Abstract
Background
Idiopathic pulmonary fibrosis (IPF) is a progressive lung disease with few treatment options. N-acetylcysteine (NAC) is a well-tolerated, inexpensive treatment with antioxidant and anti-fibrotic properties. The National Heart, Lung, and Blood Institute (NHLBI)-sponsored PANTHER (Prednisone Azathioprine and NAC therapy in IPF) trial confirmed the harmful effects of immunosuppression in IPF, and did not show a benefit to treatment with NAC. However, a post hoc analysis revealed a potential beneficial effect of NAC in a subgroup of individuals carrying a specific genetic variant, TOLLIP rs3750920 TT genotype, present in about 25% of patients with IPF. Here, we present the design and rationale for the Phase III, multi-center, randomized, double-blind, placebo-controlled Prospective Treatment Efficacy in IPF Using Genotype for NAC Selection (PRECISIONS) clinical trial.
Methods
The PRECISIONS trial will randomize 200 patients with IPF and the TOLLIP rs3750920 TT genotype 1:1 to oral N-acetylcysteine (600 mg tablets taken three times a day) or placebo for a 24-month duration. The primary endpoint is the composite of time to 10% relative decline in forced vital capacity (FVC), first respiratory hospitalization, lung transplantation, or death from any cause. Secondary endpoints include change in patient-reported outcome scores and proportion of participants with treatment-emergent adverse events. Biospecimens, including blood, buccal, and fecal will be collected longitudinally for future research purposes. Study participants will be offered enrollment in a home spirometry substudy, which explores time to 10% relative FVC decline measured at home, and its comparison with study visit FVC.
Discussion
The sentinel observation of a potential pharmacogenetic interaction between NAC and TOLLIP polymorphism highlights the urgent, unmet need for better, molecularly focused, and precise therapeutic strategies in IPF. The PRECISIONS clinical trial is the first study to use molecularly-focused techniques to identify patients with IPF most likely to benefit from treatment. PRECISIONS has the potential to shift the paradigm in how trials in this condition are designed and executed, and is the first step toward personalized medicine for patients with IPF.
Trial Registration ClinicalTrials.gov identifier: NCT04300920. Registered March 9, 2020. https://clinicaltrials.gov/ct2/show/NCT04300920
Similar content being viewed by others
Background
Idiopathic pulmonary fibrosis (IPF) is a chronic respiratory disease characterized by progressive scarring and architectural distortion of the lung parenchyma [1]. Patients with IPF have high morbidity, poor quality of life, and an average survival of 3–5 years from diagnosis [2]. There are only two Food and Drug Administration (FDA)-approved treatments for IPF in the United States, pirfenidone and nintedanib. Both drugs slow disease progression and have been linked to reduced mortality [3,4,5]. However, neither pharmacologic therapy reverses fibrosis or improves patient symptoms, and both drugs carry a significant side effect profile, making them poorly tolerated by a significant proportion of patients. Therefore, there is a continued, urgent, unmet need for more effective and better tolerated therapies for IPF.
In this protocol paper, we describe the design and rationale of the Phase III, multi-center randomized, double-blind, placebo-controlled Prospective Treatment Efficacy in IPF Using Genotype for NAC Selection (PRECISIONS) clinical trial. We highlight the novel approach of this trial to patient selection, by identifying individuals that carry a specific genetic variant, TOLLIP rs3750920 TT genotype, who are most likely to benefit from N-acetylcysteine (NAC) therapy. The PRECISIONS trial is one of the first studies to use a precision medicine approach in IPF, by testing the efficacy of a therapy in a targeted population based on a specific genotype.
Methods
Rationale
NAC is an inexpensive and well-tolerated agent for treatment of lung and other organ diseases [6]. There has been long-standing interest in the potential therapeutic properties of NAC in IPF. NAC increases production of the antioxidant glutathione and has demonstrated anti-fibrotic properties [7]. Oral administration of NAC in IPF patients leads to higher levels of glutathione in bronchoalveolar lavage fluid, and reduced markers of oxidative stress at the alveolar surface [8, 9]. The IFIGENIA trial examined the efficacy of NAC added to standard of care, which at the time included treatment with prednisone plus azathioprine, compared to standard of care alone, on disease progression in IPF. Therapy with NAC at 600 mg three times daily added to prednisone and azathioprine slowed the decline in FVC and single-breath carbon monoxide diffusing capacity (DLCO) at one year [10].
The IPFnet PANTHER trial compared the effect of NAC alone or NAC with prednisone and azathioprine versus placebo on lung function decline in IPF [11]. There was increased mortality observed in the combination therapy arm and this arm was terminated early. There was no difference in outcomes between the NAC-only arm and placebo in the overall study population [12]. However, a post hoc analysis suggested that shifting patterns in participant enrollment and characteristics over the course of the study, and specifically after the early termination of the immunosuppressive therapy arm, may have influenced the observed results. This leaves open the possibility that a subgroup of patients with IPF may benefit from NAC.
This rationale led to the candidate genotyping of polymorphisms in TOLLIP and MUC5B in the PANTHER trial cohort. A significant interaction between the TOLLIP rs3750920 genotype and NAC therapy was found [13]. Participants that carried the TOLLIP TT genotype (about 25% of the cohort) and received NAC had a significant reduction in the composite endpoint of death, transplant, hospitalization or ≥ 10% FVC decline compared with those who received placebo. In contrast, participants with the TOLLIP CC genotype (about 25% of the cohort) showed a trend toward harm from NAC treatment. Those with a CT genotype (about 50% of the cohort) had similar outcomes to those treated with placebo. These findings were validated in an additional independent cohort [13].
The TOLLIP gene encodes an ubiquitin-binding protein that regulates innate immune response by inhibiting Toll-like receptor (TLR) signaling [14]. Variants in TOLLIP have been linked with IPF susceptibility and mortality in patients of European ancestry [15]. TLRs play a critical role in the innate immune response to various pathogen-associated molecular patterns (PAMPs). Alterations in TLR expression and signaling have been linked to IPF disease progression and mortality [16,17,18]. The TOLLIP rs3750920 TT genotype results in increased expression of TOLLIP in human monocytes, which may influence TLR signaling and host response to immunomodulatory therapies [19]. These findings suggest a potential mechanism for the heterogeneity of treatment effect based on the TOLLIP genotype observed in PANTHER, and that NAC therapy may provide clinical benefit in a significant number of patients with IPF.
