the optimal stage value of BT1 cfDNA was calculated to be 15 (ng/mL), and patients of high BT1 cfDNA group had better PFS, suggesting that dynamic monitoring of cfDNA may be beneficial in predicting NSCLC outcomes. cfDNA is mainly present in serum and plasma together with histones as nucleosomes and shows high potential for diagnostic, prognostic and therapeutic monitoring [20]. In a human NSCLC xenograft mouse model, cfDNA showed a significant transient increase immediately after chemotherapy or surgery, followed by a rapid decrease [27]. The level of cfDNA in peripheral blood provided a real-time snapshot of tumor cell death as well as an indirect measure of overall tumor load, making it a reliable surrogate indicator of tumor treatment response. Compared to expensive imaging tools, cfDNA analysis is cost-effective and continuous monitoring of cfDNA may have a potential role in monitoring tumor efficacy.
The role of cfDNA and NLR in the prognosis of advanced NSCLC has been demonstrated separately [28,29,30].However, in this study, we identified a high-risk subgroup with both high cfDNA and NLR by combining the two biomarkers, and found that they had the highest risk of disease progression. The complementary effects of these two biomarkers suggest the potential of using cfDNA and NLR in combination to predict prognosis in NSCLC patients in clinical practice. This provides insight into the significance of combining multiple metrics for prediction, as the application of a single marker for prediction may not meet clinical needs. We found several independent prognostic factors such as cfDNA, NLR and tumor size in advanced NSCLC patients, which is consistent with previous research [31,32,33]. One study [34] demonstrated that monitoring cfDNA changes and pretreatment PA levels in advanced NSCLC patients receiving treatment is an accurate predictor of tumor response and PFS. Therefore, all this evidence reveals the importance for every clinician to comprehensively identify independent prognostic factors and individually evaluate the prognosis of patients.
The association between high cfDNA concentrations and poor prognosis may be related to tumor load or comorbidities. Our study is limited only to the quantification of cfDNA and should also be analyzed for tumor-specific genomic alterations in cfDNA such as EGFR[35] and KRAS [36] for more precise efficacy prediction and personalized treatment selection. In addition, advanced lung adenocarcinoma might have a higher level of heterogeneity[37], but we did not refine the patient’s treatment plan, which may have affected the accuracy of the results. We did not follow up further on patient survival and lacked Overall survival (OS) data, which should have been further analyzed. It is also important to mention here that since this retrospective study only involved a limited number of NSCLC patients, the conclusions still need to be validated by more external, multicenter and large-scale prospective studies.
In conclusion, our data suggest that high baseline cfDNA concentrations in plasma are associated with poor prognosis in advanced NSCLC. Baseline cfDNA concentrations perform poorly in assessing NSCLC efficacy and do not provide an accurate prediction of efficacy. However, increased BT3 cfDNA in PD patients may be used as an adjunct to imaging to assess efficacy. This may help in the adjustment of treatment regimens in some patients when progression is not yet clear on CT imaging.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Bray F, Laversanne M, Weiderpass E, Soerjomataram I. The ever-increasing importance of cancer as a leading cause of premature death worldwide. Cancer. 2021;127(16):3029–30.
Skřičková J, Kadlec B, Venclíček O, Merta Z. Lung cancer. Casopis lekaru ceskych. 2018;157(5):226–36.
Normanno N, Denis MG, Thress KS, Ratcliffe M, Reck M. Guide to detecting epidermal growth factor receptor (EGFR) mutations in ctDNA of patients with advanced non-small-cell lung cancer. Oncotarget. 2017;8(7):12501–16.
Song P, Wu LR, Yan YH, Zhang JX, Chu T, Kwong LN, Patel AA, Zhang DY. Limitations and opportunities of technologies for the analysis of cell-free DNA in cancer diagnostics. Nat biomedical Eng. 2022;6(3):232–45.
Sigalotti L, Covre A, Colizzi F, Fratta E. Quantitative methylation-specific PCR: a simple method for studying epigenetic modifications of cell-free DNA. Methods in molecular biology (Clifton NJ). 2019;1909:137–62.
Yang Lan L, Chen Bojiang BJ, Li Lei L. [Research progress of circulating tumor DNA methylation in non-small cell lung cancer]. Zhonghua jie he he hu xi za zhi = Zhonghua jiehe he huxi zazhi = chinese. J tuberculosis respiratory Dis. 2018;41(12):967–70.
Arathimos R, Sharp GC, Granell R, Tilling K, Relton CL. Associations of sex hormone-binding globulin and testosterone with genome-wide DNA methylation. BMC Genet. 2018;19(1):113.
