Abstract
Background
Lung cancer surgery is reported as a risk factor for chronic pulmonary aspergillosis (CPA). However, limited data are available on its clinical impact. We aimed to determine the effect of developed CPA after lung cancer surgery on mortality and lung function decline.
Methods
We retrospectively identified the development of CPA after lung cancer surgery between 2010 and 2016. The effect of CPA on mortality was evaluated using multivariable Cox proportional hazard analyses. The effect of CPA on lung function decline was evaluated using multiple linear regression analyses.
Results
During a median follow-up duration of 5.01 (IQR, 3.41–6.70) years in 6777 patients, 93 developed CPA at a median of 3.01 (IQR, 1.60–4.64) years. The development of CPA did not affect mortality in multivariable analysis. However, the decline in forced vital capacity (FVC) and forced expiratory volume in 1 second (FEV1) were greater in patients with CPA than in those without (FVC, − 71.0 [− 272.9 to − 19.4] vs. − 10.9 [− 82.6 to 57.9] mL/year, p < 0.001; FEV1, − 52.9 [− 192.2 to 3.9] vs. − 20.0 [− 72.6 to 28.6] mL/year, p = 0.010). After adjusting for confounding factors, patients with CPA had greater FVC decline (β coefficient, − 103.6; 95% CI − 179.2 to − 27.9; p = 0.007) than those without CPA. However, the FEV1 decline (β coefficient, − 14.4; 95% CI − 72.1 to 43.4; p = 0.626) was not significantly different.
Conclusion
Although the development of CPA after lung cancer surgery did not increase mortality, the impact on restrictive lung function deterioration was profound.
Similar content being viewed by others
Background
Lung cancer is the most common cause of cancer death globally, and the incidence is increasing [1, 2]. In recent decades, advances in surveillance and treatment strategies have improved the long-term survival rates of lung cancer patients [3, 4]. This clinical success has been observed mainly in early-stage lung cancer, which can be surgically resected, rather than advanced lung cancer [5, 6]. As long-term follow-up of lung cancer patients after surgery has become more attainable, the frequency of chronic pulmonary infections has increased [7].
Chronic pulmonary aspergillosis (CPA) is a slowly destructive pulmonary disease in patients with pre-existing chronic pulmonary disease [8,9,10,11]. Colonization of Aspergillus species in the residual cavities of the lung parenchyma is a sequela of chronic pulmonary disease and is responsible for CPA [12]. CPA was relatively overlooked for a long time by physicians but has recently been recognized as a serious global health burden [8, 11]. Previous studies have reported that lung cancer surgery is one of the risk factors for CPA [7, 13]. These studies showed that development of CPA was closely related to underlying pulmonary disease, postoperative pulmonary complication (PPC), and neoadjuvant/adjuvant therapy in lung cancer patients who underwent lung cancer surgery [7, 13]. In addition, the incidence of CPA increased as the postoperative survival time of patients lengthened.
However, limited data are available regarding the clinical impacts of the development of CPA after lung cancer surgery. Therefore, we aimed to investigate the clinical characteristics of CPA that occur after lung cancer surgery especially focusing on mortality and lung function decline.
Methods
Study population and data collection
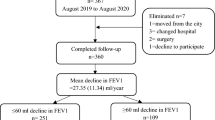
Using the Lung Cancer Surgery Registry at Samsung Medical Center (a 1979-bed referral hospital in South Korea), we retrospectively identified the development of CPA after lung cancer surgery in patients with non-small cell lung cancer (NSCLC) between January 2010 and December 2016. This study included previously published data from patients who underwent lung cancer surgery between January 2010 to December 2013 [7]. Patients with CPA at the time of surgery were excluded.
We used the electronic medical records to gather the following information: patient-related factors including age, sex, body mass index (BMI), smoking history, underlying pulmonary diseases, other comorbidities, and pulmonary function test results; cancer-related factors including histologic type, location of the tumor, and clinical/pathological stage; cancer treatment-related factors including neoadjuvant or adjuvant treatments used, surgical approach, extent of surgical resection, and PPC within 30 days after surgery [7]; CPA-related factors such as confirmation method of Aspergillus species and antifungal drug used. The tumor was staged using the 7th edition of the American Joint Committee on Cancer [14]. PPC was defined as development of intrathoracic complications during hospital stay or readmission within 30 days after surgery [15]. Patient follow-up data were last updated in August 2021.