Study overview
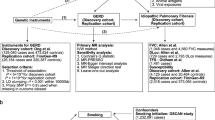
PRECISIONS is a multi-center, randomized, double-blind, placebo-controlled trial of 200 participants with TOLLIP rs3750920 TT genotype to receive NAC or placebo for a 24-month duration. The structure of the study is shown in Fig. 1. Study participants will be randomized to NAC or placebo in a 1:1 fashion, while receiving standard of care. Standard of care is defined as allowing background therapy with FDA-approved medications for IPF, such as pirfenidone or nintedanib, if taking a stable dose for at least 6 weeks prior to enrollment. Randomization will be stratified by stable concomitant IPF therapy use (pirfenidone or nintedanib) versus no pirfenidone or nintedanib use. A schematic overview of the study design is shown in Fig. 1.
Hypothesis
Patients with idiopathic pulmonary fibrosis (IPF) that have the TOLLIP rs3750920 TT genotype will exhibit improved clinical outcomes when treated with N-acetyl cysteine (NAC) compared to placebo, while receiving standard care.
Study objective
The primary objective is to compare the effect of NAC plus standard care versus matched placebo plus standard care on the time to a composite endpoint of relative decline in lung function [10% relative decline in FVC, first respiratory hospitalization, lung transplantation, or all-cause mortality] in patients diagnosed with IPF who have the TOLLIP rs3750920 TT genotype. The secondary objectives will be to examine the effect of NAC on the components of the primary composite endpoint, the rates of clinical events, change in physiology, change in health status, and change in respiratory symptoms.
Eligibility
The inclusion and exclusion criteria are listed in Table 1. Eligible participants must have a clinical diagnosis of IPF and the TOLLIP rs3750920 TT genotype. The exclusion criteria are primarily related to contraindications to receiving NAC. Participants will be recruited from approximately 25 sites in the US selected primarily from the Pulmonary Fibrosis Foundation (PFF) Care Center Network.
Determination of TOLLIP genotype
A portion of participants we plan to enroll in PRECISIONS will have confirmed TOLLIP rs3750920 TT genotype through their participation in the PFF Registry and Biorepository. Samples from PFF Registry participants who previously consented to DNA genotyping and to being directly contacted for future enrollment in clinical trials will be sent coded to the University of Virginia for TOLLIP genotyping. The Clinical Coordinating Center (CCC) will notify study sites of potential participants with confirmed TT genotype for potential enrollment into the PRECISIONS trial. Additionally, to reach a recruitment target of 200 randomized participants, we will recruit participants with IPF and unknown TOLLIP genotype. These potential participants will undergo a screening visit, in which a blood sample will be collected for genotyping after informed consent is obtained. The University of Virginia School of Medicine, with supervision from Dr. Imre Noth’s laboratory, will determine the TOLLIP rs3750920 genotype for all blood samples collected from the genotype screening visits, and will report back to sites within 14 days.
Study intervention
Participants will be randomized to NAC (600 mg tablets to be taken three times a day by mouth) or placebo for 24 months. Zambon Pharma (Vicenza, Italy) will supply the study drug and matching placebo (white, round tablets in blister packaging). The study drug distribution to enrolling institutions will be overseen by the investigational drug pharmacy at Temple University. Participants will initially receive a 4-month supply upon their randomization visit and 4-month refills at subsequent 4-month study visits.
Safety review
The most frequent adverse events related to the oral use of NAC are gastrointestinal (nausea, vomiting, diarrhea, abdominal pain, nausea, stomatitis) [20]. NAC may have a detrimental effect on the stomach’s mucosa which may be relevant to patients with active peptic ulcers. Less frequent events that have been reported include anaphylactic shock, anaphylactoid reaction, bronchospasm, angioedema, dyspnea, tachycardia, rash, pruititis, and urticaria [21]. Headache, tinnitus, pyrexia, hypotension, facial edema and hemorrhage have also been reported with oral NAC. NAC has been shown to reduce platelet aggregation, although the clinical significance of this is unknown [22].
The PANORAMA study showed that IPF patients receiving both oral NAC and pirfenidone experienced higher rates of photosensitivity rash compared to those that received pirfenidone only (9% vs. 2%). However, these rates were markedly lower than the rates reported in pooled phase III trial data (33%), suggesting that this difference in the PANORAMA study may have been driven by its smaller sample size [23]. PANORAMA and a small study from Japan that examined the efficacy and tolerability of combination therapy with inhaled NAC and pirfenidone versus pirfenidone alone had findings that suggested more rapid disease progression in the group that received combination therapy [24]. The TOLLIP TT genotype of participants in both studies was unknown and has been shown to have a very low frequency among Japanese IPF patients. In addition, differences in dosage, modes of delivery for NAC and smaller sample sizes make the collective interpretation of these studies’ findings difficult [25].
Procedures
At the enrollment study visit, the study coordinator will obtain and provide the following information:
-
Informed consent
-
Demographics
-
Eligibility Review
-
Medical History
-
Complete Physical Exam
-
Vital Signs
-
Study Site Spirometry
-
Home Spirometry Tutorial
-
DLCO
-
Quality-of-life questionnaires (Leicester Cough Questionnaire [26], EuroQoL EQ-5D [27], UCSD-SOB [28], K-BILD [29, 30], SGRQ scores [31, 32], R-Scale-PF [33])
-
COVID-19 Questionnaire (Additional file 1)
-
Blood collection (complete blood count, chemistry pane, liver function tests)
-
Pregnancy Test
-
Concomitant medication review
-
Plasma, serum, RNA, buccal, and fecal sample collection
-
Adverse Event Review and Evaluation
-
Randomization
-
Administration of study drug
-
Distribution of drug diary
Study participants will undergo follow-up study visits every 4 months. Study procedures are summarized in Table 2. Follow-up COVID-19 Questionnaire is included as Additional File 2.
