Abstract
Background
There were relatively few studies about the incidence and risk factors for bloodstream infection (BSI) in patients with severe acute respiratory distress syndrome (ARDS) supported by veno–venous extracorporeal membrane oxygenation (VV–ECMO).
Methods
Patients who were diagnosed with severe ARDS and received VV–ECMO treatment in the medical intensive care unit of China–Japan Friendship Hospital from August 2013 to March 2019 were retrospectively studied. The pathogens isolated from blood culture (BC) were identified and analyzed for drug sensitivity. The risk factors for BSI were analyzed by logistic regression.
Results
A total of 105 patients were included in this single–center retrospective cohort study. Among them, 23 patients (22%) had BSIs. 19 cases were identified as primary BSI; while the other 4 cases were as secondary BSI. A total of 23 pathogenic strains were isolated from BCs, including gram–negative (G–) bacilli in 21 (91%) cases, gram–positive (G+) cocci in 1 case, fungus in 1 case, and multidrug–resistant (MDR) organisms in 8 cases. Compared with patients without BSI, patients with BSI had a higher Murray score (odds ratio = 6.29, P = 0.01) and more blood transfusion (odds ratio = 1.27, P = 0.03) during ECMO.
Conclusions
The incidence of BSI in patients with severe ARDS supported by VV–ECMO was 22%. G– bacilli was the main pathogen, and most of them were MDR–G– bacilli (MDR–GNB). Higher Murray score and more blood transfusion may be the independent risk factors for BSI.
Similar content being viewed by others
Introduction
Veno–venous extracorporeal membrane oxygenation (VV–ECMO) is the gold standard of support in the treatment of severe refractory acute respiratory distress syndrome (ARDS). Although equipment technology has greatly improved, the incidence of complications, especially infection, remains high and might affect the clinical outcomes of patients.
According to two retrospective analyses of Extracorporeal Life Support Organization (ELSO) data by Vogel and Bizzarro, the prevalence of hospital–acquired infections in adult patients during ECMO was 21% [1], while the incidence of BSI during ECMO ranged from 3 to 18% [2,3,4,5]. Compared with that in patients supported by veno–arterial ECMO (VA–ECMO), the infection rate in patients supported by VV–ECMO was higher [6]. The reasons might be attributed to longer treatment times and more frequent exposure to antibiotics and systemic steroids during VV–ECMO.
The pathogens and risk factors for BSI vary among different patients and different modes of ECMO. Enterobacteriaceae was reported to be the most common pathogen of BSIs, accounting for 16%, and the incidence of BSI caused by Enterobacteriaceae was 4.45 cases/1000 ECMO days [2]. Renal failure [7] and blood transfusion due to anemia and thrombocytopenia were identified as risk factors for BSI in patients with cancer [8]. A study in 92 patients with VA–ECMO indicated that age and serum total bilirubin level pre–ECMO were risk factors [9]. A single–center retrospective cohort study noted that patients with BSI during VV–ECMO had a longer duration of ECMO support than patients without BSI (18 days vs. 9 days, P < 0.01) [10]. The effect of BSI on mortality in ECMO patients remains controversial. A retrospective cohort study by Steiner et al. observed a threefold increase in the risk of death in patients suffering from BSI during ECMO [11]. However, other studies revealed that although BSI increased the length of hospitalization, there was no effect on hospital mortality [1,2,3,4].
Relevant data of patients with severe pneumonia–induced ARDS who are supported by VV–ECMO are extremely limited. We therefore conducted a study to evaluate the incidence and risk factors for BSI in severe ARDS patients supported by VV–ECMO.
Methods
Study design and participants
From August 2013 to March 2019, 276 patients treated with ECMO were admitted to the MICU of China–Japan Friendship Hospital. Among them, 243 patients were supported with VV–ECMO, 30 patients with VA–ECMO and 3 patients with ECCO2R.
Inclusion criteria
-
1.
Severe ARDS (PaO2/FiO2 ≤ 100 mmHg when positive end-expiratory pressure (PEEP) ≥ 5 mmHg) due to pneumonia;
-
2.
ECMO initiation within 7 days after mechanical ventilation;
-
3.
VV–ECMO support for more than 24 h.
Exclusion criteria
-
1.