This study obtained approval from Samsung Medical Center Institutional Review Board (SMC IRB no. 2021-10-046) to review and publish information from patient records. The requirement for informed consent was waived by SMC IRB due to the retrospective nature of the study.
Diagnosis of CPA
After surgical resection, most patients were routinely followed for at least 5 years by thoracic surgeons. Pulmonologists jointly followed up patients with pre-existing or newly developed pulmonary diseases [7]. When CPA was suspected, patients were referred to pulmonologists, and further diagnostic tests were performed. CPA was diagnosed according to the European Society for Clinical Microbiology and Infectious Diseases/European Respiratory Society (ERS) criteria: (1) compatible clinical symptoms; (2) serological or microbiological evidence: positive serum Aspergillus precipitin test (Aspergillus fumigatus IgG ELISA kit; IBL International, Hamburg, Germany); isolation of Aspergillus species from a respiratory specimen, or histologic confirmation; (3) compatible radiological findings with overt progression; and (4) exclusion of alternative diagnosis [11, 16].
Spirometry and annual lung function decline
Spirometry was performed using a Vmax 22 system (SensorMedics, Yorba Linda, CA, USA) following the American Thoracic Society/ERS guidelines [17]. Absolute values of forced vital capacity (FVC) and forced expiratory volume in one second (FEV1) were obtained from a pre-bronchodilator test, and the predicted percentage values (% predicted) for FVC and FEV1 were calculated using the equation obtained by analyzing the representative value of the Korean population [18].
An obstructive pattern was defined as FEV1/FVC < 70% and predicted FEV1 < 80%, and a restrictive pattern was defined as FEV1/FVC ≥ 70% and predicted FVC < 80%. Patients with FEV1/FVC ≥ 70% and predicted FVC ≥ 80% were classified as having a normal pattern. Patients with FEV1/FVC < 70% and predicted FEV1 ≥ 80% were also classified as having a normal pattern [19]. Annual lung function decline rates (ml/year) were calculated in patients who had a pulmonary function test (PFT) at least 3 months after surgery when lung function was considered to have recovered [20]. To calculate the annual rate of decline, only patients who had at least two PFT results that were at least 6 months apart were counted. For patients with CPA, only those with follow-up PFT results after the diagnosis of CPA were included in the analysis. The lung function decline was calculated as [(last FVC [or FEV1]) – (FVC [or FEV1] at baseline)]/follow-up duration (years) [19, 21]. Rapid progression of FVC (or FEV1) decline was defined as ≥ 40 mL loss per year in FVC (or FEV1) [22, 23].
Statistical analyses
All data are expressed as the median (interquartile range, IQR) for continuous variables, and as the numbers (%) for categorical variables. Categorical variables were compared using Pearson χ2 test or Fisher’s exact test, and continuous variables were compared using Mann–Whitney U test. The Kaplan–Meier method was used to estimate the cumulative incidence of CPA and overall survival (OS) after the lung cancer surgery. The log-rank test was used to compare survival according to the development of CPA [7].
The effect of CPA on mortality was evaluated using multivariable Cox proportional hazard analyses. Three models were used: model 1 was adjusted for clinical baseline characteristics; model 2 was adjusted for variables with lung cancer-related factors; model 3 was adjusted for all the preceding variables.
The effect of CPA on lung function decline was evaluated using multiple linear regression analyses. Five models were constructed: model 1 was adjusted for baseline FVC [L] in FVC decline and for baseline FEV1 [L] in FEV1 decline; model 2 was adjusted for selected variables with p < 0.20 in univariate analyses with consideration of multicollinearity; model 3 was adjusted for variables that were generally considered to influence the decline of lung function; model 4 was adjusted for variables related to lung cancer treatment and variables considered to be related to the development of CPA; and, finally, model 5 was adjusted for all the preceding variables.
All tests were two-sided, and a p value < 0.05 was considered significant. We used PASW Statistics 27 (SPSS Inc., Chicago, IL, USA) for analysis.