Home spirometry
Home spirometry has been shown to be feasible and practical in tracking IPF progression for patients [34]. PRECISIONS participants will be asked to perform spirometry at home three times a week (Monday, Wednesday and Friday) in the morning using hand-held, vertically mounted turbine spirometers (GoSpiro®, Monitored Therapeutics Inc, Dublin, OH, USA). Participants and study investigators will be blinded to the results of home spirometry for the duration of the study. Participants will receive feedback on quality of the maneuver by the GoSpiro® avatar. Home spirometry data will undergo quality control and quality assurances by the University of California Los Angeles (UCLA) Exercise Physiology Research Laboratory, under the supervision of Dr. Christopher Cooper, in coordination with the data coordinating center. Participation in the home spirometry study will be optional. Participants will be asked reasons for choosing not to participate in home spirometry, and will be surveyed about their experience with home spirometry during and at the end of the study (Additional files 3 and 4).
Primary endpoints and endpoint adjudication
The primary endpoint of the PRECISIONS trial will be the time to a composite of 10% relative FVC decline, first respiratory hospitalization, lung transplantation, or all-cause mortality. Each of the events comprising the primary composite endpoint are clinically meaningful. FVC decline and hospitalizations have been linked to disease progression and subsequent time to death [35, 36]. Lung transplantation is a surrogate marker of disease progression and severity. Respiratory hospitalizations will be determined by a blinded clinical events adjudication committee.
Secondary endpoints
Secondary endpoints include the following:
-
Time to 10% relative decline in FVC% predicted, first respiratory hospitalization, lung transplantation, or death from any cause
-
Time to death from any cause
-
Time to first respiratory hospitalization
-
Time to 10% relative decline in FVC
-
Time to lung transplantation
-
Time to 10% relative decline in FVC% predicted
-
Time to first all-cause hospitalization
-
Annualized rate of respiratory hospitalizations
-
Annualized rate of non-elective, all-cause hospitalizations
-
Proportion of participants undergoing lung transplantation during follow-up
-
Change in FVC % predicted from randomization to 12 and 24 months
-
Change in FVC from randomization to 12 and 24 months
-
Change in DLCO uncorrected, from randomization to 12 and 24 months
-
Change in patient-reported outcomes scores from randomization to 12 and 24 months (Leicester Cough Questionnaire, EuroQoL EQ-5D, UCSD-SOB, K-BILD, SGRQ scores)
-
Proportion of participants with and number of treatment- emergent adverse events, serious adverse events, adverse events leading to discontinuation, unanticipated problems
Exploratory endpoints
Exploratory endpoints are related to home spirometry and include the following:
-
Time to 10% relative decline in home spirometry FVC
-
Time to 10% relative decline in home spirometry FVC % predicted
-
Change in home spirometry FVC % predicted from randomization to 12 and 24 months
-
Change in home spirometry FVC from randomization to 12 and 24 months
-
Comparison between a 10% relative decline captured by home spirometry with the relative change in FVC captured by the next on-site FVC measurement
Data and safety monitoring board
Safety and oversight will be under the direction of a Data and Safety Monitoring Board (DSMB) that is appointed by the NHLBI. The DSMB will be composed of members with the appropriate expertise for this study. Members of the DSMB will be independent from the study conduct and without conflict of interest. The DSMB will meet at least semiannually to assess the safety and efficacy data for each study arm. They will review the study protocol, monitor all aspects of the study (recruitment, adverse events, protocol adherence, data quality, attrition, demographic and baseline characteristics), and recommend protocol modifications, including early study termination. Any proposed changes to the study protocol will also be reviewed by the DSMB.
Statistical analysis plan
Continuous variables will be summarized using descriptive statistics including, n, means, standard deviations, medians, 25th and 75th percentiles, and minimum and maximum. Categorical variables will be presented as counts and percentages. Demographic and baseline summaries will be provided by treatment group and overall. Two-sided hypotheses will be tested for endpoints at the 5% level. SAS software (SAS Institute, Inc., Cary, NC, USA) and R freeware will be used to perform the analyses.
Analysis of the primary endpoint
The primary analysis plan will be based on a modified intent-to-treat design applied to participants who have received at least one dose of study medication. A two-sample log-rank test will be used to assess whether assignment to a 24-month course of NAC is superior to placebo for the primary composite endpoint (time to 10% relative decline in FVC, first respiratory hospitalization, lung transplantation, or death from any cause). Participants will be censored at 2 years of follow-up or at loss to follow-up, whichever occurs first.
Secondary analyses include assessing treatment differences in the composite endpoint graphically using Kaplan–Meier estimates. The effect of NAC versus placebo will be explored in subgroups of interest with results tabulated (estimated hazard ratio by subgroup, corresponding 95% confidence interval and p-value) as well as displayed via forest plots. Pre-specified subgroups include concomitant therapy usage, sex, race/ethnicity, smoking history, dichotomized age, dichotomized baseline FVC (L and % predicted), dichotomized baseline DLCO (ml/min/mm Hg and % predicted) and any respiratory hospitalizations in the previous year. Multivariable analyses of the primary endpoint will be conducted based on whether proportionality of hazards holds. Cox proportional hazards models will be used if the hazards are proportional, otherwise restricted mean regression will be used. Variables adjusted for in the multivariable model will include concomitant therapy (pirfenidone or nintedanib vs. none), age, sex, baseline FVC, and baseline DLCO. These variables are potential confounders and are commonly included in multivariable analyses of IPF studies. In addition, we will adjust for home spirometry participation if the participation of home spirometry is below 90% of randomized study participants. The adjusted hazards ratio, 95% confidence interval, and p-value will be used to assess the impact of NAC versus placebo on the primary composite endpoint from the multivariable model.
If there is more than 10% loss to-follow-up, sensitivity analyses will be performed based on multiple imputation of the primary outcome. If more than 10% of participants having missing adjustment variables for the secondary analyses of the primary endpoint, then sensitivity analyses multiply imputing the missing predictors will be performed. In the event more than 10% of participants switch concomitant therapy use after randomization (ex. participant who was not using pirfenidone at start of trial starts using pirfenidone mid-way through the trial), a sensitivity analyses will be performed that uses inverse weighting methodology.
