Abstract
Background
The use of antibiotics in mild to severe acute exacerbations of chronic obstructive pulmonary disease (COPD) remains controversial.
Aim
To explore in-hospital antibiotic use in severe acute exacerbations of COPD (AECOPD), to analyze determinants of in-hospital antibiotic use, and to investigate its association with hospital length of stay (LOS) and in-hospital mortality.
Methods
A retrospective, observational study was conducted in Ghent University Hospital. Severe AECOPD were defined as hospitalizations for AECOPD (ICD-10 J44.0 and J44.1) discharged between 2016 and 2021. Patients with a concomitant diagnosis of pneumonia or ‘pure’ asthma were excluded. An alluvial plot was used to describe antibiotic treatment patterns. Logistic regression analyses identified determinants of in-hospital antibiotic use. Cox proportional hazards regression analyses were used to compare time to discharge alive and time to in-hospital death between antibiotic-treated and non-antibiotic-treated AECOPD patients.
Results
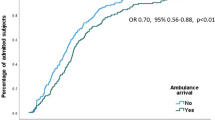
In total, 431 AECOPD patients (mean age 70 years, 63% males) were included. More than two-thirds (68%) of patients were treated with antibiotics, mainly amoxicillin-clavulanic acid. In multivariable analysis, several patient-related variables (age, body mass index (BMI), cancer), treatment-related variables (maintenance azithromycin, theophylline), clinical variables (sputum volume and body temperature) and laboratory results (C-reactive protein (CRP) levels) were associated with in-hospital antibiotic use independent of sputum purulence, neutrophil counts, inhaled corticosteroids and intensive care unit of which CRP level was the strongest determinant. The median hospital LOS was significantly longer in antibiotic-treated patients (6 days [4–10]) compared to non-antibiotic-treated patients (4 days [2–7]) (p < 0.001, Log rank test). This was indicated by a reduced probability of hospital discharge even after adjustment for age, sputum purulence, BMI, in-hospital systemic corticosteroid use and forced expiratory volume in one second (FEV1) (adjusted hazard ratio 0.60; 95% CI 0.43; 0.84). In-hospital antibiotic use was not significantly associated with in-hospital mortality.
Conclusions
In this observational study in a Belgian tertiary hospital, in-hospital antibiotic use among patients with severe AECOPD was determined by the symptom severity of the exacerbation and the underlying COPD severity as recommended by the guidelines, but also by patient-related variables. Moreover, in-hospital antibiotic use was associated with a longer hospital stay, which may be linked to their disease severity, slower response to treatment or 'harm' due to antibiotics.
Trial registration
Number: B670201939030; date of registration: March 5, 2019.
Similar content being viewed by others
Background
Chronic obstructive pulmonary disease (COPD) is a common lung disease characterized by persistent respiratory symptoms and airflow limitation [1]. A major contributor to the overall severity of COPD and the associated burden on health-care systems worldwide, is acute exacerbations of COPD (AECOPD) [1, 2]. AECOPD are episodes of worsening of respiratory symptoms, leading to a change in medication (mild-moderate) or hospitalization (severe) [1, 2]. Exacerbations arise as a result of environmental triggers and/or respiratory tract infections, encompassing viral and bacterial infections [3]. A meta-analysis including 118 studies demonstrated that half of the infections in AECOPD patients are bacterial [4], and might require active antibiotic treatment [5]. However, the use of antibiotics in mild to severe exacerbations remains controversial [6]. Pooled results of four randomized controlled trials in a 2018 Cochrane meta-analysis did not show that the currently used antibiotics significantly reduce the risk of treatment failure, duration of hospital admission or mortality in hospitalized patients, excluding patients in intensive care [6]. Moreover, any antibiotic use, particularly unnecessary or inappropriate use, contributes to the selection of resistant pathogens [7].
The conflicting evidence called for research in biomarkers which could help in selecting patients who benefit from antibiotic treatment to limit overuse. One of the most frequently discussed markers that can be used to guide antibiotic therapy is sputum purulence [6]. The Global Initiative for Chronic Obstructive Lung Disease (GOLD) guidelines recommend antibiotic treatment if patients have at least two of three cardinal symptoms, including increased sputum purulence [1]. This recommendation is based on the results of the historical randomized crossover trial by Anthonisen et al. [8], and is supported by a more recent meta-analysis showing a moderate level of evidence that purulent sputum during AECOPD increased the probability of potentially pathogenic bacteria by two-fold [9]. In Belgium, the Infectiology Guide from the Belgian Society for Infectiology and Clinical Microbiology recommends considering the underlying COPD severity in addition to the Anthonisen criteria [10]. Other studies have indicated the use of C-reactive protein (CRP) to guide antibiotic therapy [11]. A randomized controlled trial including patients hospitalized with an AECOPD reported a significantly lower antibiotic use in the CRP-guided (cut-off ≥ 50 mg/L) group (31.7%) than in the GOLD-guided (increased sputum purulence in combination with dyspnea and/or sputum volume) group (46.2%), without an increase in adverse events [12]. CRP-guided treatment is implemented in the Belgian outpatient guidelines due to a more recent revision in 2021 [13], but not yet in the Belgian inpatient [10], and GOLD guidelines [1].
In addition to the decision to initiate antibiotic therapy, the choice of an appropriate antibiotic is of great importance [7]. According to the GOLD 2022 guidelines, the initial empirical therapy could be an aminopenicillin with clavulanic acid, a macrolide or tetracycline, based on the local bacterial resistance pattern [1]. However, GOLD warns for the presence of gram-negative bacterial infections in patients with frequent exacerbations, severe airflow limitation and/or need for mechanical ventilation [1]. The antimicrobial drug recommended by the Belgian Infectiology Guide based on national resistance is amoxicillin-clavulanic acid, but another antibiotic is preferred in case of immunoglobulin E (IgE) mediated penicillin allergy (moxifloxacin) or risk factors for Pseudomonas aeruginosa infection (anti-pseudomonal antibiotic such as piperacillin-tazobactam). The total recommended duration of antibiotic therapy was 5 days and rotation of antibiotics was not recommended [10].