Results
Study population and development of CPA after lung cancer surgery
In 6789 patients who underwent lung cancer surgery for NSCLC, 12 who were diagnosed with CPA and lung cancer at the same time or had a previous history of CPA were excluded from this study (Fig. 1). Finally, of 6777 patients, 93 developed CPA at a median of 3.01 (IQR, 1.60–4.64) years after lung cancer surgery. The cumulative incidences of CPA after lung cancer surgery were 0.3%, 0.8%, 1.3%, and 3.0% at 1, 3, 5, and 10 years, respectively (Fig. 2 A).
The median age of the study population was 63 years (IQR, 56–69 years), and 61.3% were male (Table 1). More than half of the patients (57.8%) were either ex- or current smokers. Most of the patients (69.0%) were in clinical stage I, and adenocarcinoma (70.5%) was the most common tumor histology. Neoadjuvant or adjuvant therapy was received by 647 (9.5%) and 1845 (27.4%) patients, respectively. Video-assisted thoracoscopic surgery was performed in 62.4%, and most patients (76.1%) underwent lobectomy. PPCs within 30 days occurred in 17.1% of patients.
Characteristics of patients with CPA and details of treatment
The detailed clinical characteristics of 93 patients who developed CPA are summarized in Additional file 1: Table S1. In most cases, the Aspergillus species was confirmed by a serologic test. Chronic cavitary pulmonary aspergillosis was the most common type (74.2%), followed by subacute invasive aspergillosis (20.4%). Of all 93 patients, 22 (23.7%) did not receive antifungal drugs, and of 71 patients who received antifungal drugs, only 45 patients were able to complete the antifungal treatment for a median of 11.1 (IQR, 6.0–16.7) months.
Effect of CPA on mortality
Patients were followed up for a median of 5.01 (IQR, 3.41–6.70) years to assess survival, and the 5-year survival rate was 76.8% (Fig. 2B). The OS of patients who developed CPA was 90.3%, 76.3%, 62.8%, and 48.4% at 1, 3, 5, and 10 years, respectively. In a crude model, development of CPA showed an unfavorable effect on OS with a hazard ratio (HR) of 1.69 (95% confidence interval [CI], 1.23–2.32, p = 0.001) (Table 2). However, the effect of CPA on mortality was reversed in models 1 to 3 through additional adjustment of several variables. Finally, in model 3, the adjusted HR of CPA on mortality was 0.73 (95% CI 0.53–1.03, p = 0.060).
Effect of CPA on lung function decline
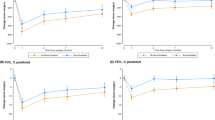
A total of 1842 patients, 50 with CPA and 1792 without CPA, had PFT results that satisfied the criteria of this study (Table 3). At baseline, FEV1 [L] was not significantly different between patients with CPA and those without (1.96 vs. 2.09 L, p = 0.116); however, FVC [L] was lower in patients with CPA than in those without (2.70 vs. 2.97 L, p = 0.003). After a median of 3.7 (IQR, 1.8–4.4) years, both FVC (2.35 vs. 3.04 L, p < 0.001) and FEV1 (1.76 vs. 1.99 L, p = 0.001) were lower in patients with CPA than in those without. In addition, the annual declines in FVC (− 71.0 vs. − 10.9 mL/year, p < 0.001) (Fig. 3 A) and FEV1 (− 52.9 vs. − 20.0 mL/year, p = 0.010) (Fig. 3B) were also greater in patients with CPA than in those without. Also, the proportions of patients with rapid progression of FVC and FEV1 decline were higher in patients with CPA than in those without (FVC, 72.0% vs. 39.0%, p < 0.001; FEV1, 54.0% vs. 39.0%, p = 0.032).
Lung function decline according to the development of CPA. Solid lines show median values, and dotted lines show interquartile ranges. A Histograms of annual FVC decline (mL/year) and B Histograms of annual FEV1 decline (mL/year) with or without CPA development. CPA Chronic pulmonary aspergillosis, FVC Forced vital capacity, FEV1 Forced expiratory volume in 1 s
From baseline spirometry in patients with CPA, the proportion of patients with a restrictive pattern was higher than that of those without (62.0% vs. 28.7%, p < 0.001). In patients with CPA, 3 of 7 (42.9%) with a normal pattern and 4 of 12 (33.3%) with an obstructive pattern at baseline changed to a restrictive pattern at the last spirometry (Additional file 1: Fig. S1). On the other hand, in patients without CPA, only 97 of 770 (12.6%) patients with a normal pattern and 45 of 508 (8.9%) patients with an obstructive pattern at baseline changed to a restrictive pattern. Finally, the proportion of patients with a restrictive pattern remained higher in patients with CPA than in those without CPA (66.0% vs. 27.6%, P < 0.001).