Analysis of secondary and exploratory endpoints
The analyses for time-to-event secondary and exploratory endpoints will be similar to those outlined for the primary endpoint. Rate endpoint analyses will be based on univariable and multivariable zero-inflated negative binomial regression models. Analyses of trajectory endpoints will use a repeated measure approach that uses mixed models and incorporates all available assessments from randomization through 24 months. In order to examine the sensitivity and specificity of home spirometry values in predicting subsequent primary and secondary time-to-event outcomes, an exploratory receiver operating characteristic analysis will be performed.
Sample size and power calculation
It is anticipated that the event rate in the placebo arm will be highly dependent on the proportion of patients enrolled at different Gender, Age, and Physiology (GAP) scores [37]. The GAP index score will likely be heavily weighted towards 3 given the availability of pirfenidone and nintedanib. Assuming an exponential distribution for the composite endpoint comparable to that from the PANTHER study, we conservatively estimate a placebo yearly event rate to be 24% for the primary composite endpoint in participants not taking concomitant therapy [11]. We have also taken into account concomitant IPF therapy and conservatively estimate that 65% of the study population will be taking one of the two anti-fibrotic medications approved for treatment. We estimate the placebo event rate among participants taking concomitant IPF medications will be lower at a 12% yearly even rate. The estimate of the magnitude of the treatment effect is based on the PANTHER pharmaco-genetic study of the TOLLIP TT genotype, in which the primary endpoint was also a composite endpoint (decline of at least 10% in FVC, lung transplantation, hospitalization, and/or time to death) [13]. The hazard ratio was 0.14 in the PANTHER cohort and 0.23 in the validation cohort, both of which are large effect estimates. Given the retrospective and observational nature of this study, we took a very conservative approach in estimating the treatment effect. The estimate effect size for PRECISIONS is a 66.7% event reduction between NAC and placebo. Based on these estimates and a 10% dropout rate, we plan to enroll 200 randomized participants, which will provide 90.1% power assuming a two-sided type-I error rate of 0.05. Table 3 summarizes the power estimates for various event rates and treatment effects.
An interim sample size adjustment is also incorporated into the statistical analysis plan. Upon enrollment of at least 25 placebo participants with at least 12 months of follow-up, an interim sample size adjustment analysis will be performed. If the observed one-year event rate in this group of placebo participants is below 16%, the sample size will be increased by 40 participants to maintain at least 80% power for the study assuming a 66.7% event rate reduction in the NAC arm compared with placebo. No type I error would be spent as part of this analysis because the adjustment would not take into account the event rate in the NAC arm. This approach is a precaution that protects study power in the case of the placebo arm event rate being below the range anticipated in the design of the trial.
Additional objectives and ancillary studies
In addition to the clinical trial, a major objective of PRECISIONS is to apply blood-based ‘omics technologies and provide novel insights in the pathogenesis of pulmonary fibrosis and different types of interstitial lung disease (ILD). In collaboration with the PFF and its biorepository, PRECISIONS will perform whole genome sequencing, proteomic analyses, transcriptional profiling and other ‘omics-powered analyses on blood samples from approximately 2000 ILD patients. Accomplishing this objective may enable identification of subgroups of patients who are more responsive to specific therapies based on the underlying pathobiology, and lead to future precision medicine trials not only for IPF but also other types of ILD. This is an opportunity for investigators to leverage the data generated from PRECISIONS to catalyze new diagnostic and therapeutic developments for pulmonary fibrosis. Ancillary study and publications committees have been created to ensure a structured process in adjudicating study proposals from researchers requesting data (including biospecimens) that ensures plausibility and appropriate resource allocation.
Discussion
Personalizing medicine for patients with IPF
Precision medicine approaches have been adopted in the treatment of malignancy and cardiovascular disease, with an increasing number of therapies prescribed based on an individual’s clinical risk and genetic profile [38]. This approach has been lacking in pulmonary fibrosis. Patients with IPF have highly heterogenous clinical trajectories, and prognosis for each individual patient is difficult to predict with currently available clinical and molecular tools. Multiple genetic and environmental factors have been linked to susceptibility and progression of IPF [1]. These key differences among patients with IPF suggest that subgroups of patients may respond differently to treatments. The gap between basic/translational research and its application to the clinical care of IPF patients has narrowed, driven by collaborative efforts among researchers to advance the understanding of mechanisms that drive progressive lung fibrosis, and disprove ineffective treatments. PRECISIONS is rooted in a plausible mechanism that links dysregulated innate immunity to pulmonary fibrosis based on pre-clinical models, identification of genes critical in regulating immunity (i.e., TOLLIP), and pharmaco-genetic studies that suggest a potential benefit of NAC in IPF patients with the TOLLIP TT genotype. This study demonstrates the feasibility of designing an IPF clinical trial that uses a precision medicine approach and may ultimately identify an effective therapy for a subgroup of patients with this disease.
Leveraging the PFF care center network for clinical trials and research
Since the TOLLIP TT genotype is only present in about 25% of patients with IPF, reaching our enrollment target of 200 participants requires screening of about 800 patients. We expect that 75% of patients with IPF will not meet the genotype requirement. To mitigate this high screen failure rate, we are leveraging existing blood samples from subjects enrolled in the PFF Patient Registry and Biorepository. The PFF Patient Registry and Biorepository prospectively enrolls participants who have been diagnosed with IPF and other interstitial lung diseases at more than 70 Care Center Network sites across the United States. The purpose of the PFF Registry and Biorepository is to increase opportunities for clinical investigation in pulmonary fibrosis by collecting clinical data and biological samples of consented patients with pulmonary fibrosis [39]. Patient recruitment for PRECISIONS will occur at approximately 25 sites selected from PFF Care Center Network, based on broad geographic coverage and participation in the Biorepository. It is hoped that PRECISIONS can provide a paradigm for how future studies can leverage the infrastructure of the PFF Care Center Network and the PFF Patient Registry to execute targeted trials in a broad and diverse population of patients with pulmonary fibrosis.