Age < 18 years old;
-
2.
VA–ECMO and ECCO2R.
Methods and parameters of ECMO
All patients were punctured with the Seldinger technique. The centrifugal pump and CPB pipeline were from the ROTAFLOW and PLS CPB pipeline systems of MAQUET Company in Sweden. Puncture catheters were acquired from MAQUET or Medtronic (USA). The femoral vein was the drainage end and the jugular vein was the reflux end. Peripheral ECMO cannulas were almost always sutured at the insertion site along the ECMO line to fix the cannulas with tube–holder devices or sutured directly to the skin and covered with sterile dressing.
Plan for prevention and treatment of nosocomial infections
Since 2013, we have implemented a standardized nosocomial infection prevention plan, which includes using an alcohol disinfectant to wash hands, changing dressings at the puncture point of the ECMO cannula every three days, and preventing ventilator–associated pneumonia (wearing isolation clothing before any operation; keeping patients in a 30–45° semi reclined position; administering chlorhexidine at least twice a day for oral care; and performing airtight endotracheal intubation). No antibiotic prophylaxis or anti–infective treatment was administered to ECMO patients with definite infection.
Blood culture collection protocol
Blood collectors disinfected their hands before collection and wore disposable gloves or sterile gloves of appropriate size. After decontamination of the skin and lids of bottles with 75% ethanol, natural drying was performed for 60 s. Blood (10 mL) from the peripheral vein was collected in one aerobic and anaerobic bottle and shaken vigorously to mix well.
Data collection
The clinical trial observation form (CRF) of ECMO patients was completed and entered into the electronic database. The variables included age, sex, body mass index (BMI), past medical history, immunosuppressive status, causes of ARDS, main indications for ECMO, severity of disease before ECMO [including Acute Physiology and Chronic Health Evaluation II (APACHE II), Sequential Organ Failure Assessment (SOFA), Predicting Death for Severe ARDS on VV⁃ECMO (PRESERVE), Respiratory ECMO Survival Prediction (RESP), and Acute Lung Injury scores]. In addition, related laboratory tests were performed during ECMO. Ventilator parameters, mechanical ventilation time before ECMO, ECMO duration, acute kidney injury (AKI) during ECMO, continuous renal replacement therapy (CRRT) during ECMO, massive hemorrhage during ECMO, type of blood product and volume of blood transfused during ECMO, length of intensive care unit (ICU) stay, total length of hospital stay and outcomes of patients were recorded.
Definition of BSI
BSI was defined as two separate blood cultures (BCs) positive for the same pathogenic organism in addition to signs of infection, including leukocytosis, leukopenia, fever or hypothermia, according to the definitions of the Centers for Disease Control/National Healthcare Safety Network [12,13,14]. Corynebacterium spp., Bacillus spp., Cutibacterium spp., coagulase–negative Staphylococci (CoNS), viridians group Streptococci, Aerococcus spp. and Micrococcus spp. were considered common skin contaminants unless the same bacterial strain was isolated from two separate BCs within 48 h of each other [14].
BCs were performed when BSI was suspected between 24 h after ECMO and within 48 h after ECMO withdrawal, and the causative organism of BSI was recorded. When the organism isolated from the BCs was unrelated to infection at another site or when the BSI was related to catheter use, the infection was classified as primary BSI [12]; when the organism isolated from BC was identical to an organism identified from another site, the infection was classified as secondary BSI. Only the first BSI was recorded.
A sudden increase in the leukocyte count, purulent secretion in the airway, the drainage of pus from an open wound, fever, new pulmonary consolidation or diffuse exudative shadows in both lungs on dynamic chest radiography, low perfusion, insufficient oxygen delivery, etc., are all manifestations of BSI. It is recommended that patients receiving ECMO support for more than 2 weeks should be regularly cultured to monitor for BSI.
Definition of a massive hemorrhage event
Significant bleeding was defined as a hemoglobin decrease of more than 20 g/L within 24 h, blood loss of more than 20 ml/kg/d or red blood cell (RBC) demand more than 10 ml/kg within 24 h. Retroperitoneal hemorrhage, pulmonary hemorrhage, central nervous system hemorrhage, or hemorrhage requiring surgical intervention were also considered massive hemorrhage events.