The results of univariate and multiple linear regression analyses of lung function decline are summarized in Additional file 1: Table S2 and Table 4. The crude model showed that development of CPA affected the decline in FVC (β coefficient, − 132.5; 95% CI − 206.0 to − 58.9; p < 0.001) but not that in FEV1 (β coefficient, − 49.1; 95% CI − 105.6 to 7.4; p = 0.088). Even after adjustment of numerous clinical variables throughout the 5 models, patients with CPA had a greater FVC decline (model 5; β coefficient, − 103.6; 95% CI − 179.2 to − 27.9; p = 0.007) than those without CPA. However, the decline in FEV1 (model 5; β coefficient, − 14.4; 95% CI − 72.1 to 43.4; p = 0.626) did not show a significant difference in the development of CPA after variables were adjusted.
Discussion
After lung cancer surgery, 93 of 6777 patients developed CPA at a median of 3 years. The OS was lower at 5 years in patients with CPA than in those without (62.8% vs. 77.0%). However, the development of CPA did not significantly affect mortality in multivariate analysis. Meanwhile, a restrictive pattern was dominant in patients with CPA, and the FVC decline was greater in patients with CPA than in those without, even after adjustment for possible confounders.
CPA is known to rarely occur in healthy lungs, but most commonly develops in a pre-existent bronchopulmonary (or less usually, pleural cavity) disorder such as sequelae of mycobacterial diseases, chronic obstructive pulmonary disease, prior pneumothorax, treated lung cancer, fibrocystic sarcoidosis, and pneumoconiosis [11]. Our cohort has structural problems of the lungs and immunosuppression problems related to chemotherapy and/or radiotherapy, so the pathophysiology for CPA development seems to be sufficient. In addition, as the treatment outcomes for lung cancer are improving, chronic infectious disease such as CPA will eventually become a bigger problem than now. However, since there is still very little data on this, we performed these analyses with our cohort and we believe this is the novelty of our study.
A few studies have investigated the mortality and risk factors of CPA. The survival rates in CPA patients in those studies varied widely, which might have resulted from different sample sizes, underlying comorbidities, and CPA subtypes [24,25,26,27,28]. A study with a relatively large cohort of 387 CPA patients who were referred to the National Aspergillosis Center in the UK, reported that survival rates were 86%, 62%, and 47% at 1, 5, and 10 years, respectively [25]. However, in the study, only about 20% of the patients were lung cancer survivors, and there was no specific information about lung cancer treatment. The effect of CPA on mortality in lung cancer patients, particularly those who have undergone lung cancer surgery, is rarely reported. Tamura et al. reported the incidence and prognosis of CPA in patients who underwent lobectomy for lung cancer [13]. However, the 1-year survival rate of their study (47.0%) was lower than that in our study (90.3%). The poorer outcome of their study was possibly affected by the smaller sample size (CPA patients/total study population, 17/475 patients), older age (median 68 years), less recent data (conducted between 2000 and 2009), and inclusion of only lobectomy cases in comparison to our study. In addition, their result did not describe the clinical information of the patients including CPA subtypes and pre- or postoperative treatment. Factors not presented in the study might differ from those in our study, and these differences might have affected mortality on both studies. Future studies conducted in diverse population groups on the effects of CPA in lung cancer patients who underwent surgery are needed to build more robust evidence.
In this study, multivariate analyses of the effect of CPA on OS in the crude model indicated higher mortality in patients with CPA than in those without (HR 1.69, P = 0.001), but when several variables were adjusted, the HRs were opposite that of the crude model (model 3; HR 0.73, P = 0.060). We interpret that the development of CPA might lead to these unexpected consequences for mortality due to the moderating effect between some variables. In previous studies, variables that could affect the development of CPA were lower BMI, ever smoker, underlying interstitial lung disease (ILD), and PPC [7, 13]. However, these variables can also influence the poor OS of lung cancer patients after surgery [29, 30]. Therefore, we suggest that the effect of CPA on OS decreased as these variables were adjusted because they affected both survival analysis and CPA development, that is, they were more common in patients with CPA than in those without.