To the best of our knowledge, studies that investigated the determinants of antibiotic use for AECOPD were performed in primary care [14, 15], or did not investigate laboratory results such as CRP [16]. Only two retrospective studies investigated the real-life effect of (guideline-recommended) antibiotics in patients hospitalized with AECOPD on outcomes, both performed in the United States [17, 18]. Since data on antibiotic use of severe AECOPD in Belgium are lacking, we aimed to describe the antibiotic use including initial choice of antibiotic in severe AECOPD patients, to analyze determinants of in-hospital antibiotic use, and to investigate its associations with important outcomes such as length of stay (LOS) and in-hospital mortality.
Methods
Study design
Antibiotic use for treatment of severe AECOPD was investigated in a retrospective, observational study in Ghent University Hospital, a Belgian tertiary hospital with more than 1000 beds.
Data sources
This study was conducted using three data sources. First, the Minimal Hospital Data (MHD) registration database from Ghent University Hospital was used to select hospitalizations for AECOPD. Additionally, patient characteristics were collected from the electronic patient records (EPR). Finally, the use of systemic antibiotics and corticosteroids was collected from the hospital pharmacy data.
Study population
Inpatients above the age of 40 hospitalized in regular ward or the intensive care unit (ICU), who were discharged between January (Jan.) 1st, 2016, and Jan. 1st, 2022, and who received an International Classification of Diseases 10th Revision (ICD-10) code for an AECOPD present on hospital admission, were included in this study. The selection of the ICD-10 codes from the ICD-10 codebook consists of COPD with acute lower respiratory infection (J44.0) or COPD with (acute) exacerbation (J44.1) as verified admission diagnosis. These codes are co-determining in the assignment of the All Patient Refined Diagnosis Related Groups (APR-DRG) 140 (COPD) through the 3 M APR-DRG algorithm. Patients with a concomitant diagnosis of pneumonia or ‘pure’ asthma were excluded based on the ICD-10 codes (J13-J18 and J45, respectively) or diagnosis in EPR. We only analyzed the index hospitalization, defined as the patient’s first hospitalization during the study period to exclude related events.
Assessment and analysis of drug use
Information was retrieved using the Anatomical Therapeutic Chemical (ATC) codes for antibacterials for systemic use (J01) and corticosteroids for systemic use (H02). Antibiotic-treated patients were defined as persons with at least one dispensing of an antibacterial for systemic use during hospitalization, independent of use before the hospitalization. Prevalence of in-hospital antibiotic use was defined as the number of antibiotic-treated patients divided by the number of patients hospitalized for an AECOPD.
Antibiotic treatment patterns were captured at the ATC 5th level (chemical substance) and categorized into the mainly used antibiotics: azithromycin with/without another antibiotic on the same day (J01FA10 w/wo other), amoxicillin-clavulanic acid (J01CR02 w/wo other), piperacillin-tazobactam (J01CR05 w/wo other), and moxifloxacin (J01MA14 w/wo other). If more than one of the previously mentioned antibiotics was dispensed on the same day, it was classified under the antibiotic with the highest antibiotic spectrum index [19]. Other antibiotics were categorized as “other”. Antibiotic treatment patterns from admission (= day 0) to discharge or until day 10 were visualized using an alluvial plot generated using R 1.4. The number of dispensed antibiotic units was converted into defined daily doses adjusted to the Belgian situation in hospitals (DDAs, version 2019). A proposal of DDAs was made by Sciensano based on the Belgian Infectiology Guide and a validation was performed by the Flemish Association of Hospital Pharmacists working group on antibiotics and the Belgian Antibiotic Policy Coordination Committee (BAPCOC) working group Hospital Medicine. Median DDAs per hospitalization were used to estimate the days of antibiotic treatment.
Outcomes
Outcomes were hospital LOS and in-hospital mortality. Hospital LOS was defined as the number of days between the hospital admission date and the hospital discharge date. In-hospital mortality was defined as death occurring during hospital stay.
Covariables assessment
Covariables were classified as 1) patient-related variables, 2) hospitalization-related variables, 3) clinical variables, 4) treatment-related variables, and 5) laboratory test results.
Patient-related variables were extracted from the MHD (age on admission and sex) or collected from the EPR: height (centimeters) and weight (kilograms) to calculate the body mass index (BMI, kg/m2). Frequent comorbidities were classified using the Elixhauser Comorbidity Software Refined for ICD-10-CM Reference File, v2022.1 (Agency for Healthcare Research and Quality).
Hospitalization-related variables were extracted from the MHD. The hospital discharge dates were categorized into winter months from Jan. to March and October (Oct.) to December (Dec.), and summer months from April to June and July to September (Sept.).
Clinical variables were collected from the EPR. Stable (most recent before hospitalization) forced expiratory volume in one second (FEV1) was used to categorize the patients into GOLD stage I (≥ 80%), II (50–79%), III (30–49%) or IV (< 30%) [1], and supplemented with GOLD stage written in the EPR if no spirometry was available before hospital admission. The Anthonisen criteria on admission (increased dyspnea, increased sputum volume or purulence) were collected, and the exacerbations were categorized into type 1 (all three cardinal symptoms), type 2 (two cardinal symptoms) and type 3 (one cardinal symptom) [8]. Body temperature was defined as the highest body temperature within 48 h after admission. Smoking status was collected through EPR and categorized into never, former or current smoker. Recent hospitalization was defined as hospitalization within 3 months before. IgE mediated penicillin allergy was defined as: itching, angioedema, urticaria, hypotension, anaphylaxis within 1–72 h.
Treatment-related variables were collected from the EPR (maintenance medication or treatment before hospitalization) or hospital pharmacy data (treatment during hospitalization). Recent or frequent antibiotics was defined as antibiotics within 3 months before or more than 4 courses during the preceding year, respectively.
Furthermore, laboratory test results on admission were collected. More information about the laboratory tests can be found elsewhere [20]. Due to the high percentage of missing values of white blood cell differentiation on admission, it was supplemented with data from other time points.