Definition of immunosuppressed status
Immunosuppressed status was defined as organ transplantation, immunosuppressant usage, or use of prednisolone at a dosage of over 15 mg for longer than 1 month [15].
Statistical methods
Data were analyzed using SPSS (version 25.0) software (SPSS Inc., Chicago, IL, USA). Normally distributed continuous variables are expressed as the \(\overline{x }\)±standard deviation (SD) and were compared using the t test. Nonnormally distributed continuous variables are expressed as medians and quartiles and were compared using the Wilcoxon rank–sum test. Categorical variables were compared using the x2 test. P values < 0.05 were considered significant.
Variables with significant differences and that were considered to be of clinical significance were entered into the logistic regression analysis. Logistic regression analysis with 95% confidence intervals (95% CIs) and odds ratios (ORs) was used to evaluate independent risk factors for BSI.
Results
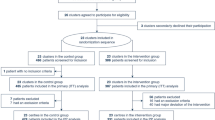
A total of 276 patients were screened for enrollment, and 171 patients who did not meet the eligibility criteria were excluded. The flow of this study is presented in a diagram (Fig. 1).
Flow diagram of this study. From August 2013 to March 2019, 276 patients receiving ECMO were admitted to the MICU. Among them, 243 patients were supported with VV–ECMO, 30 patients with VA–ECMO and 3 patients with ECCO2R. A total of 105 patients who met the inclusion criteria were enrolled. According to the BSI criteria, 23 patients were diagnosed with BSI, while the other 82 patients were diagnosed with non–BSI. Of the 23 patients with BSI, 7 survived, and 16 died
Incidence of and pathogens causing BSI
BSI occurred in 23 of 105 patients (22%). The time interval between the initiation of ECMO support and BSI was 6.5 days (median; interquartile range [IQR]: 4.0, 18.5). There were 19(83%) cases of primary BSI and 4 (17%) cases of secondary BSI. Among the primary BSIs, 4 cases were catheter–related infections (CRIs). The cause of secondary BSI was pneumonia. In total, 21 strains of G––rods were isolated, including Acinetobacter baumannii in 11 cases [carbapenem–resistant Acinetobacter baumannii (CRAB) in 6 cases and multidrug–resistant Acinetobacter baumannii (MDRAB) in 5 cases], Burkholderia cepacian in 5 cases, Pseudomonas aeruginosa in 2 cases [multidrug–resistant Pseudomonas aeruginosa (MDRPA) in 1 case], and Klebsiella pneumoniae in 3 cases [carbapenem–resistant Enterobacteriaceae (CRE) in 2 cases and multidrug–resistant Enterobacteriaceae (MDRE) in 1 case]. One strain of G+ cocci (Enterococcus faecium) and 1 strain of fungi (Candida parapsilosis) were identified. The rate of resistance of Acinetobacter baumannii to cephalosporins and carbapenems was 54.5%, and the rate of resistance to sulfamethoxazole trimethoprim was 81.8%. Only 7 of these infection patients survived, and the mortality rate (70%) was high.
Comparison between the BSI group and the non–BSI group (Table 1 )
A total of 105 patients were included, with an average age of 49 (± 16) years; 76 patients (72%) were male. The most common cause of severe pneumonia was viral pneumonia (51%), followed by bacterial pneumonia (29%). Before ECMO, the APACHE II score was 18 (± 6) points, the SOFA score was 9 (± 4) points, the PRESERVE score was 5 (± 3) points, the RESP score was 2 (–1–4) points and the Murray score was 3 (± 1) points.
Compared with the non–BSI group, the BSI group a higher proportion of males (91% vs. 67%, P = 0.02), a higher proportion of patients with hypertension (52% vs. 28%, P = 0.03), and a higher Murray score [4 (± 1) vs. 3 (± 1), P = 0.02]. In addition, higher rates of blood transfusion (74% vs. 49%, P = 0.03), packed RBCs (pRBCs) [6 (2–8) vs. 2 (0–4) u, P = 0.001], fresh frozen plasma (FFP) [1200 (400–2400) vs. 700 (0–1200) ml, P = 0.02] and platelet (Plt) [1 (0–5) vs. 0 (0–1) u, P = 0.01] transfusion were identified in the BSI group. The number (and proportion) of patients with AKI receiving CRRT in the BSI group was significantly higher than that in the non–BSI group (70% vs. 43%, P = 0.02). There was no significant difference in age, BMI, smoking history, past medical history, ECMO duration, ICU stay, hospital stay or mortality rate between the two groups.