In our study, the restrictive pattern was more dominant in patients with CPA at both baseline and the last PFTs compared to patients without CPA. In addition, patients with CPA had lower FVC and FEV1 values at both baseline and last PFTs and had significantly greater FVC decline than those without. A restrictive pattern of spirometry is associated with space-occupying lesions and ILD [31]. The patients with CPA had more frequent ILD, history of thoracotomy, and PPC than those without CPA, which were common factors in the development of CPA and the restrictive pattern [7]. Therefore, it seems natural that patients with CPA after lung cancer surgery showed restrictive patterns at both baseline and the last PFTs. CPA itself might contribute to the restrictive patterns and FVC decline as it is a slow-progressing disease that destroys the lung parenchyma.
A recent study conducted with the general population using a national dataset reported that patients with a restrictive pattern related to poor quality of life (QOL) and lower high-intensity physical activity compared with patients with normal spirometry [32]. Thus, CPA development after lung cancer surgery might be associated with impaired QOL and low physical activity due to restrictive pulmonary function. One Chinese study with a general population cohort reported that impaired pulmonary function was significantly associated with future decreased QOL [33]. In addition, Al-Shair et al. assessed lung function and patient-reported outcome (PRO) in patients with CPA and reported that fatigue, dyspnea, and poor lung function were strongly associated with impaired health status on multivariate analysis [34]. Therefore, the development of CPA after lung cancer surgery leads to worsening of lung function that can be associated with the low QOL in lung cancer patients.
This study has several limitations. First, this is a study of a single institution and there might have been a selection bias. Second, Republic of Korea is a country with an intermediate incidence of tuberculosis, which is one of the predisposing factors for CPA [35], and this might limit the generalization of our results. Third, only 30% (2026/6684) of patients without CPA and 57% (53/93) of patients with CPA had PFT clinical information. Follow-up PFTs after lung cancer surgery were not routinely performed in patients with little or no dyspnea and occasionally could not be performed in patients with a poor general condition. If the PFTs were conducted in all patients, lung function decline would have shown a more marked difference between patients with CPA and those without. Lastly, PRO data were not obtained in our study population. If there were serially measured PROs with PFT results, the effect of CPA development on the QOL should have been clearer. Despite the limitations, to the best of our knowledge, this is the first study to report the effects of CPA on lung function decline and survival outcomes in lung cancer patients who underwent lung cancer surgery. It was also noteworthy that we analyzed a large number of patients with a long-term follow-up period.
Conclusion
CPA is a chronic pulmonary infectious disease that can occur during follow-up after lung cancer resection surgery. Although the development of CPA after lung cancer surgery did not affect the OS, it could unfavorably contribute to the decline in lung function. Since the diagnosis of CPA is not simple and can be overlooked, patients who have risk factors for CPA after surgery should be monitored carefully for the development of CPA and managed by expert pulmonologists.
Availability of data and materials
The data of this study are available from the corresponding author upon reasonable request.
Abbreviations
- BMI:
-
Body mass index
- CI:
-
Confidence interval
- COPD:
-
Chronic obstructive pulmonary disease
- CPA:
-
Chronic pulmonary aspergillosis
- FEV1 :
-
Forced expiratory volume in 1 s
- FVC:
-
Forced vital capacity
- HR:
-
Hazard ratio
- ILD:
-
Interstitial lung disease
- IQR:
-
Interquartile range
- NSCLC:
-
Non-small cell lung cancer
- OS:
-
Overall survival
- PFT:
-
Pulmonary function test
- PPC:
-
Postoperative pulmonary complications
- PRO:
-
Patient-reported outcome
- QOL:
-
Quality of life
References
Bade BC, Dela Cruz CS. Lung cancer 2020: epidemiology, etiology, and prevention. Clin Chest Med. 2020;41(1):1–24.
Fitzmaurice C, Allen C, Barber RM, Barregard L, Bhutta ZA, Brenner H, et al. Global, regional, and national cancer incidence, mortality, years of life lost, years lived with disability, and disability-adjusted life-years for 32 cancer groups, 1990 to 2015: a systematic analysis for the global burden of disease study. JAMA Oncol. 2017;3(4):524–48.
