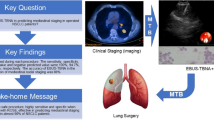
Abstract
Background
Endobronchial ultrasound-guided transbronchial needle aspiration (EBUS-TBNA) is established as the preferred method of mediastinal lymph node (LN) staging in non-small cell lung cancer (NSCLC). Selective (targeted) LN sampling is most commonly performed however studies in early stage NSCLC and locally advanced NSCLC confirm systematic EBUS-TBNA evaluation improves accuracy of mediastinal staging. This study aims to establish the rate of detection of positron emission tomography (PET)-occult LN metastases following systematic LN staging by EBUS-TBNA, and to determine the utility of systematic mediastinal staging for accurate delineation of radiation treatment fields in patients with locally advanced NSCLC.
Methods
Consecutive patients undergoing EBUS-TBNA for diagnosis/staging of locally advanced NSCLC will be enrolled in this international multi-centre single arm study. Systematic mediastinal LN evaluation will be performed, with all LN exceeding 6 mm to be sampled by TBNA. Where feasible, endoscopic ultrasound staging (EUS-B) may also be performed. Results of minimally invasive staging will be compared to FDG-PET. The primary end-point is proportion of patients in whom systematic LN staging identified PET-occult NSCLC metastases. Secondary outcome measures include (i) rate of nodal upstaging, (ii) false positive rate of PET for mediastinal LN assessment, (iii) analysis of clinicoradiologic risk factors for presence of PET-occult LN metastases, (iv) impact of systematic LN staging in patients with discrepant findings on PET and EBUS-TBNA on target coverage and dose to organs at risk (OAR) in patients undergoing radiotherapy.
Discussion
With specificity of PET of 90%, guidelines recommend tissue confirmation of positive mediastinal LN to ensure potentially early stage patients are not erroneously denied potentially curative resection. However, while confirmation of pathologic LN is routinely sought, the exact extent of mediastinal LN involvement in NSCLC in patient with Stage III NSCLC is rarely established. Studies examining systematic LN staging in early stage NSCLC report a significant discordance between PET and EBUS-TBNA. In patients with locally advanced disease this has significant implications for radiation field planning, with risk of geographic miss in the event of PET-occult mediastinal LN metastases. The SEISMIC study will examine both diagnostic outcomes following systematic LN staging with EBUS-TBNA, and impact on radiation treatment planning.
Trial registration
ACTRN12617000333314, ANZCTR, Registered on 3 March 2017.
Similar content being viewed by others
Background
Endobronchial ultrasound-guided transbronchial needle aspiration (EBUS-TBNA) is a widely accepted technique for minimally invasive sampling of mediastinal lymph nodes (LN). It is the preferred method for staging of patients presenting with suspected locally advanced lung cancer due to its excellent diagnostic accuracy and safety profile [1]. Non-invasive imaging with positron emission tomography (PET), generally performed pre-procedurally, is used to complete clinical staging. Stage III NSCLC is suspected following demonstration of fluoro-deoxyglucose (FDG) avidity in mediastinal LN.
Accuracy of PET for detection of metastatic LN in NSCLC patients is imperfect [2] and consequently international clinical practice guidelines mandate biopsy confirmation of PET-positive mediastinal LN [1, 3, 4]. In this scenario, “selective” sampling with EBUS-TBNA is generally targeted to pathologic (FDG-avid) stations with the primary intent of confirming a pathologic diagnosis. If metastatic involvement is confirmed at any site no further sites are examined. Confirmation of metastatic involvement commonly results in a recommendation of definitive chemoradiotherapy. Despite the known imperfect accuracy of PET for evaluation of mediastinal LN in NSCLC, radiation target volumes are most commonly based on PET-identified extent of disease [5].
Systematic mediastinal LN staging in early stage NSCLC prior to surgical resection is recommended in patients with risk factors for post-operative up-staging [3]. Systematic EBUS-TBNA staging in early stage NSCLC may identify PET-occult mediastinal LN metastases in up to 1 in 14 patients. Our single centre pilot study confirmed that discordant findings between PET-CT and EBUS-TBNA regarding extent of mediastinal LN involvement are seen in a significant proportion of patients with clinical stage III NSCLC, with both up-staging (i.e. detection of PET-occult LN metastases) and down-staging observed [6]. Follow-on studies also confirmed that EBUS-TBNA prior to curative-intent radiotherapy significantly improves coverage of subclinical disease through detection of PET-occult metastases. Identification of false-positive lymph node involvement in highly selected cases may reduce radiation dose to organs at risk (OAR) [7].