Independent risk factors for BSI in ARDS–VV–ECMO patients (Table 2 )
Variables with a P value < 0.05 and relevant variables reported in previous studies, including male sex, history of hypertension, Murray score, transfusion, CRRT, and ECMO duration, were included in the multiple logistic regression analysis. The Murray score (odds ratio = 6.29, P = 0.01) and blood transfusion (odds ratio = 1.27, P = 0.03) were independent risk factors for BSI.
Discussion
Many studies evaluating BSI during ECMO have been conducted in patients receiving VA–ECMO [9, 10, 12]. Among the few studies involving ARDS patients supported by VV–ECMO [1, 16], the etiology of ARDS variable and included interstitial lung diseases, radioactive pneumonia, diffuse alveolar hemorrhage, trauma and other diseases in addition to severe pneumonia. This study retrospectively analyzed the incidence and risk factors for BSI in patients with severe ARDS associated with pulmonary infection receiving VV–ECMO support. The etiology of ARDS was relatively singular, excluding other confounding factors.
The incidence of BSI was 22% in our study, which was similar to that in other studies [1, 16]. Our study showed that the most common pathogenic microorganism of BSI in severe ARDS patients with VV–ECMO was G– bacilli, mainly Acinetobacter baumannii, Burkholderia cepacia and Klebsiella pneumoniae, consistent with the findings of some single–center studies [4, 17]. However, other researchers revealed that Candida (13%, the average incidence was 2.33/1000 ECMO days) and Staphylococcus aureus (7–10%, the average incidence was 1.20–2.37/1000 ECMO days) were the most common [18] isolates from BCs in patients receiving ECMO. We also observed that the incidence of BSI caused by MDR bacteria, especially multidrug–resistant G– bacteria (MDR–GNB), was high during VV–ECMO support (34%). The high rate of antibiotic resistance is worrisome and can be attributed to frequent exposure to broad–spectrum antibiotics, acquired or primary immune impairment, prolonged hospitalization and mechanical ventilation [19, 20].
Although the association between blood transfusion and nosocomial infection in critically ill patients has been confirmed [21, 22], whether there is a potential association between blood transfusion and BSI in ECMO patients remains uncertain. Our study showed that blood transfusion was an independent risk factor for BSI, which was consistent with previous studies. Soo [23] et al. noted that the total units of transfused blood (aOR, 1.01; 95% CI, 1.00–1.02) was independently associated with BSI in patients receiving VV–ECMO for respiratory failure. Biologically, transfusion of pRBCs may increase the risk of BSI by interfering with the cytokine profile of the host. Several studies have shown that pRBCs contain multiple proinflammatory cytokines that, when infused into a susceptible subject, could potentially tip the balance and lead to the dysregulation of multiple cascades [24]. Thus, transfusion directly promotes inflammation, as demonstrated in studies that observed elevated levels of interleukin–6 in a recipient following pRBC administration [25]. On the other hand, residual donor white blood cells (WBCs) could promote T–cell activation [26], which in turn could result in subtle changes in the host's immune status, predisposing him or her to infection. Both cellular and humoral immunity are adversely affected by blood transfusion [27]. Following pRBC transfusion, decreased production of interleukin–2 and increased production of prostaglandin–E2 have been documented. A decrease in CD4 helper cells, interleukin–2 receptor–positive helper cells, and natural killer cells occurs, as well as an increase in B cells and CD8 suppressor cells. Some immune functions return to normal within hours following pRBC transfusion, but evidence suggests that long–term or permanent alterations in immune function may occur [27].