Whitson BA, Groth SS, Duval SJ, Swanson SJ, Maddaus MA. Surgery for early-stage non-small cell lung cancer: a systematic review of the video-assisted thoracoscopic surgery versus thoracotomy approaches to lobectomy. Ann Thorac Surg. 2008;86(6):2008–16 discussion 16–8.
Jonas DE, Reuland DS, Reddy SM, Nagle M, Clark SD, Weber RP, et al. Screening for lung cancer with low-dose computed tomography: updated evidence report and systematic review for the US preventive services task force. JAMA. 2021;325(10):971–87.
Sherwood JT, Brock MV. Lung cancer: new surgical approaches. Respirology. 2007;12(3):326–32.
Blandin Knight S, Crosbie PA, Balata H, Chudziak J, Hussell T, Dive C. Progress and prospects of early detection in lung cancer. Open Biol. 2017;7(9).
Shin SH, Kim BG, Kang J, Um SW, Kim H, Kim HK, et al. Incidence and risk factors of chronic pulmonary aspergillosis development during long-term follow-up after lung cancer surgery. J Fungi (Basel). 2020;6(4):271.
Denning DW, Pleuvry A, Cole DC. Global burden of chronic pulmonary aspergillosis as a sequel to pulmonary tuberculosis. Bull World Health Organ. 2011;89(12):864–72.
Smith NL, Denning DW. Underlying conditions in chronic pulmonary aspergillosis including simple aspergilloma. Eur Respir J. 2011;37(4):865–72.
Page ID, Byanyima R, Hosmane S, Onyachi N, Opira C, Richardson M, et al. Chronic pulmonary aspergillosis commonly complicates treated pulmonary tuberculosis with residual cavitation. Eur Respir J. 2019;53(3).
Denning DW, Cadranel J, Beigelman-Aubry C, Ader F, Chakrabarti A, Blot S, et al. Chronic pulmonary aspergillosis: rationale and clinical guidelines for diagnosis and management. Eur Respir J. 2016;47(1):45–68.
Gago S, Denning DW, Bowyer P. Pathophysiological aspects of aspergillus colonization in disease. Med Mycol. 2019;57(Supplement_2):S219–27.
Tamura A, Suzuki J, Fukami T, Matsui H, Akagawa S, Ohta K, et al. Chronic pulmonary aspergillosis as a sequel to lobectomy for lung cancer. Interact Cardiovasc Thorac Surg. 2015;21(5):650–6.
Edge SB, Compton CC. The american Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol. 2010;17(6):1471–4.
Miskovic A, Lumb AB. Postoperative pulmonary complications. Br J Anaesth. 2017;118(3):317–34.
Jhun BW, Jeon K, Eom JS, Lee JH, Suh GY, Kwon OJ, et al. Clinical characteristics and treatment outcomes of chronic pulmonary aspergillosis. Med Mycol. 2013;51(8):811–7.
Miller MR, Hankinson J, Brusasco V, Burgos F, Casaburi R, Coates A, et al. Standardisation of spirometry. Eur Respir J. 2005;26(2):319–38.
Choi JK, Paek D, Lee JO. Normal predictive values of spirometry in Korean population. Tuberc Respir Dis. 2005;58(3):230–42.
Park HY, Jeong BH, Chon HR, Jeon K, Daley CL, Koh WJ. Lung function decline according to clinical course in nontuberculous mycobacterial lung disease. Chest. 2016;150(6):1222–32.
Bolliger CT, Wyser C, Roser H, Solèr M, Perruchoud AP. Lung scanning and exercise testing for the prediction of postoperative performance in lung resection candidates at increased risk for complications. Chest. 1995;108(2):341–8.
Lange P, Celli B, Agustí A, Boje Jensen G, Divo M, Faner R, et al. Lung-function trajectories leading to chronic obstructive pulmonary disease. N Engl J Med. 2015;373(2):111–22.
Park HY, Churg A, Wright JL, Li Y, Tam S, Man SF, et al. Club cell protein 16 and disease progression in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2013;188(12):1413–9.
Vestbo J, Edwards LD, Scanlon PD, Yates JC, Agusti A, Bakke P, et al. Changes in forced expiratory volume in 1 second over time in COPD. N Engl J Med. 2011;365(13):1184–92.
Jewkes J, Kay PH, Paneth M, Citron KM. Pulmonary aspergilloma: analysis of prognosis in relation to haemoptysis and survey of treatment. Thorax. 1983;38(8):572–8.