Statistical analysis
Data analyses were performed using IBM SPSS Statistics® version 25. P values < 0.05 were considered significant. In order to examine baseline characteristics, descriptive statistics were applied. Continuous data were described as mean with standard deviation (SD) if normally distributed and as median with interquartile range (Q1-Q3) if not. Categorical variables were described as counts (n) with percentages. An independent samples T-test, Mann–Whitney test, X2-test or Fisher’s Exact test were used to test differences in characteristics between AECOPD patients treated with or without antibiotics, respectively. Among antibiotic-treated patients, subgroup analyses were performed to test the differences in characteristics between AECOPD patients initially treated with the first choice amoxicillin-clavulanic acid or another antibiotic. Logistic regression analyses identified factors associated with in-hospital antibiotic use. A manual forward selection process was used to add factors with a p < 0.15.
We constructed cumulative survival curves using the Kaplan–Meier method and used Log rank test to identify significant differences. Cox proportional hazards regression analyses were used to compare time to discharge alive and time to in-hospital death between antibiotic-treated patients and non-antibiotic-treated patients. The proportional hazard assumption was checked graphically. For time to discharge alive as outcome, patients were censored at date of in-hospital death or transfer to another hospital. An HR of less than one indicates that the probability of hospital discharge was reduced and thus the LOS was longer. The following covariables were considered as potential confounders: age [21], sex, BMI, FEV1, GOLD stage, sputum purulence, pH, smoking status, respiratory failure, recent hospitalization, maintenance theophylline and in-hospital systemic corticosteroid (SCS) use [22]. Model 1 was adjusted for age, model 2 was adjusted for covariates which changed the point estimate by more than 5%, and model 3 was additionally adjusted for FEV1. Sensitivity analyses were performed 1) after exclusion of antibiotic users before admission since they could initially be classified as no in-hospital antibiotic users, and 2) after exclusion of late initiated antibiotic users (i.e. from day 2) to verify the robustness of the results. Subgroup analyses were performed 1) for the mainly used antibiotics (see 2.4), and 2) whether or not in-hospital SCS were used. Propensity scores (PS) were calculated. Variables related to in-hospital antibiotic use and LOS (age, BMI and FEV1) were included in the PS. Patients were matched with a match tolerance of 0.2.
For in-hospital death as outcome, patients were censored at date of discharge alive. The following covariables were considered as potential confounders for in-hospital death: age, sex, BMI, FEV1, comorbidities, pH, CRP, respiratory failure, sputum purulence and in-hospital SCS use [23]. Model 1 was adjusted for age, model 2 was adjusted for covariates which changed the point estimate by more than 5%, and model 3 was additionally adjusted for FEV1.
Results
Study population
The study population is represented in Fig. 1. In total, 627 patients were discharged at least once between Jan. 1st, 2016 and Jan. 1st, 2022. Of these patients, 143 were excluded based on MHD and 53 on EPR. Clinical characteristics were collected for 431 patients during index hospitalization of which eight out of ten patients had COPD with acute exacerbation as verified admission diagnosis. The majority were hospitalized at the regular ward (n = 384, 89%). Only 47 patients (11%) were hospitalized at the ICU, of which 39 on the admission day.
Prevalence of in-hospital antibiotic use
Of all 994 hospitalizations (see Fig. 1), more than half were discharged during winter months (Oct.-March, n = 546, 55%). During the index hospitalization, more than two-thirds of patients were treated with antibiotics (n = 293/431, 68%). The proportion of patients receiving antibiotics discharged in the summer months (April-Sept., 61%) was lower compared to patients discharged in winter months (Oct.-March, 73%, Chi2, p = 0.009). This was mainly driven by a lower prevalence in July-Sept. (n = 51/89, 57%) compared to Jan.-March (n = 115/149, 77%). Patients discharged in April-June (OR 0.55, 95% CI 0.31 to 0.96) and mainly in July-Sept. (OR 0.40, 95% CI 0.22 to 0.70) were less likely to receive antibiotic treatment compared to patients discharged in Jan.-March. Patients discharged in 2021 were less likely to receive antibiotic treatment (OR 0.45, 95% CI 0.23 to 0.88) compared to patients discharged in 2016 after adjustment for discharge quarter.
The majority of antibiotic-treated patients initially received empirical therapy (n = 246, 84.0%) (Table S1). Median (Q1-Q3) antibiotic consumption was 5.50 (3.38–8.20) DDAs per hospitalization (Table S1). Figure 2 illustrates the antibiotic treatment pattern per day during hospitalization of AECOPD patients with at least one antibiotic dispensing (n = 293). The majority of patients received amoxicillin-clavulanic acid w/wo other (J01CR02) as initial antibiotic therapy of which 132 on the admission day (= day 0, 45.1%). The following antibiotics were dispensed approximately equally on the admission day: piperacillin-tazobactam w/wo other (J01CR05, n = 20, 6.8%) and moxifloxacin w/wo other (J01MA14, n = 21, 7.2%). A minority got azithromycin w/wo other on the admission day (J01FA10, n = 11, 3.8%). Independent of dispensing day, 188 patients (64.2%) received amoxicillin-clavulanic acid as initial antibiotic therapy.
Alluvial plot showing the antibiotic treatment patterns of treated patients (n = 293) from one antibiotic to another from admission (= day 0) to discharge or until day 10. The height of each node represents the number of patients receiving the specified treatment (e.g. the majority started on amoxicillin-clavulanic acid and remained until discharge)
Baseline characteristics of included patients
In total, 431 AECOPD patients were included. Of these patients, the majority were men (n = 273, 63%) and the mean age at index hospitalization was 70 ± 11 years. Nine out of ten patients were transferred from home. The majority of the antibiotic-treated patients received an antibiotic on the day of admission (n = 196, 67%) or the day after admission (n = 61, 21%).