This prospective international multi-centre study will examine the use of systematic mediastinal LN staging in patients with locally advanced (stage III) NSCLC. Aims of the study include:
-
1.
To determine the rate of detection of PET-occult LN metastases in patients with locally advanced (Stage III) NSCLC
-
2.
To identify the proportion of patients with potentially false positive involvement of mediastinal LN on PET
-
3.
To identify clinical risk predictors for presence of PET-occult LN metastases
-
4.
In patients with discrepant findings on PET and EBUS-TBNA, to compare target coverage and radiation dose to OARs using target volumes based on PET alone versus PET and EBUS-TBNA.
Importance of comprehensive mediastinal lymph node staging in early stage NSCLC
The importance of accurate LN staging in early stage NSCLC is well established. Guidelines recommend systematic LN staging at the time of curative intent surgery [4, 8, 9], both to ensure complete resection (R0) of all disease, and to ensure accurate disease staging for optimal guidance of adjuvant therapy recommendations. The importance of accurate LN staging may be illustrated by poorer survival outcomes in patients undergoing surgery where resection is considered incomplete, due either to lack of systematic nodal dissection, or positivity of the highest mediastinal node removed [10, 11]. Critically, 5-year survival in patients where the highest LN removed was positive was markedly worse than in patients with presence of carcinoma in situ at the bronchial resection margin (29 vs 40%) [10]. The International Association for the Study of Lung Cancer identify the significance of have described the significance of LN staging for defining the residual tumour descriptor in resected NSCLC [12], and assigned patients with metastatic involvement of the highest mediastinal LN resected as having an unknown residual tumour status (R(un)), with this group experiencing significantly worse median survival than in patients in whom adequate nodal staging is completed.
Variability in adequacy of LN examination/sampling for resected NSCLC has been suggested to explain a large proportion of intercontinental survival differences [11]. Consequently, the National Comprehensive Cancer Network recommends a minimum of 3 or more mediastinal nodal stations, the American College of Surgeons Commission on Cancer recommends 10 total LN regardless of station, and the Union for International Cancer Control seventh edition recommends 6 total nodes, 3 from N1 and 3 from N2 stations are sampled [9].
Accuracy of EBUS-TBNA for systematic LN staging in early stage NSCLC is established, though is recognized to be inferior to that observed for patients with radiologically pathologic mediastinal LN [13] EBUS-TBNA may demonstrate PET-occult disease in up to 10% of NSCLC patients with a radiologically normal mediastinum, and up to 29% of patients staged cN1 on PET/CT [14,15,16]. International guidelines recommend EBUS-TBNA staging pre-operatively in patients with radiologic features associated with an increased risk of post-operative pathologic upstaging [3]
Comprehensive mediastinal lymph node staging in locally advanced NSCLC
In contrast to early stage NSCLC where intra-operative LN staging is recommended to achieve completion of pathologic staging, current International guidelines recommend radiation target volumes be constructed based on PET-identified extent of disease [5, 17] In this setting, any PET-occult LN metastases would not be included in the radiation field [18] and potentially result in a higher likelihood of regional failure. Equally, inclusion of false positive LN may result in unnecessary increased radiation dose to critical structures, with consequent increased risk of organ toxicity [7]
Incomplete staging of the mediastinum in patients with locally advanced NSCLC is analogous to R(un) staging described above in patients undergoing resection—while many patients will have the entirety of their disease encompassed by radiation fields, an unknown proportion (estimated 5–29% based on previously published studies [6, 15, 19] will have disease located outside the radiation field. Patients whose disease by definition has demonstrated metastatic potential to LN may even be expected to have a higher rate of presence of PET-occult disease—a higher rate of postoperative upstaging to pN2 is observed in patients with cN1 disease, and a similar higher rate of pN2 disease has been observed following systematic EBUS-TBNA mediastinal staging in NSCLC [15]
Similar to studies in early stage NSCLC, our single centre pilot study [6], consistent with prior retrospective studies [19, 20] confirmed a significant rate of detection of PET-occult LN metastases in NSCLC following systematic mediastinal staging with EBUS-TBNA. Critically, our follow-up study confirmed that this has major implications for radiotherapy planning and probability of tumour control following curative-intent radiation [7] predominantly due to reduction of geographic miss in these patients.