Utility of home spirometry in future IPF trials
Endpoints in IPF clinical trials have traditionally used FVC obtained at in-person study site visits. Home spirometry has been shown to be feasible and practical in tracking disease severity and progression in patients with IPF [34, 40]. There has been significant interest in using FVC measured by home spirometry as a clinical endpoint in IPF trials. This approach may potentially allow for more frequent measurements, more precise assessments of changes in FVC, and reduced sample sizes required for clinical trials. However, the utility of home spirometry in IPF clinical trials remains uncertain [41, 42]. The home spirometry substudy of PRECISIONS provides an opportunity to investigate the most effective protocols and appropriate analysis plans that may shape future clinical trials to use home spirometry as a primary endpoint. By making the substudy optional, we will also investigate the characteristics of those patients who opt into the study, and examine barriers to home spirometry in this patient population. Data obtained from home spirometry will provide valuable information on potentially incorporating this procedure in future clinical trials.
Future studies
The rationale and design of the PRECISIONS trial was largely shaped by data from the IPFnet PANTHER trial and other cohorts of IPF patients. The usage of stored biospecimens from clinical trials is critical in expanding our knowledge of IPF, identifying novel treatments, and determining which subgroups of IPF patients may benefit by investigative therapies. Therefore, biospecimens will be collected and stored from participants in the PRECISIONS trial for future research purposes. Blood will be collected at each study visit and will include plasma, serum, RNA, and cellular samples. Buccal and fecal samples will also be collected at each study visit. This extensive and serial collection of biospecimens will facilitate further research in IPF.
Support
PRECISIONS is primarily funded by the National Lung Heart and Blood Institute (grant number UH3HL145266 and U24HL145265), National Institutes of Health. Additional funding sources include the Three Lakes Foundation, which is a philanthropic organization with the mission of advancing care for IPF patients by supporting efforts to improve time to diagnosis and accelerate new treatments (https://threelakesfoundation.org/). The Pulmonary Fibrosis Foundation is an integral collaborator for this clinical trial as PRECISIONS has the potential to demonstrate the utility of partnering with prospective cohorts to ensure clinical trial enrichment (https://pulmonaryfibrosis.org/).
In summary, PRECISIONS takes a novel approach to IPF clinical trials by targeting a subgroup of patients with IPF and a specific genotype that may benefit from NAC therapy based on prior pharmaco-genetic studies. In addition, the collection of various biological specimens, exploratory utility of home spirometry, and comprehensive clinical event data will provide substantial resources for future investigations of IPF and other types of ILD.
Availability of data and materials
Not applicable.
Abbreviations
- CCC:
-
Clinical Coordinating Center
- DLCO:
-
Diffusing capacity for carbon monoxide
- DSMB:
-
Data and Safety Monitoring Board
- FDA:
-
Food and Drug Administration
- FVC:
-
Forced vital capacity
- GAP:
-
Gender, age, and physiology
- ILD:
-
Interstitial lung disease
- IPF:
-
Idiopathic pulmonary fibrosis
- K-BILD:
-
King’s brief interstitial lung disease
- NAC:
-
N-Acetylcysteine
- NHLBI:
-
National Heart Lung and Blood Institute
- PAMP:
-
Pathogen-associated molecular pattern
- PANTHER:
-
Prednisone azathioprine and NAC therapy in IPF
- PFF:
-
Pulmonary Fibrosis Foundation
- PRECISIONS:
-
Prospective Treatment Efficacy in IPF Using Genotype for NAC Selection
- SGRQ:
-
St. George’s Respiratory Questionnaire
- TLR:
-
Toll-like receptor
- UCLA:
-
University of California Los Angeles
- UCSD-SOB:
-
University of California San Diego Shortness of Breath
References
Lederer DJ, Martinez FJ. Idiopathic pulmonary fibrosis. N Engl J Med. 2018;378(19):1811–23.
Raghu G, Chen SY, Yeh WS, Maroni B, Li Q, Lee YC, Collard HR. Idiopathic pulmonary fibrosis in US Medicare beneficiaries aged 65 years and older: incidence, prevalence, and survival, 2001–11. Lancet Respir Med. 2014;2(7):566–72.
King TE Jr, Bradford WZ, Castro-Bernardini S, Fagan EA, Glaspole I, Glassberg MK, Gorina E, Hopkins PM, Kardatzke D, Lancaster L, et al. A phase 3 trial of pirfenidone in patients with idiopathic pulmonary fibrosis. N Engl J Med. 2014;370(22):2083–92.
Richeldi L, du Bois RM, Raghu G, Azuma A, Brown KK, Costabel U, Cottin V, Flaherty KR, Hansell DM, Inoue Y, et al. Efficacy and safety of nintedanib in idiopathic pulmonary fibrosis. N Engl J Med. 2014;370(22):2071–82.
Behr J, Prasse A, Wirtz H, Koschel D, Pittrow D, Held M, Klotsche J, Andreas S, Claussen M, Grohe C et al: Survival and course of lung function in the presence or absence of antifibrotic treatment in patients with idiopathic pulmonary fibrosis: long-term results of the INSIGHTS-IPF registry. Eur Respir J 2020;56(2).
Smilkstein MJ, Douglas DR, Daya MR. Acetaminophen poisoning and liver function. N Engl J Med. 1994;331(19):1310–1 (author reply 1311–1312).
Rushworth GF, Megson IL. Existing and potential therapeutic uses for N-acetylcysteine: the need for conversion to intracellular glutathione for antioxidant benefits. Pharmacol Ther. 2014;141(2):150–9.
Behr J, Maier K, Degenkolb B, Krombach F, Vogelmeier C. Antioxidative and clinical effects of high-dose N-acetylcysteine in fibrosing alveolitis: adjunctive therapy to maintenance immunosuppression. Am J Respir Crit Care Med. 1997;156(6):1897–901.
Meyer A, Buhl R, Magnussen H. The effect of oral N-acetylcysteine on lung glutathione levels in idiopathic pulmonary fibrosis. Eur Respir J. 1994;7(3):431–6.
Demedts M, Behr J, Buhl R, Costabel U, Dekhuijzen R, Jansen HM, MacNee W, Thomeer M, Wallaert B, Laurent F, et al. High-dose acetylcysteine in idiopathic pulmonary fibrosis. N Engl J Med. 2005;353(21):2229–42.