Baseline characteristics of patients treated with or without antibiotics are presented in Table 1. AECOPD patients using in-hospital antibiotics differed from patients not using in-hospital antibiotics regarding patient-related variables (age, BMI, diabetes mellitus, cancer and chronic kidney disease), clinical variables (FEV1% predicted, sputum volume and purulence, body temperature, recent hospitalization and bronchiectasis), treatment-related variables (maintenance inhaled corticosteroids (ICS), theophylline and azithromycin) and laboratory results (CRP levels, peripheral blood leucocyte, neutrophil and eosinophil counts, and arterial blood pH). Remarkably, 17 COPD patients were neither treated with antibiotics nor SCS, but only with bronchodilators during hospitalization. Seven of them were treated with antibiotics or SCS before admission, three were newly diagnosed COPD, and two had a do not resuscitate code (ICD Z66) ≥ 2.
Baseline characteristics of patients initially treated with amoxicillin-clavulanic acid or another antibiotic are presented in Table S1. Deviation from the first choice amoxicillin-clavulanic acid was significantly associated with clinical variables and treatment-related variables. Regarding clinical variables, the proportion of patients with a penicillin allergy label, recent hospitalization, prior Pseudomonas aeruginosa isolation and no empiric treatment for AECOPD were significantly higher in patients treated with another antibiotic. Regarding treatment-related variables, the use of ICS and antibiotics before admission (mainly amoxicillin-clavulanic acid) were significantly associated with the use of another antibiotic than amoxicillin-clavulanic acid.
Determinants of in-hospital antibiotic use and choice
The results of the univariable and multivariable regression analysis tabulating the determinants of in-hospital antibiotic use and amoxicillin-clavulanic acid as initial therapy are summarized in Table 2 and Table S2, respectively. CRP level was the strongest determinant for in-hospital antibiotic use. Results from the multivariable logistic regression model showed that several patient-related variables (age, BMI, cancer), treatment-related variables (maintenance azithromycin, theophylline), clinical variables (sputum volume and body temperature) and laboratory results (CRP levels) were associated with in-hospital antibiotic use independent of sputum purulence, neutrophil counts, ICS and ICU. No empiric AECOPD treatment was the strongest determinant for deviation from amoxicillin-clavulanic acid.
Outcomes
In total, the median (Q1-Q3) hospital LOS was 6 days (3–9). As shown in Fig. 3, the median hospital LOS was significantly longer in patients treated with antibiotics (6 days [4-10]) compared to patients not treated with antibiotics (4 days [2-7]) (p < 0.001, Log rank test). The age-adjusted hazard on discharge was 40% lower for antibiotic-treated patients (Table 3). The results did not change meaningfully after adjusting for possible confounders (BMI, in-hospital SCS use, sputum purulence and FEV1), after propensity score (including age, BMI and FEV1) adjustment (n = 126, HR: 0.63; 95% CI 0.43; 0.92) or after exclusion of patients late initiated antibiotic treatment (i.e. from day 2) (Table S3, sensitivity analysis 2). The sensitivity analysis after exclusion of antibiotic users before admission, resulted in a weaker association with time to discharge (model 2: HR 0.66; 95% CI 0.50; 0.87), and was no longer significant in model 3 (Table S3).
The results of the subgroupanalyses are presented in Table 3. Subgroup analyses by initial in-hospital antibiotic indicated that the hazard on discharge was significantly lower for patients treated with piperacillin-tazobactam or other antibiotics compared to amoxicillin-clavulanic acid. Subgroup analyses by in-hospital SCS use indicated that the hazard on discharge was significantly lower for all the three groups in model 1, but only for the subgroups receiving antibiotics with or without SCS in model 2 (Fig. 3B and Table 3).
In total, 25/431 AECOPD patients (5.8%) died during index hospitalization. The observed in-hospital mortality was 6.1% among those treated with antibiotics compared with 5.1% among those not treated with antibiotics. In-hospital antibiotic use was not significantly associated with in-hospital mortality (Table 4). Results of the sensitivity analyses are presented in Table S4.
Discussion
Prevalence of in-hospital antibiotic use
This retrospective study observed that more than two-thirds (68%) of AECOPD patients were treated with antibiotics during index hospitalization. The observed prevalence of in-hospital antibiotic use (68%) is higher than the expected prevalence of 50% bacterially triggered AECOPD according to available literature [4]. In contrast, this antibiotic prevalence was lower in comparison to 86% (75.6% in Belgium) of admissions treated with an antibiotic according to a European COPD audit between 2010 and 2011 [16]. However, the inclusion of the patients in the European COPD audit was during winter months some years ago [24], and in our study we also observed a higher proportion of patients receiving antibiotics at the beginning of the study and in the winter months (73%). Whether this seasonal variation indicates that proportionally more virally triggered infections are treated with antibiotics [25], or that viral pathogens trigger secondary bacterial infections due to a shift in the microbiome should be further investigated, because respiratory multiplex polymerase chain reaction (PCR) or 16S microbiome analyses were not performed in these patients.
In our study, more than half of the patients received amoxicillin-clavulanic acid as initial therapy which is the preferred antibiotic according to the GOLD guidelines [1], and the Belgian Infectiology Guide [10]. More specifically, amoxicillin-clavulanic acid is recommended for mild or moderate COPD, or severe to very severe COPD patients without risk factors for infection by Pseudomonas aeruginosa if initial intravenous treatment is necessary [10]. The following antibiotics were dispensed approximately equally as initial therapy: piperacillin-tazobactam and moxifloxacin. The prevalence of piperacillin-tazobactam use (8.2%) was above the expected prevalence of P. aeruginosa in unselected outpatients (4%), but in line with the expected prevalence in COPD patients with advanced airflow obstruction (8–13%) [26]. The prevalence of moxifloxacin use (10.9%) is in line with the prevalence of reported penicillin allergy, but higher than the prevalence of truly allergic patients [27].