Most patients who receive ECMO support are in critical condition and are often complicated with AKI, with an incidence of 70.3%–84.4%. Approximately 60% of ECMO patients require CRRT [28]. In this study, 50% of ECMO patients received CRRT, while as many as 70% of patients with BSI required CRRT. The rate of CRRT in VV–ECMO patients with BSI was 48–83% in other studies [1, 10, 16, 17]. An injured kidney may compromise the immune response via systemic release of leukocytes from the kidneys and renal tubular cells. Such changes in the host immune response may be associated with nosocomial infection [29, 30]. CRRT in AKI may be an independent risk factor for BSI, which may be related to potential multisystem disorders, systemic inflammation, hormone imbalance [31, 32], prolonged duration of ECMO or CRRT–related operation in VV–ECMO patients [10].
Immunosuppression is a risk factor for infection. Nosocomial infection has been shown to be common in immunocompromised patients receiving ECMO support [19, 33, 34]. However, in some previous studies, there was no significant difference in immunosuppressive status between the BSI group and the non–BSI group [1, 16, 17], which was consistent with the results of our study. Our study found that immunosuppressive status did not increase the risk of BSI.
A previous study demonstrated that ECMO duration of more than 250 h significantly increased the incidence of BSI [5, 33]. However, some researchers believe that a prolonged duration of ECMO might be an adverse effect of BSI rather than a risk factor for BSI [16]. In our study, there was no association between ECMO duration and BSI.
Kutleša M [1] et al. reported that the incidence of massive hemorrhage was 34% in patients receiving VV–ECMO for ARDS, and massive hemorrhage was independently associated with BSI. The most common sites of massive hemorrhage are the gastrointestinal tract (GIT), urinary tract (UT), abdominal and thoracic cavities. Bleeding from the GIT, UT and nasal cavity can enhance the translocation of colonizing bacteria from those sites to the bloodstream. Furthermore, any significant bleeding requires urgent treatment with multiple invasive procedures, which might disrupt the mucosal barrier and increase the risk of infection. Finally, major bleeding episodes increase the duration of ECMO, which also increases the chances of acquiring BSI [4]. In our study, the incidence of massive hemorrhage events in the BSI group was 35%, and the most common types were GIT hemorrhage, cerebral hemorrhage and alveolar hemorrhage. We found no difference in bleeding events between the BSI group and the non–BSI group. The possible reasons were the strict aseptic techniques applied in all invasive operations and the administration of broad–spectrum antibiotics, which reduced the occurrence of BSI to some extent.
Our study has several limitations. It was conducted in a single clinical center and thus may lack general representativeness in terms of disease types and population. Our results should be confirmation in large–scale, prospective multicenter studies. In addition, the small number of BSI patients may lead to unreliable statistical conclusions. To accurately determine the source of BSI, strain homology analysis should be performed, but we did not conduct this analysis. However, this is one of the few studies conducted in BSI in patients with severe pneumonia–associated ARDS who received ECMO. This study systematically analyzed the incidence and risk factors in these patients.
Conclusion
G–bacilli are the main pathogens causing BSIs in patients with severe ARDS supported by VV–ECMO, and most of them are MDR–GNB. The Murray score and blood transfusion may be independent risk factors for BSI.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.
References
Kutleša M, Santini M, Krajinović V, et al. Nosocomial blood stream infections in patients treated with venovenous extracorporeal membrane oxygenation for acute respiratory distress syndrome. Minerva Anestesiol. 2017;83(5):493–501. https://doi.org/10.23736/S0375-9393.17.11659-7.
Hsu MS, Chiu KM, Huang YT, et al. Risk factors for nosocomial infection during extracorporeal membrane oxygenation. J Hosp Infect. 2009;73(3):210–6. https://doi.org/10.1016/j.jhin.2009.07.016.
Kim HS, Park S, Ko HH, et al. Different characteristics of bloodstream infection during venoarterial and venovenous extracorporeal membrane oxygenation in adult patients. Sci Rep. 2021;11:9498. https://doi.org/10.1038/s41598-021-89108-4.
Aubron C, Cheng AC, Pilcher D, et al. Factors associated with outcomes of patients on extracorporeal membrane oxygenation support: a 5–year cohort study. Crit Care. 2013;17(2):R73. https://doi.org/10.1186/cc12681.
Sun HY, Ko WJ, Tsai PR, et al. Infections occurring during extracorporeal membrane oxygenation use in adult patients. J Thorac Cardiovasc Surg. 2010;140(5):1125-32.e2. https://doi.org/10.1016/j.jtcvs.2010.07.017.