Idiopathic Pulmonary Fibrosis Clinical Research N, Raghu G, Anstrom KJ, King TE, Jr., Lasky JA, Martinez FJ: Prednisone, azathioprine, and N-acetylcysteine for pulmonary fibrosis. N Engl J Med 2012;366(21):1968–77.
Idiopathic Pulmonary Fibrosis Clinical Research N, Martinez FJ, de Andrade JA, Anstrom KJ, King TE, Jr., Raghu G: Randomized trial of acetylcysteine in idiopathic pulmonary fibrosis. N Engl J Med 2014;370(22):2093–101.
Oldham JM, Ma SF, Martinez FJ, Anstrom KJ, Raghu G, Schwartz DA, Valenzi E, Witt L, Lee C, Vij R, et al. TOLLIP, MUC5B, and the response to N-acetylcysteine among individuals with idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2015;192(12):1475–82.
Capelluto DG. Tollip: a multitasking protein in innate immunity and protein trafficking. Microbes Infect. 2012;14(2):140–7.
Noth I, Zhang Y, Ma SF, Flores C, Barber M, Huang Y, Broderick SM, Wade MS, Hysi P, Scuirba J, et al. Genetic variants associated with idiopathic pulmonary fibrosis susceptibility and mortality: a genome-wide association study. Lancet Respir Med. 2013;1(4):309–17.
O’Dwyer DN, Armstrong ME, Trujillo G, Cooke G, Keane MP, Fallon PG, Simpson AJ, Millar AB, McGrath EE, Whyte MK, et al. The Toll-like receptor 3 L412F polymorphism and disease progression in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2013;188(12):1442–50.
Samara KD, Antoniou KM, Karagiannis K, Margaritopoulos G, Lasithiotaki I, Koutala E, Siafakas NM. Expression profiles of Toll-like receptors in non-small cell lung cancer and idiopathic pulmonary fibrosis. Int J Oncol. 2012;40(5):1397–404.
Trujillo G, Meneghin A, Flaherty KR, Sholl LM, Myers JL, Kazerooni EA, Gross BH, Oak SR, Coelho AL, Evanoff H, et al. TLR9 differentiates rapidly from slowly progressing forms of idiopathic pulmonary fibrosis. Sci Transl Med. 2010;2(57):57ra82.
Shah JA, Vary JC, Chau TT, Bang ND, Yen NT, Farrar JJ, Dunstan SJ, Hawn TR. Human TOLLIP regulates TLR2 and TLR4 signaling and its polymorphisms are associated with susceptibility to tuberculosis. J Immunol. 2012;189(4):1737–46.
Company Core Safety Information for NAC oral formulation. In.: Zambon SpA; July 25, 2008.
N-Acetyl-cysteine (NAC) Oral formulations, Fluimucil. In., 5 edn. Bresso, Milan, Italy: ZAMBON SpA; 2009.
Chirkov YY, Horowitz JD. N-Acetylcysteine potentiates nitroglycerin-induced reversal of platelet aggregation. J Cardiovasc Pharmacol. 1996;28(3):375–80.
Noble PW, Albera C, Bradford WZ, Costabel U, du Bois RM, Fagan EA, Fishman RS, Glaspole I, Glassberg MK, Lancaster L, et al. Pirfenidone for idiopathic pulmonary fibrosis: analysis of pooled data from three multinational phase 3 trials. Eur Respir J. 2016;47(1):243–53.
Sakamoto S, Kataoka K, Kondoh Y, Kato M, Okamoto M, Mukae H, Bando M, Suda T, Yatera K, Tanino Y et al: Pirfenidone plus inhaled N-acetylcysteine for idiopathic pulmonary fibrosis: a randomised trial. Eur Respir J 2021;57(1).
Podolanczuk AJ, Noth I, Raghu G: Idiopathic pulmonary fibrosis: prime time for a precision-based approach to treatment with N-acetylcysteine. Eur Respir J 2021;57(1).
Leconte S, Ferrant D, Dory V, Degryse J. Validated methods of cough assessment: a systematic review of the literature. Respiration. 2011;81(2):161–74.
King P: The EuroQoL instrument: an index of health-related quality of life. In: Quality of life and pharmacoeconomics in clinical trials. edn. Edited by Spilker B. Philadelphia (PA): Lippincott-Raven Publishers; 1996: 191–201.
Eakin EG, Resnikoff PM, Prewitt LM, Ries AL, Kaplan RM. Validation of a new dyspnea measure: the UCSD Shortness of Breath Questionnaire. University of California, San Diego. Chest. 1998;113(3):619–24.
Patel AS, Siegert RJ, Brignall K, Gordon P, Steer S, Desai SR, Maher TM, Renzoni EA, Wells AU, Higginson IJ, et al. The development and validation of the King’s Brief Interstitial Lung Disease (K-BILD) health status questionnaire. Thorax. 2012;67(9):804–10.
Sinha A, Patel AS, Siegert RJ, Bajwah S, Maher TM, Renzoni EA, Wells AU, Higginson IJ, Birring SS. The King’s Brief Interstitial Lung Disease (KBILD) questionnaire: an updated minimal clinically important difference. BMJ Open Respir Res. 2019;6(1): e000363.
Barr JT, Schumacher GE, Freeman S, LeMoine M, Bakst AW, Jones PW. American translation, modification, and validation of the St. George’s Respiratory Questionnaire Clin Ther. 2000;22(9):1121–45.
Jones PW, Quirk FH, Baveystock CM. The St George’s Respiratory Questionnaire. Respir Med. 1991;85 Suppl B:25–31 (discussion 33–27).
Scallan C, Strand L, Hayes J, Kadura S, Collins B, Ho L, Spada C, Canestaro W, Kolb M, Raghu G: R-Scale for Pulmonary Fibrosis (PF): a simple, visual tool for the assessment of health-related quality of life. Eur Respir J 2021.
Russell AM, Adamali H, Molyneaux PL, Lukey PT, Marshall RP, Renzoni EA, Wells AU, Maher TM. Daily home spirometry: an effective tool for detecting progression in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2016;194(8):989–97.
du Bois RM, Weycker D, Albera C, Bradford WZ, Costabel U, Kartashov A, King TE Jr, Lancaster L, Noble PW, Sahn SA, et al. Forced vital capacity in patients with idiopathic pulmonary fibrosis: test properties and minimal clinically important difference. Am J Respir Crit Care Med. 2011;184(12):1382–9.