Determinants of in-hospital antibiotic use and choice
In-hospital antibiotic use was determined by characteristics for which antibiotics are recommended by the guidelines or literature. The airflow limitation was more severe (indicated by lower FEV1) in patients treated with antibiotics. However, in almost half of patients, no spirometric values were available, which was comparable to the European COPD audit [16]. Moreover, the univariable association with FEV1 was no longer significant after inclusion of azithromycin maintenance treatment suggesting that maintenance treatment could be used as a proxy for the underlying COPD severity. Sputum purulence and sputum volume were associated with in-hospital antibiotic use, while no association was observed with dyspnea. This finding may indicate that sputum volume is used as an indicator to start antibiotics, although sputum purulence is the strongest predictor of bacterial infection among the Anthonisen criteria [15]. CRP level was the strongest determinant of in-hospital antibiotic use for which there is moderate evidence to differentiate bacterial AECOPD [11]. Moreover, patients treated with an antibiotic had a significantly lower peripheral blood eosinophil and higher neutrophil count. Previous studies have showed that the airway microbiome differs between neutrophilic and eosinophilic COPD patients [28]. However, the univariable associations with eosinophils and blood leucocyte counts in our study disappeared after including CRP level in the multivariable model, which was supported by the findings of a meta-analysis to summarize biomarkers reporting that blood leucocyte count is not a useful biomarker [11].
In-hospital antibiotic use was also determined by patient-related variables, although not included in the Belgian guidelines. Patients treated with an antibiotic were significantly older and had a lower BMI. Higher age was previously described as a predictor for antibiotic prescribing in general practice [14], and was associated with antibiotic prescription in the European COPD audit [16]. Older age is also a factor taken into account in the guideline of the Dutch Association of Physicians in Chest Medicine and Tuberculosis (NVALT) to start antibiotics more quickly in hospitalized COPD patients [29]. More recently, a subgroup analysis of a meta-analysis observed that the prevalence of bacterial infection in COPD patients increased over age and was lower in studies with a higher proportion of males [4]. However, we did not observe an association with sex which was consistent with Llor and colleagues in general practice [14]. A possible explanation of the observed association with lower BMI is that patients with a BMI ≤ 22 kg/m2 were more likely to yield bacterial isolates in sputum in a Greek prospective observational study [30].
Despite the recommendation of antibiotic treatment for patients with acute and chronic respiratory failure in the Belgium Infectiology Guide [10], or requiring mechanical ventilation according to the GOLD 2022 guidelines [1], we observed no significant association between in-hospital antibiotic use and respiratory failure defined as an ICD-10 code for respiratory failure (J96), the need for ventilatory support or hospitalization on ICU. A strong beneficial effect for patients admitted to the ICU needing mechanical ventilation is evidenced by a statistically significant effect on mortality [6]. However, this was based on only one randomized controlled trial, performed more than 20 years ago in which patients did not receive corticosteroids [6, 31].
Antibiotic choice was determined by guideline recommended indications to deviate from the first choice antibiotic amoxicillin-clavulanic acid (no empirical AECOPD treatment, penicillin allergy and recent hospitalization). The risk factors for infection by Pseudomonas aeruginosa for which we observed a significant univariable association were recent hospitalization, recent antibiotics and prior Pseudomonas aeruginosa isolation of which only recent hospitalization was included in the multivariable model. Patients with a penicillin allergy label were less likely to be treated with amoxicillin-clavulanc acid. To avoid overuse of second-line treatment, it is important that patients with an IgE penicillin allergy are correctly labeled. In 2017, 6.1% of patients reported an antibiotic allergy (of which 90% penicillin) at Ghent University Hospital surgical day hospitalization [32]. However, based on a prospective observational study in a Belgian outpatient population, 91% of the participants could be safely delabeled [33].
Antibiotic choice was also determined by antibiotic use before admission and azithromycin maintenance treatment which are not included in guidelines. Antibiotic use before admission was significantly associated with dispensing of another antibiotic than amoxicillin-clavulanic acid which could be due to clinical failure on initiated amoxicillin-clavulanic treatment by the general practitioner. The association between antibiotic prescription before and during admission observed in the European COPD audit was only a trend in our study suggesting that the need to continue antibiotics is questioned [16]. Therefore, Antimicrobial Stewardship interventions should not only target in-hospital antibiotic use, but also antibiotic use in primary care. Azithromycin maintenance therapy was not only a determinant of in-hospital antibiotic use, but also of another antibiotic than amoxicillin-clavulanic acid. This could be due to continuation of the chronic immunomodulatory treatment in hospital [1, 34] or by the more severe underlying COPD.
We focused on demographic, clinical and treatment-related variables and laboratory test results at the individual patient level. However, we could not exclude that variables related to the prescriber, the hospital and the policy level might also have influenced the in-hospital antibiotic use or choice.
Outcomes
The median LOS in our study (6 days) was 1 day shorter compared to the median LOS (7 days) in a European COPD audit performed in 2010–2011 [22]. In our study, we observed that the LOS was longer for patients receiving antibiotic therapy. This finding was consistent with this European COPD audit which observed that antibiotic use was strongly associated with an increased risk of a LOS longer than the median [22]. LOS was similar between the antibiotic and placebo group in a meta-analysis of four inpatient randomized controlled trials [6]. In contrast, we also observed a longer LOS for patients treated with antibiotics compared to patients not receiving antibiotics nor corticosteroids in the subgroup analysis. Another American retrospective study observed no significant differences in hospital LOS between the GOLD guideline-recommended or inappropriate antibiotic therapy [18]. Subgroup analyses by initial antibiotic indicated that the hazard on discharge was significantly lower for piperacillin-tazobactam and other antibiotics compared to amoxicillin-clavulanic acid. A recent American retrospective, multicenter cohort study observed less treatment failure (initiation of new antibiotic and readmission) and shorter LOS in the azithromycin group compared to the beta-lactam group [35]. However, the most commonly prescribed beta-lactam was ceftriaxone [35], and not amoxicillin-clavulanic acid as in our study. Moreover, azithromycin was probably not used as treatment in our study, but as chronic immunomodulatory treatment [1, 34].