Vogel AM, Lew DF, Kao LS, et al. Defining risk for infectious complications on extracorporeal life support. J Pediatr Surg. 2011;46(12):2260–4. https://doi.org/10.1016/j.jpedsurg.2011.09.013.
Vanholder R, Van Loo A, Dhondt AM, et al. Influence of uraemia and haemodialysis on host defence and infection. Nephrol Dial Transplant. 1996;11(4):593–8. https://doi.org/10.1093/oxfordjournals.ndt.a027346.
Hanna HA, Raad I. Blood products: a significant risk factor for long–term catheter–related bloodstream infections in cancer patients. Infect Control Hosp Epidemiol. 2001;22(3):165–6. https://doi.org/10.1086/501885.
Silvetti S, Ranucci M, Pistuddi V, et al. Bloodstream infections during post–cardiotomy extracorporeal membrane oxygenation: Incidence, risk factors, and outcomes. Int J Artif Organs. 2019;42(6):299–306.
Menaker J, Galvagno S, Rabinowitz R, et al. Epidemiology of blood stream infection in adult extracorporeal membrane oxygenation patients: a cohort study. Heart Lung. 2019;48(3):236–9. https://doi.org/10.1016/j.hrtlng.2019.01.004.
Steiner CK, Stewart DL, Bond SJ, et al. Predictors of acquiring a nosocomial bloodstream infection on extracorporeal membrane oxygenation. J Pediatr Surg. 2001;36(3):487–92. https://doi.org/10.1053/jpsu.2001.21609.
Seidelman JL, Lewis SS, Huslage K, et al. To be a CLABSI or not to be a CLABSI–that is the question: the epidemiology of BSI in a large ECMO population. Infect Control Hosp Epidemiol. 2018;39(3):362–5. https://doi.org/10.1017/ice.2017.320.
Garner JS, Jarvis WR, Emori TG, et al. CDC definitions for nosocomial infections, 1988. Am J Infect Control. 1988;16(3):128–40. https://doi.org/10.1016/0196-6553(88)90053-3.
Horan TC, Andrus M, Dudeck MA. CDC/NHSN surveillance definition of health care–associated infection and criteria for specific types of infections in the acute care setting. Am J Infect Control. 2008;36(5):309–32. https://doi.org/10.1016/j.ajic.2008.03.002.
Yun JH, Hong S-B, Jung S–H, et al. Epidemiology and clinical characteristics of bloodstream infection in patients under extracorporeal membranous oxygenation. J Intensive Care Med. 2021;36(9):1053–60. https://doi.org/10.1177/0885066620985538.
Wang JR, Huang JY, Hu W, et al. Bloodstream infections in patients undergoing extracorporeal membrane oxygenation. Pak J Med Sci. 2020;36(6):1171–6. https://doi.org/10.12669/pjms.36.6.2882.
Biffi S, Di Bella S, Scaravilli V, et al. Infections during extracorporeal membrane oxygenation: epidemiology, risk factors, pathogenesis and prevention. Int J Antimicrob Agents. 2017;50(1):9–16.
Grasselli G, Scaravilli V, Di Bella S, et al. Nosocomial infections during extracorporeal membrane oxygenation: incidence, etiology, and impact on patients’ outcome. Crit Care Med. 2017;45(10):1726–33. https://doi.org/10.1097/CCM.0000000000002652.
Glater-Welt LB, Schneider JB, Zinger MM, et al. Nosocomial bloodstream infections in patients receiving extracorporeal life support: variability in prevention practices: a survey of the extracorporeal life support organization members. J Intensive Care Med. 2016;31(10):654–69. https://doi.org/10.1177/0885066615571540.
Graves TA, Cioffi WG, Jr Mason AD, et al. Relationship of transfusion and infection in a burn population. J Trauma. 1989;29(7):948–52.
Edna TH, Bjerkeset T. Association between blood transfusion and infection in injured patients. J Trauma. 1992;33(5):659–61.
Na SJ, Chung CR, Choi HJ, et al. Blood stream infection in patients on venovenous extracorporeal membrane oxygenation for respiratory failure. Infect Control Hosp Epidemiol. 2018;39(7):871–4. https://doi.org/10.1017/ice.2018.90.