Ley B, Swigris J, Day BM, Stauffer JL, Raimundo K, Chou W, Collard HR. Pirfenidone reduces respiratory-related hospitalizations in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2017;196(6):756–61.
Ley B, Ryerson CJ, Vittinghoff E, Ryu JH, Tomassetti S, Lee JS, Poletti V, Buccioli M, Elicker BM, Jones KD, et al. A multidimensional index and staging system for idiopathic pulmonary fibrosis. Ann Intern Med. 2012;156(10):684–91.
Collins FS, Varmus H. A new initiative on precision medicine. N Engl J Med. 2015;372(9):793–5.
Wang BR, Edwards R, Freiheit EA, Ma Y, Burg C, de Andrade J, Lancaster L, Lindell K, Nathan SD, Raghu G, et al. The pulmonary fibrosis foundation patient registry: rationale, design, and methods. Ann Am Thorac Soc. 2020;17(12):1620–8.
Johannson KA, Vittinghoff E, Morisset J, Lee JS, Balmes JR, Collard HR: Home monitoring improves endpoint efficiency in idiopathic pulmonary fibrosis. Eur Respir J 2017;50(1).
Maher TM, Corte TJ, Fischer A, Kreuter M, Lederer DJ, Molina-Molina M, Axmann J, Kirchgaessler KU, Samara K, Gilberg F, et al. Pirfenidone in patients with unclassifiable progressive fibrosing interstitial lung disease: a double-blind, randomised, placebo-controlled, phase 2 trial. Lancet Respir Med. 2020;8(2):147–57.
Noth I, Cottin V, Chaudhuri N, Corte TJ, Johannson KA, Wijsenbeek M, Jouneau S, Michael A, Quaresma M, Rohr KB et al: Home spirometry in patients with idiopathic pulmonary fibrosis: data from the INMARK trial. Eur Respir J 2021;58(1).
Acknowledgements
We acknowledge Dr. Matthew Craig, Dr. Neil Aggarwal, and Dr. Barry Schmetter from the NHLBI for their support during the design and conduct of this trial.
The following collaborators on the PRECISIONS study team contributed to study conception and data acquisition:
Elizabeth Freiheit, Adam Martin-Schwarze, Ashley Szparza, Tanvi Naik, Rex Edwards, Gordon Bernard, Deborah Barnbaum, Joao de Andrade, Daren Knoell, Peter Lindenauer, Andre Rogatko, Marinella Temprosa, Shwu-Fan Ma, Emma Strickland, Jamie Sheth, Joyce Lee, Cheryl Nickerson-Nutter, David Lebo, Elizabeth Belloli, Candace Flaherty, Timothy Whelan, Max Lento, Amy Case, Ugonna Nwosu, Matthew Kottmann, Gerard Criner, Julie Juhas, Joshua Mooney, Jeanette Smith, Andrew Limper, Shannon Daley, Tessy Paul, Yousef Althulth, Chad Newton, Rhoda Annoh Gordon, Mary Strek, Spring Maleckar, Hyun Kim, Mandi DeGrote, Reba Blissell, Robert Kaner, Elizabeth Peters, Alicia Morris, Mark Hamblin, Carime Ward, Ryan Boente, Meghan Willig, Nitin Bhatt, Benjamin Hood, Cathleen Wilson, Sachin Chaudhary, Heidi Erickson, Haylie Lengel, Daniel Dilling, Sydney Montesi, Caroline Fromson, Toby Maher, Anoop Nambiar, Hilda Pomroy, Mary Beth Scholand, Chloe Kirkpatrick, Lisa Lancaster, Jim Del Greco, Stephen Sam Weigt, Eileen Callahan
All authors reviewed the manuscript and are responsible for the conduct of the study.
Data Coordinating Center (University of Michigan) – Cathie Spino, Kevin Flaherty, Susan Murray, Elizabeth Freiheit, Adam Martin-Schwarze, Ashley Szparza, Tanvi Naik, Rex Edwards. Data and Safety Monitoring Board – Gordon Bernard, Deborah Barnbaum, Joao de Andrade, Daren Knoell, Peter Lindenauer, Andre Rogatko, Marinella Temprosa. Biobank Core (University of Virginia) – Imre Noth, Shwu-Fan Ma, Emma Strickland. Clinical Events Committee – Jamie Sheth. Pulmonary Fibrosis Foundation – Joyce Lee. Three Lakes Foundation – Cheryl Nickerson-Nutter. Temple University Current Good Manufacturing Practices Services – David Lebo. University of Michigan – Elizabeth Belloli, Candace Flaherty. Medical University of South Carolina – Timothy Whelan, Max Lento. Piedmont Healthcare – Amy Case, Ugonna Nwosu. University of Rochester Medical Center – Matthew Kottmann. Temple University – Gerard Criner, Julie Juhas. Stanford University – Joshua Mooney, Jeanette Smith. Mayo Clinic – Andrew Limper, Shannon Daley. University of Virginia – Tessy Paul, Yousef Althulth. University of Texas Southwestern – Chad Newton, Rhoda Annoh Gordon. University of Chicago – Mary Strek, Spring Maleckar. University of Minnesota – Hyun Kim, Mandi DeGrote. University of Washington – Ganesh Raghu, Reba Blissell. Weill Cornell Medicine – Anna Podolanczuk, Robert Kaner, Elizabeth Peters, Alicia Morris. University of Kansas Medical Center – Mark Hamblin, Carime Ward. Indiana University – Ryan Boente, Meghan Willig. Ohio State University – Nitin Bhatt, Benjamin Hood. University of Arizona – Cathleen Wilson, Sachin Chaudhary, Heidi Erickson. University of Colorado – Joyce Lee, Haylie Lengel. Loyola University – Daniel Dilling. Massachusetts General Hospital – Sydney Montesi, Caroline Fromson. University of Southern California – Toby Maher. University of Texas San Antonio – Anoop Nambiar, Hilda Pomroy. University of Utath – Mary Beth Scholand, Chloe Kirkpatrick. Vanderbilt University – Lisa Lancaster, Jim Del Greco. University of California Los Angeles – Stephen Sam Weigt, Eileen Callahan.