Possible explanations for our findings are a more severe AECOPD, slower response to treatment or ‘harm’ due to antibiotics. First, the association could be due to a more severe AECOPD or patient condition [22]. This hypothesis seems less probable, since (propensity score) adjusted analyses yielded similar effect estimates. However, unmeasured confounders may still be present. Secondly, the association may be due to a slower response to treatment of causative phenotype of AECOPD. Patients with a respiratory infectious phenotype had longer LOS and higher COPD Assessment Test score than non-infectious patients in a previous study [36], and patients with eosinophilic AECOPD had a shorter LOS [37]. It is possible that patients not treated with antibiotics with an eosinophilic exacerbation (suggested by the higher eosinophil count in this group) more rapidly respond to (corticosteroid) treatment. Finally, patients treated with (prolonged) antibiotic therapy could in theory experience (more) adverse effects requiring a longer hospital stay. The Cochrane meta-analysis showed that antibiotic-treated patients had more (but not statistical significant) frequent adverse effects [6]. This explanation seems to contribute only partially, since only a few patients had an ICD-10 code for a possible adverse effect or were switched to another antibiotic because of adverse drug effects [data not shown]. The LOS was not significantly longer in those on antibiotics with an ICD-10 code for a possible adverse effect (n = 12; 8.5 days) compared to those on antibiotics without record of a possible adverse effect (n = 281, 6 days, Mann–Whitney, p = 0.307).
In this study, 5.8% died during index hospitalization compared to the overall short-term cumulative incidence of death of 3.6% (1.8–20.4%) observed in a systematic review of 17 studies [23]. We observed no association between antibiotic therapy and in-hospital mortality. Another large retrospective cohort study throughout the United States observed that antibiotic treatment was associated with a 40% risk reduction of in-hospital mortality [17]. Since patients starting on antibiotics after the third hospital day were grouped with controls who were not treated with antibiotics, results might have favored the (early initiating) antibiotic group because late treatment is likely associated with clinical deterioration [17]. We have grouped late initiating patients to the antibiotics group to avoid immortal time bias as the patients must survive the first two days to receive late antibiotic therapy and did a sensitivity analysis excluding these patients which confirmed no significant association with mortality.
Strengths and limitations
A first strength of our study is the long study period of 6 years. Other observational studies investigating the association between antibiotic use and outcomes had shorter study periods. Moreover, in addition to the MHD and hospital pharmacy data, we also collected clinical data which led to a very rich dataset.
Our study had some limitations. First, as this study was a real-world observational study, COPD patients were not randomly treated, but upon the clinical judgment of the clinician which could have led to bias by the symptom severity of the AECOPD. Second, the study was a single center study, only involving Ghent University Hospital, a tertiary care center, institution-specific confounders may exist and the results may not be generalizable to all COPD patients with severe acute exacerbations. Third, as this was a retrospective study, this research relied on routinely collected health data. As a result, incomplete medical records or errors in medical records could not be ruled out, which could lead to information bias. For example, white blood cell differentiation was only available on admission in almost half of the cases and was supplemented with results at other time points whereby eosinophil levels may be influenced by SCS therapy. Some potential misclassification of patients with clinically confirmed AECOPD may have occurred by including patients based on J44 ICD-10 codes [38]. However, we tried to limit misclassification by excluding pneumonias and ‘pure' asthma exacerbations. In a sensitivity analysis only excluding patients with a concomitant diagnosis of pneumonia, but not asthma, the antibiotic prevalence was 67% (compared to 68%).
We were not able to investigate long-term outcomes. Readmission would be underestimated in our study due to readmission to other hospitals in the region. Moreover, we could not exclude the possibility that some dispensed antibiotics were prescribed for a co-infection instead of the initial COPD exacerbation. This highlights the need for the inclusion of an indication-based coded field in electronic health records. We aimed to estimate the days of antibiotic treatment as best as possible by using DDAs instead of DDDs. Still, DDAs will overestimate the actual length of antibiotic therapy for patients receiving combination therapy and underestimate the actual length of antibiotic therapy for patient receiving further antibiotic therapy before or after admission.
Future perspectives
An upcoming randomized, double-blind, placebo-controlled study (ABACOPD) targeting to include 980 patients with moderate AECOPD receiving state-of-the-art treatment will be the first study to investigate whether use of placebo is not inferior to antibiotic treatment [39]. The results of this study may help to further optimize the risk–benefit ratio of antibiotics for patients with a severe COPD exacerbation. However, the first results showed that the study failed to demonstrate non-inferiority of placebo to sultamicillin across all AECOPD patients, but suggests that antibiotic therapy could be withheld in GOLD stage I- II patients [40].
Our study highlights the need for Antibiotic Stewardship and more evidence about “SMART” biomarkers to support clinicians whether or not to prescribe antibiotics for AECOPD. Most of the approaches to define a bacterial AECOPD fail to discriminate between airway colonization and the cause of the infection when detecting bacteria. In the case of co-infections it is even more difficult to differentiate between the relative effect of the isolated pathogens [11]. Future guidelines should guide clinicians not only in the choice of antibiotic, but also the use to increase the benefit-risk ratio and include recommendations regarding laboratory results and patient-related variables.
Conclusion
In summary, this observational study in a Belgian tertiary hospital demonstrated that several patient-, hospitalization-, and treatment- related variables, clinical variables and laboratory results were associated with in-hospital antibiotic use in severe AECOPD patients. AECOPD patients treated with antibiotics were associated with a longer LOS compared to AECOPD patients not treated with antibiotics, which may be linked to their disease severity, slower response to treatment or ‘harm’ due to antibiotics.