Biedler AE, Schneider SO, Seyfert U, et al. Impact of alloantigens and storage–associated factors on stimulated cytokine response in an in vitro model of blood transfusion. Anesthesiology. 2002;97(5):1102–9.
Vamvakas EC, Blajchman MA. Deleterious clinical effects of transfusion–associated immunomodulation: fact or fiction? Blood. 2001;97(5):1180–95.
Landers DF, Hill GE, Wong KC, et al. Blood transfusion–induced immunomodulation. Anesth Analg. 1996;82(1):187–204.
Shorr AF, Jackson WL, Kelly KM, et al. Transfusion practice and blood stream infections in critically ill patients. Chest. 2005;127(5):1722–8.
Antonucci E, Lamanna I, Fagnoul D, et al. The impact of renal failure and renal replacement therapy on outcome during extracorporeal membrane oxygenation therapy. Artif Organs. 2016;40(8):746–54.
Lee DW, Faubel S, Edelstein CL. Cytokines in acute kidney injury (AKI). Clin Nephrol. 2011;76(3):165–73.
Grigoryev DN, Liu M, Hassoun HT, et al. The local and systemic inflammatory transcriptome after acute kidney injury. J Am Soc Nephrol. 2008;19(3):547–58.
Villa G, Katz N, Ronco C. Extracorporeal membrane oxygenation and the kidney. Cardiorenal Med. 2015;6(1):50–60.
Mitra S, Ling RR, Tan CS, et al. Concurrent use of renal replacement therapy during extracorporeal membrane oxygenation support: a systematic review and meta-analysis. J Clin Med. 2021;10(2):241.
Schmidt M, Schellongowski P, Patroniti N, et al. Six-month outcome of immunocompromised patients with severe acute respiratory distress syndrome rescued by extracorporeal membrane oxygenation: an international multicenter retrospective study. Am J Respir Crit Care Med. 2018;197(10):1297–307.
Abrams D, Grasselli G, Schmidt M, et al. ECLS–associated infections in adults: what we know and what we don’t yet know. Intensive Care Med. 2020;46(2):182–91.
Manerikar A, Watanabe S, Kandula V, et al. Indwelling central venous catheters drive bloodstream infection during veno–venous extracorporeal membrane oxygenation support. ASAIO J. 2022;68(6):859–64.
Acknowledgements
The authors thank the clinicians and nurses in the medical intensive care unit of China–Japan Friendship Hospital.
Funding
Liuting YANG was supported by a grant from the Non-profit Central Research Institute Fund of Chinese Academy of Medical Sciences (2020-PT320-005).
Author information
Authors and Affiliations
Contributions
All authors made substantial contributions to the conception and design of the study or to the data acquisition, analysis or interpretation; reviewed and approved the final manuscript; and significantly contributed to this study. Liuting YANG, Sichao Gu, and Min Li contributed equally to this work. Dr. Zhan takes full responsibility for the integrity of the submission and publication and was involved in the study design. Liuting Yang, Sichao Gu, and Min Li wrote the main manuscript text; Yingying Feng prepared the figures; and Xu Huang, Yi Zhang, Ye Tian, and Xiaojing Wu prepared Tables 1 and 2. All authors reviewed the manuscript and take responsibility for the integrity of the data and the accuracy of the data analysis. Dr. Linna Huang was responsible for data verification and analysis.
Corresponding authors
Ethics declarations
Ethical approval and consent to participate
The study was conducted in accordance with the principles of the Declaration of Helsinki, and the study protocol was approved by the Ethics Committee of the China–Japan Friendship Hospital. Because of the retrospective nature of the study, the requirement for informed consent was waived by the Ethics Committee of the China–Japan Friendship Hospital.
Consent for publication
We confirm that all steps/methods were performed in accordance with relevant guidelines and regulations. Informed consent for the publication of identifiable information/images in an open access journal was obtained from all study participants.
Competing Interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Yang, L., Li, M., Gu, S. et al. Risk factors for bloodstream infection (BSI) in patients with severe acute respiratory distress syndrome (ARDS) supported by veno–venous extracorporeal membrane oxygenation (VV–ECMO). BMC Pulm Med 22, 370 (2022). https://doi.org/10.1186/s12890-022-02164-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12890-022-02164-y