Yuan Z, Wang X, Geng X, Li Y, Mu J, Tan F, Xue Q, Gao S, He J. Liquid biopsy for esophageal cancer: is detection of circulating cell-free DNA as a biomarker feasible? Cancer communications (London, England) 2021, 41(1):3–15.
Zhang C, Chao F, Wang S, Han D, Chen G. Cell-free DNA as a Promising Diagnostic biomarker in prostate Cancer: a systematic review and Meta-analysis. J Oncol. 2022;2022:1505087.
Gianni C, Palleschi M, Merloni F, Di Menna G, Sirico M, Sarti S, Virga A, Ulivi P, Cecconetto L, Mariotti M et al. Cell-free DNA fragmentomics: a promising biomarker for diagnosis, prognosis and prediction of response in breast Cancer. Int J Mol Sci 2022, 23(22).
Breitbach S, Sterzing B, Magallanes C, Tug S, Simon P. Direct measurement of cell-free DNA from serially collected capillary plasma during incremental exercise. J Appl Physiol (Bethesda Md: 1985). 2014;117(2):119–30.
Tsai NW, Lin TK, Chen SD, Chang WN, Wang HC, Yang TM, Lin YJ, Jan CR, Huang CR, Liou CW, et al. The value of serial plasma nuclear and mitochondrial DNA levels in patients with acute ischemic stroke. Clin Chim Acta. 2011;412(5–6):476–9.
Mok CH. Zoster-like disease in infants and young children. N Engl J Med. 1971;285(5):294.
Cabel L, Riva F, Servois V, Livartowski A, Daniel C, Rampanou A, Lantz O, Romano E, Milder M, Buecher B, et al. Circulating tumor DNA changes for early monitoring of anti-PD1 immunotherapy: a proof-of-concept study. Annals of oncology: official journal of the European Society for Medical Oncology. 2017;28(8):1996–2001.
Ren S, Ren XD, Guo LF, Qu XM, Shang MY, Dai XT, Huang Q. Urine cell-free DNA as a promising biomarker for early detection of non-small cell lung cancer. J Clin Lab Anal. 2020;34(8):e23321.
Chen Z, Hu Z, Sui Q, Huang Y, Zhao M, Li M, Liang J, Lu T, Zhan C, Lin Z, et al. LncRNA FAM83A-AS1 facilitates tumor proliferation and the migration via the HIF-1α/ glycolysis axis in lung adenocarcinoma. Int J Biol Sci. 2022;18(2):522–35.
Shen W, He J, Hou T, Si J, Chen S. Common pathogenetic mechanisms underlying aging and Tumor and Means of Interventions. Aging and disease. 2022;13(4):1063–91.
Aunan JR, Cho WC, Søreide K. The Biology of Aging and Cancer: a brief overview of Shared and Divergent Molecular Hallmarks. Aging and disease. 2017;8(5):628–42.
Ruiz E, Kandil E, Alhassan S, Toraih E, Errami Y, Elmageed ZYA, Zerfaoui M. An integrative multi-omics analysis of the Molecular Links between Aging and aggressiveness in thyroid cancers. Aging and disease. 2023;14(3):992–1012.
Gautschi O, Bigosch C, Huegli B, Jermann M, Marx A, Chassé E, Ratschiller D, Weder W, Joerger M, Betticher DC, et al. Circulating deoxyribonucleic acid as prognostic marker in non-small-cell lung cancer patients undergoing chemotherapy. J Clin Oncol. 2004;22(20):4157–64.
Beau-Faller M, Gaub MP, Schneider A, Ducrocq X, Massard G, Gasser B, Chenard MP, Kessler R, Anker P, Stroun M, et al. Plasma DNA microsatellite panel as sensitive and tumor-specific marker in lung cancer patients. Int J Cancer. 2003;105(3):361–70.
van der Drift MA, Hol BE, Klaassen CH, Prinsen CF, van Aarssen YA, Donders R, van der Stappen JW, Dekhuijzen PN, van der Heijden HF, Thunnissen FB. Circulating DNA is a non-invasive prognostic factor for survival in non-small cell lung cancer. Lung Cancer. 2010;68(2):283–7.
Fournié GJ, Courtin JP, Laval F, Chalé JJ, Pourrat JP, Pujazon MC, Lauque D, Carles P. Plasma DNA as a marker of cancerous cell death. Investigations in patients suffering from lung cancer and in nude mice bearing human tumours. Cancer Lett. 1995;91(2):221–7.
Sirera R, Bremnes RM, Cabrera A, Jantus-Lewintre E, Sanmartín E, Blasco A, Del Pozo N, Rosell R, Guijarro R, Galbis J, et al. Circulating DNA is a useful prognostic factor in patients with advanced non-small cell lung cancer. J Thorac Oncol. 2011;6(2):286–90.