Funding
This work was supported by grants UH3HL145266 and U24HL145265 from the National Heart, Lung, and Blood Institute (NHLBI), Three Lakes Foundation, and Pulmonary Fibrosis Foundation. The study protocol has undergone full external peer review by the funding bodies as part of the peer review process. The funders had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Author information
Authors and Affiliations
Consortia
Contributions
FJM, IM, GR, CS, KRF, SM, and JMO conceptualized the study. FJM, IM, GR, CAS, KRF, SM, CBC and JAL designed the protocol. AJP and JSK drafted the main manuscript text. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The protocol was reviewed and approved by an independent DSMB. All participating sites used a single Institutional Review Board (sIRB) to conduct the ethical review, in accordance with NIH policy. The Biomedical Research Alliance of New York (BRANY) was the sIRB for this study (Protocol # 19-02-406). BRANY approved the study. All participants will sign an inform consent to participate in the clinical trial.
Consent for publication
Not applicable.
Competing interests
Dr. Podolanczuk grants from NIH (NHLBI K23HL140199), grants from American Lung Association, consulting fees from Regeneron, Roche, Imvaria, Boehring Ingelheim, and personal fees from National Association for Continuing Medical Education and EBSCO/DynaMed. Dr. Kim reports grants from NIH (NHLBI K23 award 150301) during the conduct of the study; he also reports grant funding from the Pulmonary Fibrosis Foundation outside the submitted work, and servin on a data and safety monitoring board (unpaid) for convalescent plasma trial for COVID-19. Dr. Cooper reports grants from NIH/NHLBI, Foundation NIH and the COPD Foundation, during the conduct of the study; he also reports personal fees from PulmonX, GlaxoSmithKline, Chiesi, NUVAIRA, MGC Diagnostics and Horizon Therapeutics, outside the submitted work. Dr. Lasky reports grants from Boehringer Ingelheim, personal fees from Boehringer Ingelheim, Veracyte, United Therapeutics, participation on an advisory board for Galecto, and a leadership role in the Pulmonary Fibrosis Foundation. Dr. Murray reports grant funding from the NIH (U24 HL145265) during the conduct of the study. Dr. Oldham reports grants from National Institutes of Health and an issued patent related to this work, along with personal fees from Boehringer Ingelheim, Roche/Genentech, AmMax Bio, Lupin pharmaceuticals and Endeavor BioMedicines unrelated to this work. Dr. Raghu reports receiving research grants from the National Institutes of Health for this trial. Dr Raghu reports receiving fees for serving as an ad hoc discussant from Bristol Myers Squibb and United Therapeutics, receiving consulting fees from Veracyte, receiving fees for reviewing investigator initiated research grant proposals from Boehringer Ingelheim, providing unpaid consulting services for Biogen, Bellerophon Therapeutics, Blade Therapeutics, FibroGen, Nitto, Roche/Genentech, and Novartis, serving (unpaid) on a data and safety monitoring board for Avalyn, and No other potential conflict of interest relevant to this manuscript. Dr. Flaherty reports grants from Boehringer Ingelheim, royalties from UpToDate, consulting fees from Roche/Genentech, Bellerophonm, Respivant, Shionogi, DevPro, Astra Zeneca, Pure Health, Horizon, Fibrogen, Sun Pharmaceuticals, Pliant, United Therapeutics, Arrowhead, Lupin, Polarean, PureTech, Trevi, CSL Behring, Daewong, Dispersol, Immumet, NeRRe Therapeutics, Insilco; he is also the Steering Committee Chair for the Pulmonary Fibrosis Foundation. Dr. Spino reports grants from NHLBI (1R01HL136682, 1U24HL154946, 1R61HL15840, and 1U24HL145265). Dr. Noth reports grant funding from NIH (NHLBI UG3HL145266), royalties from UpToDate, Licensing fees for Protein Markers in IPF, consulting fees from Boehringer Ingelheim and Sanofi, patents for FVC gene signature predictor, pending patent for PCSK6, and serving on data safety monitoring board for Yale COVID trials. Dr. Martinez reports funding from Afferent/Merck, Bayer, Biogen, Nitto, Novartis, Patara/Respivant, Promedior/Roche, Veracyte for serving on a steering committee for ILD studies, consulting fees from Abvie, Boehringer Ingelheim, BMS, Bridge Biotherapeutics, Csl Behring, DevPro, Genentech, IQVIA, Sanofi, Shionogi, twoXAR, Veracyte, honoraria from United Therapeutics, support for attending meetings from Boehringer Ingelheim, Csl Behring, Patara/Respivant, and participation on data safety monitoring boards for Biogen, and Boehringer Ingelheim.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1:
Baseline COVID-19 Questionnaire. COVID-19 questionnaire to be completed by all study participants at the baseline visit. PRECISIONS logo created by the PRECISIONS study team for the PRECISIONS study. Written permission obtained from the Data Coordinating Center.
Additional file 2:
COVID-19 Questionnaire Follow-Up. COVID-19 questionnaire to be completed by all study participants at each follow-up visit. PRECISIONS logo created by the PRECISIONS study team for the PRECISIONS study. Written permission obtained from the Data Coordinating Center.
Additional file 3:
Home Spirometry Survey. Home spirometry survey to be completed at visit 3 and visit 5 by study participants who agreed to enroll in the home spirometry substudy. PRECISIONS logo created by the PRECISIONS study team for the PRECISIONS study. Written permission obtained from the Data Coordinating Center.
Additional file 4:
Home Spirometry Exit Survey. Home spirometry survey to be completed at visit 7 or early termination visit by study participants who agree to enroll in the home spirometry substudy. PRECISIONS logo created by the PRECISIONS study team for the PRECISIONS study. Written permission obtained from the Data Coordinating Center.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Podolanczuk, A.J., Kim, J.S., Cooper, C.B. et al. Design and rationale for the prospective treatment efficacy in IPF using genotype for NAC selection (PRECISIONS) clinical trial. BMC Pulm Med 22, 475 (2022). https://doi.org/10.1186/s12890-022-02281-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12890-022-02281-8