Availability of data and materials
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- AB:
-
Antibiotics
- AECOPD:
-
Acute exacerbations of COPD
- APR-DRG:
-
All Patient Refined Diagnosis Related Groups
- ATC:
-
Anatomical Therapeutic Chemical
- BAPCOC:
-
Belgian Antibiotic Policy Coordination Committee
- BMI:
-
Body mass index
- CI:
-
Confidence interval
- Co-amoxiclav:
-
Amoxicillin-clavulanic acid
- COPD:
-
Chronic obstructive pulmonary disease
- CRP:
-
C-reactive protein
- CT:
-
Computed tomography
- DDA:
-
Defined daily doses adjusted
- EPR:
-
Electronic patient records
- FEV1 :
-
Forced expiratory volume in one second
- GOLD:
-
Global Initiative for Chronic Obstructive Lung Disease
- HR:
-
Hazard ratio
- ICD:
-
International Classification of Diseases
- ICS:
-
Inhaled corticosteroids
- ICU:
-
Intensive care unit
- Ig:
-
Immunoglobulin
- Jan.:
-
January
- LOS:
-
Length of stay
- MHD:
-
Minimal Hospital Data
- MWF:
-
Monday, Wednesday, and Friday
- n:
-
Counts
- NVALT:
-
Dutch Association of Physicians in Chest Medicine and Tuberculosis
- Oct.:
-
October
- Pip-tazo:
-
Piperacillin-tazobactam
- PS:
-
Propensity scores
- PCR:
-
Polymerase chain reaction
- Q1-Q3:
-
Interquartile range
- SCS:
-
Systemic corticosteroid
- Sept.:
-
September
- SD:
-
Standard deviation
- w/wo:
-
With/without
References
Global Initiative for Chronic Obstructive Lung Disease. Global Strategy for the Diagnosis, Management and Prevention of COPD: 2022 Report 2022 Available from: https://goldcopd.org/wp-content/uploads/2021/12/GOLD-REPORT-2022-v1.1-22Nov2021_WMV.pdf.
Wedzicha JA, Seemungal TA. COPD exacerbations: defining their cause and prevention. Lancet (London, England). 2007;370(9589):786–96.
Sethi S, Murphy TF. Infection in the pathogenesis and course of chronic obstructive pulmonary disease. N Engl J Med. 2008;359(22):2355–65.
Moghoofei M, AzimzadehJamalkandi S, Moein M, Salimian J, Ahmadi A. Bacterial infections in acute exacerbation of chronic obstructive pulmonary disease: a systematic review and meta-analysis. Infection. 2020;48(1):19–35.
Wilson R, Sethi S, Anzueto A, Miravitlles M. Antibiotics for treatment and prevention of exacerbations of chronic obstructive pulmonary disease. J Infect. 2013;67(6):497–515.
Vollenweider DJ, Frei A, Steurer-Stey CA, Garcia-Aymerich J, Puhan MA. Antibiotics for exacerbations of chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2018;10:CD010257.
Miravitlles M, Anzueto A. Chronic respiratory infection in patients with chronic obstructive pulmonary disease: what is the role of antibiotics? Int J Mol Sci. 2017;18(7):1344.
Anthonisen NR, Manfreda J, Warren CP, Hershfield ES, Harding GK, Nelson NA. Antibiotic therapy in exacerbations of chronic obstructive pulmonary disease. Ann Intern Med. 1987;106(2):196–204.
Chen K, Pleasants KA, Pleasants RA, Beiko T, Washburn RG, Yu Z, et al. A systematic review and meta-analysis of sputum purulence to predict bacterial infection in COPD exacerbations. COPD. 2020;17(3):311–7.
The Belgian Society for Infectiology and Clinical Microbiology. Acute infectious exacerbation of bronchitis and tracheobronchitis in (adolescents) and adults with COPD 2019. Available from: https://www.bvikm.org/documenten.
Hoult G, Gillespie D, Wilkinson TMA, Thomas M, Francis NA. Biomarkers to guide the use of antibiotics for acute exacerbations of COPD (AECOPD): a systematic review and meta-analysis. BMC Pulm Med. 2022;22(1):194.
Prins HJ, Duijkers R, van der Valk P, Schoorl M, Daniels JMA, van der Werf TS, et al. CRP-guided antibiotic treatment in acute exacerbations of COPD in hospital admissions. Eur Respir J. 2019;53(5):1802014.
Belgian Antibiotic Policy Coordination Commission. Belgische gids voor anti-infectieuze behandeling in de ambulante praktijk. 2021. Available from: https://www.health.belgium.be/sites/default/files/uploads/fields/fpshealth_theme_file/belgische_gids_bapcoc_nl_2021_a4_2.pdf.
Llor C, Bjerrum L, Munck A, Hansen MP, Cordoba GC, Strandberg EL, et al. Predictors for antibiotic prescribing in patients with exacerbations of COPD in general practice. Ther Adv Respir Dis. 2013;7(3):131–7.
Gillespie D, Butler CC, Bates J, Hood K, Melbye H, Phillips R, et al. Associations with antibiotic prescribing for acute exacerbation of COPD in primary care: secondary analysis of a randomised controlled trial. Br J Gen Pract. 2021;71(705):e266–72.
Lopez-Campos JL, Hartl S, Pozo-Rodriguez F, Roberts CM. European CAt. Antibiotic Prescription for COPD Exacerbations Admitted to Hospital: European COPD Audit. PLoS One. 2015;10(4):e0124374.
Stefan MS, Rothberg MB, Shieh MS, Pekow PS, Lindenauer PK. Association between antibiotic treatment and outcomes in patients hospitalized with acute exacerbation of COPD treated with systemic steroids. Chest. 2013;143(1):82–90.
Petite SE, Murphy JA. Systemic corticosteroid and antibiotic use in hospitalized patients with chronic obstructive pulmonary disease exacerbation. Ann Pharmacother. 2019;53(2):144–50.
Gerber JS, Hersh AL, Kronman MP, Newland JG, Ross RK, Metjian TA. Development and application of an antibiotic spectrum index for benchmarking antibiotic selection patterns across hospitals. Infect Control Hosp Epidemiol. 2017;38(8):993–7.
Klinische Biologie UZ gent. UZ Gent laboratoriumgids [03/08/2022]. Available from: http://labgids.gent/.
Wang Y, Bos JH, Boezen HM, Alffenaar JC, van Boven JFM, Schuiling-Veninga CCM, et al. Influence of age on real-life effects of doxycycline for acute exacerbations among COPD outpatients: a population-based cohort study. BMJ Open Respir Res. 2020;7(1):e000535.
Ruparel M, Lopez-Campos JL, Castro-Acosta A, Hartl S, Pozo-Rodriguez F, Roberts CM. Understanding variation in length of hospital stay for COPD exacerbation: European COPD audit. ERJ Open Res. 2016;2(1):00034–2015.
Singanayagam A, Schembri S, Chalmers JD. Predictors of mortality in hospitalized adults with acute exacerbation of chronic obstructive pulmonary disease. Ann Am Thorac Soc. 2013;10(2):81–9.