Hyun MH, Sung JS, Kang EJ, Choi YJ, Park KH, Shin SW, Lee SY, Kim YH. Quantification of circulating cell-free DNA to predict patient survival in non-small-cell lung cancer. Oncotarget. 2017;8(55):94417–30.
Kapeleris J, Müller Bark J, Ranjit S, Irwin D, Hartel G, Warkiani ME, Leo P, O’Leary C, Ladwa R, O’Byrne K, et al. Prognostic value of integrating circulating tumour cells and cell-free DNA in non-small cell lung cancer. Heliyon. 2022;8(7):e09971.
Rago C, Huso DL, Diehl F, Karim B, Liu G, Papadopoulos N, Samuels Y, Velculescu VE, Vogelstein B, Kinzler KW, et al. Serial assessment of human tumor burdens in mice by the analysis of circulating DNA. Cancer Res. 2007;67(19):9364–70.
Tissot C, Toffart AC, Villar S, Souquet PJ, Merle P, Moro-Sibilot D, Pérol M, Zavadil J, Brambilla C, Olivier M, et al. Circulating free DNA concentration is an independent prognostic biomarker in lung cancer. Eur Respir J. 2015;46(6):1773–80.
Ai B, Liu H, Huang Y, Peng P. Circulating cell-free DNA as a prognostic and predictive biomarker in non-small cell lung cancer. Oncotarget. 2016;7(28):44583–95.
Syrigos K, Fiste O, Charpidou A, Grapsa D. Circulating tumor cells count as a predictor of survival in lung cancer. Crit Rev Oncol Hematol. 2018;125:60–8.
Palomar-Abril V, Soria-Comes T, Tarazona Campos S, Martín Ureste M, Giner Bosch V, Maestu Maiques IC. Impact of age on inflammation-based scores among patients diagnosed with Stage III Non-Small Cell Lung Cancer. Oncology. 2020;98(8):528–33.
Zhong Y, Fan Q, Zhou Z, Wang Y, He K, Lu J. Plasma cfDNA as a potential biomarker to evaluate the efficacy of Chemotherapy in Gastric Cancer. Cancer Manage Res. 2020;12:3099–106.
Lee JH, Long GV, Boyd S, Lo S, Menzies AM, Tembe V, Guminski A, Jakrot V, Scolyer RA, Mann GJ, et al. Circulating tumour DNA predicts response to anti-PD1 antibodies in metastatic melanoma. Annals of oncology: official journal of the European Society for Medical Oncology. 2017;28(5):1130–6.
Chen J, Shao J, Zhang X, Wei S, Cai H, Wang G. Monitoring early dynamic changes of plasma cell-free DNA and pretreatment pre-albumin to predict chemotherapy effectiveness and survival outcomes in advanced non-small cell lung cancer. Annals of translational medicine. 2022;10(5):253.
Xing S, Hu K, Wang Y. Tumor Immune Microenvironment and Immunotherapy in Non-Small Cell Lung Cancer: Update and New Challenges. Aging and disease. 2022;13(6):1615–32.
Nygaard AD, Garm Spindler KL, Pallisgaard N, Andersen RF, Jakobsen A. The prognostic value of KRAS mutated plasma DNA in advanced non-small cell lung cancer. Lung Cancer. 2013;79(3):312–7.
Chen Z, Huang Y, Hu Z, Zhao M, Li M, Bi G, Zheng Y, Liang J, Lu T, Jiang W, et al. Landscape and dynamics of single tumor and immune cells in early and advanced-stage lung adenocarcinoma. Clin translational Med. 2021;11(3):e350.
Acknowledgements
We thank the editor and reviewers for relevant and helpful comments on the manuscript.
Funding
This study was funded by the National Natural Science Foundation of China (No. 82273162).
Author information
Authors and Affiliations
Contributions
(I) Conception and design: YF and GRZ; (II) Administrative support: GRZ; (III) Provision of study materials or patients: GRZ, HHS and SDW; (IV) Collection and assembly of data: WWP, YL, HHS and SDW; (V) Data analysis and interpretation: WWP and YL; (VI) Manuscript writing: WWP and YL; (VII) Final approval of manuscript: All authors.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
This study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by Ethics Committee of Jiangsu Cancer Hospital (2023–034). The need for written informed consent was waived by the Ethics Committee of Jiangsu Cancer Hospital due to retrospective nature of the study.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.







Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Peng, WW., Liu, Y., Sha, HH. et al. Relationship between plasma circulating cell-free DNA concentration and treatment outcomes including prognosis in patients with advanced non-small cell lung cancer. BMC Pulm Med 23, 348 (2023). https://doi.org/10.1186/s12890-023-02586-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12890-023-02586-2