Lowes D, Al-Shair K, Newton PJ, Morris J, Harris C, Rautemaa-Richardson R, et al. Predictors of mortality in chronic pulmonary aspergillosis. Eur Respir J. 2017;49(2).
Nam HS, Jeon K, Um SW, Suh GY, Chung MP, Kim H, et al. Clinical characteristics and treatment outcomes of chronic necrotizing pulmonary aspergillosis: a review of 43 cases. Int J Infect Dis. 2010;14(6):e479-82.
Aguilar-Company J, Martín MT, Goterris-Bonet L, Martinez-Marti A, Sampol J, Roldán E, et al. Chronic pulmonary aspergillosis in a tertiary care centre in Spain: a retrospective, observational study. Mycoses. 2019;62(9):765–72.
Maitre T, Cottenet J, Godet C, Roussot A, Abdoul Carime N, Ok V, et al. Chronic pulmonary aspergillosis: prevalence, favouring pulmonary diseases and prognosis. Eur Respir J. 2021;58(2).
Endo S, Ikeda N, Kondo T, Nakajima J, Kondo H, Yokoi K, et al. Model of lung cancer surgery risk derived from a Japanese nationwide web-based database of 78,594 patients during 2014–2015. Eur J Cardiothorac Surg. 2017;52(6):1182–9.
Onaitis MW, Furnary AP, Kosinski AS, Kim S, Boffa D, Tong BC, et al. Prediction of long-term survival after lung cancer surgery for elderly patients in the society of thoracic surgeons general thoracic surgery database. Ann Thorac Surg. 2018;105(1):309–16.
Backman H, Eriksson B, Hedman L, Stridsman C, Jansson SA, Sovijärvi A, et al. Restrictive spirometric pattern in the general adult population: methods of defining the condition and consequences on prevalence. Respir Med. 2016;120:116–23.
Chung SJ, Kim HI, Yang B, Kim T, Sim YS, Kang HK, et al. Impact of the severity of restrictive spirometric pattern on nutrition, physical activity, and quality of life: results from a nationally representative database. Sci Rep. 2020;10(1):19672.
Xie G, Li Y, Shi P, Zhou B, Zhang P, Wu Y. Baseline pulmonary function and quality of life 9 years later in a middle-aged chinese population. Chest. 2005;128(4):2448–57.
Al-Shair K, Muldoon EG, Morris J, Atherton GT, Kosmidis C, Denning DW. Characterisation of fatigue and its substantial impact on health status in a large cohort of patients with chronic pulmonary aspergillosis (CPA). Respir Med. 2016;114:117–22.
Hayes GE, Novak-Frazer L. Chronic pulmonary aspergillosis-where are we? And where are we going? J Fungi (Basel). 2016;2(2).
Acknowledgements
The authors would like to thank the Statistics and Data Center, Research Institute for Future. Medicine of Samsung Medical Center for the statistical analysis and advice.
Funding
No funding source.
Author information
Authors and Affiliations
Contributions
B-HJ is the guarantor of the manuscript content. Study design and conceptualization: B-HJ, B-GK, and YSC; administrative support: YSC and B-HJ; provision of study materials or patients: YSC, SHS, KL, S-WU, HK, YJJ, JL, JHC, HKK, JK, YMS, and B-HJ; collection and assembly of data: B-GK; data analysis and interpretation: B-GK, YSC, and B-HJ; manuscript writing: B-GK and B-HJ; contribution to intellectual content and revision of the manuscript: YSC, SHS, KL, S-WU, HK, YJJ, JL, JHC, HKK, JK, and YMS; final approval of manuscript: all of the authors. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study was conducted in accordance with the Declaration of Helsinki. This study obtained approval from Samsung Medical Center Institutional Review Board (SMC IRB No. 2021-10-046) to review and publish information from patient records. The requirement for informed consent was waived by SMC IRB due to the retrospective nature of the study.
Consent for publication
Not applicable.
Competing interests
None of the authors has a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1.
Online Supplement.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Kim, BG., Choi, Y.S., Shin, S.H. et al. Mortality and lung function decline in patients who develop chronic pulmonary aspergillosis after lung cancer surgery. BMC Pulm Med 22, 436 (2022). https://doi.org/10.1186/s12890-022-02253-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12890-022-02253-y