Lopez-Campos JL, Hartl S, Pozo-Rodriguez F, Roberts CM. European CAt. European COPD Audit: design, organisation of work and methodology. Eur Respir J. 2013;41(2):270–6.
Versporten A, Gyssens IC, Pulcini C, Monnier AA, Schouten J, Milanic R, et al. Metrics to assess the quantity of antibiotic use in the outpatient setting: a systematic review followed by an international multidisciplinary consensus procedure. J Antimicrob Chemother. 2018;73(suppl_6):vi59–66.
Lieberman D, Lieberman D. Pseudomonal infections in patients with COPD: epidemiology and management. Am J Respir Med. 2003;2(6):459–68.
Drug TB. Penicillin allergy-getting the label right. BMJ. 2017;358: j3402.
Wang Z, Locantore N, Haldar K, Ramsheh MY, Beech AS, Ma W, et al. Inflammatory endotype-associated airway microbiome in chronic obstructive pulmonary disease clinical stability and exacerbations: a multicohort longitudinal analysis. Am J Respir Crit Care Med. 2021;203(12):1488–502.
the Dutch Association of Physicians in Chest Medicine and Tuberculosis (NVALT). Diagnostiek en behandeling COPD-longaanval in het ziekenhuis, 2017. Available from: https://richtlijnendatabase.nl/richtlijn/behandeling_copd-longaanval_in_het_ziekenhuis/antibiotica_bij_copd.html.
Tsimogianni AM, Papiris SA, Kanavaki S, Stathopoulos GT, Sotiropoulou C, Manali ED, et al. Predictors of positive sputum cultures in exacerbations of chronic obstructive pulmonary disease. Respirology (Carlton, Vic). 2009;14(8):1114–20.
Nouira S, Marghli S, Belghith M, Besbes L, Elatrous S, Abroug F. Once daily oral ofloxacin in chronic obstructive pulmonary disease exacerbation requiring mechanical ventilation: a randomised placebo-controlled trial. Lancet (London, England). 2001;358(9298):2020–5.
Lapeere H. Penicilline allergie 2019. Available from: https://www.uzgent.be/nl/home/Lists/PDFs%20zorgaanbod/Apotheek_Allergie%20en%20antibiotica.pdf.
Van Gasse AL, Oulkadi R, Mousati Z, Ebo DG, Chiriac AM, Van Der Poorten MM, et al. Prevalence of self-reported and confirmed penicillin allergy in a Belgian outpatient population. Allergy. 2020;75(8):2111–5.
Vermeersch K, Gabrovska M, Aumann J, Demedts IK, Corhay JL, Marchand E, et al. Azithromycin during Acute COPD Exacerbations Requiring Hospitalization (BACE): a Multicentre, Randomized, Double-blind, Placebo-controlled Trial. Am J Respir Crit Care Med. 2019; 200(7):857–68.
Baalbaki N, Giuliano C, Hartner CL, Kale-Pradhan P, Johnson L. Azithromycin Versus Beta-lactams in Hospitalized Patients with Acute Exacerbations of COPD. J Gen Intern Med. 2022;37(16):4183–8.
Dai MY, Qiao JP, Xu YH, Fei GH. Respiratory infectious phenotypes in acute exacerbation of COPD: an aid to length of stay and COPD assessment test. Int J Chron Obstruct Pulmon Dis. 2015;10:2257–63.
Bafadhel M, Greening NJ, Harvey-Dunstan TC, Williams JE, Morgan MD, Brightling CE, et al. Blood eosinophils and outcomes in severe hospitalized exacerbations of COPD. Chest. 2016;150(2):320–8.
Echevarria C, Steer J, Bourke SC. Coding of COPD Exacerbations and the Implications on Clinical Practice, Audit and Research. COPD. 2020;17(6):706–10.
Rohde GG, Koch A, Welte T, group As. Randomized double blind placebo-controlled study to demonstrate that antibiotics are not needed in moderate acute exacerbations of COPD–the ABACOPD study. BMC Pulm Med. 2015;15:5.
Rohde GG, Häckl S, Koch A, Kolditz M, Timmermann H, Andreas S, et al. Randomized double blind placebo-controlled study to demonstrate that antibiotics are not needed in moderate acute exacerbations of COPD - Results from the ABACOPD study. Eur Respir J. 2022;60(suppl 66):1727.
Acknowledgements
The authors would like to thank Stefanie Vermeersch, clinical research coordinator at Department of Respiratory Medicine; Laura Van Hecke and Louise Devlieger, former master students from the Faculty of Pharmaceutical Sciences Ghent University for their contributions to this research.
Funding
This research received no external funding.
Author information
Authors and Affiliations
Contributions
N.D.B. composed the statistical dataset on Minimal Hospital Data. F.B. composed the statistical dataset on the dispensing data. C.V.H. collected additional patient characteristics from the electronic patient records. A.V. performed analyses and wrote a first version of the manuscript under supervision of L.L. E.V.B was the Principal Investigator at Ghent University Hospital. All authors critically reviewed the manuscript. The author(s) read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study has been performed in accordance with the Declaration of Helsinki. The study protocol was approved by the Medical Ethics Committee of Ghent University Hospital (B670201939030) and, due to its retrospective nature, informed consent was waived.
Consent for publication
Not applicable.
Competing interests
E.V.B. is co-author of the Belgian outpatient and inpatient guidelines for AECOPD. L.L. reports honoraria for a lecture from the Institute for Continuing Study of Pharmacists (IPSA) and a fee for external expert consultation from AstraZeneca, outside the submitted work. A.V. reports a honorarium for a lecture from the Flemish Association of Hospital Pharmacists (VZA), outside the submitted work. The other authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Vanoverschelde, A., Van Hoey, C., Buyle, F. et al. In-hospital antibiotic use for severe chronic obstructive pulmonary disease exacerbations: a retrospective observational study. BMC Pulm Med 23, 138 (2023). https://doi.org/10.1186/s12890-023-02426-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12890-023-02426-3