EBUS is now considered standard of care in management of lung cancer. The utility of Endoscopic Ultrasound (EUS) in mediastinal staging of lung cancer has long been established, with complementary reach of the techniques allowing a greater number of LN stations to be assessed via minimally invasive means. More recently use of the endobronchial video-bronchoscope has allowed “complete” mediastinal staging. EUS using the bronchoscope (EUS-B) can be performed by pulmonologists in the same procedure as EBUS-TBNA [21, 22] and multiple studies have confirmed that the combined use of EBUS-TBNA and EUS-FNA significantly improves sensitivity in detecting mediastinal nodal metastases in NSCLC [13, 23] Consequently, where possible, European Respiratory Society and European Society of Thoracic Surgeons guidelines on staging of lung cancer recommend both techniques be employed [3]
Methods/design
Study design The SEISMIC study (Systematic EndoscopIc Staging of Mediastinum to determine Impact on radiotherapy for locally advanced lung Cancer) is a prospective international multi-centre single arm interventional study.
Setting seven international tertiary lung cancer services.
Intervention systematic LN staging with EBUS-TBNA will be performed at the time of staging EBUS as previously described [6] At centres where expertise and experience allows, endoscopic staging will comprise both EBUS and EUS-B [22] mediastinal examination and sampling.
Patient population patients with known or suspected NSCLC and suspected or known mediastinal metastases (i.e. cN2/3 disease), based upon CT and PET findings. Inclusion and Exclusion criteria are recorded in Box 1.
Box 1: Inclusion and exclusion criteria | |
|---|---|
Inclusion criteria • Patients with known/suspected NSCLC • Patients with suspected or known mediastinal metastases (i.e. cN2/3 disease) • Patients are potential candidates for radical dose or high-dose palliative conventional radiotherapy (± chemotherapy) | Exclusion criteria • Medical co-morbidities preclude bronchoscopy • Medical co-morbidities or known pathologic extent of disease precludes consideration of radiotherapy • Age < 18 years |
Outcomes the primary study outcome is proportion of patients in who PET-occult mediastinal LN metastases are detected by systematic LN staging with EBUS-TBNA/(EUS-B-FNA).
Secondary outcomes include:
-
Proportion of patients with endoscopy-demonstrated benign LN at sites of FDG-avidity on PET/CT
-
Multi-regression analysis of clinic- radiologic factors to identify risk factors for endoscopic detection of PET-occult disease
-
Difference in planned radiation dose to lymph nodes and adjacent critical organs in treatment plans based on FDG-PET/CT only vs FDG-PET/CT & EBUS-TBNA in patients where EBUS-TBNA staging demonstrates discrepant extent of mediastinal involvement compared with FDG-PET/CT.
-
Radiomics analysis to identify risk factors for EBUS-PET discrepancy regarding extent of mediastinal LN involvement.
-
Incidence of detection of PET-occult disease by EUS-B-FNA
-
Development of a radiomics signature to predict nodal status as defined by EBUS
-
Outcomes of surgical staging (where performed) in patients with PET + /EBUS– findings
Data collection
All patients’ demographic and clinical data will be collected including gender, age, smoking status, whether symptomatic disease, and histology.
Radiologic features from PET-CT scans include primary tumour size, peripheral vs central position (“concentric” lines, inner one-third, centre of the tumour, as per Casal et al. [24]) of primary tumour, N-stage as determined by CT chest, N-stage as determined by FDG-PET, and highest LN station involved on radiologic imaging.
EBUS imaging features; including whether endobronchial tumour was seen [25] and which PET negative nodes were identified, which nodes were sampled, station, sonographic features of LN (size, LN margin, shape, echogenicity, central hilum visible, necrosis sign visible), number of LN sampled, whether EUS-B was performed and EUS-B features, procedure time and procedural complications.
Additionally, collection of data will include; final N-stage as determined by EBUS-TBNA/EUS-B, highest LN station involved by NSCLC, and any discrepancy between PET and EBUS-TBNA staging.
Performance of systematic LN staging
PET-CT
Integrated PET-CT scans will be performed before recruitment into the study and performance of EBUS staging bronchoscopy. Pre-treatment staging established according to the 8th edition of the Lung Cancer Stage Classification, the TNM descriptors for which are reviewed in detail elsewhere [26]
Endoscopic staging procedure
Endoscopic staging will be performed according to the following methodology:
-
Visual examination of the following LN stations is required in all cases – 2R, 2L, 4R, 4L, 7. In centres where EUS-B is utilized, endoscopic examination also requires visualization of stations 4L, 5, 7, 8, 9.
-
Any visible LN exceeding 6 mm in these stations will be sampled via EBUS-TBNA and/or EUS-B-FNA. Rapid on-site cytology examination (ROSE) of TBNA/FNA aspirates will be used where available to confirm adequate specimens/diagnostic tissue is obtained before proceeding to the next LN station.
-
Systematic sampling should commence at the highest echelon mediastinal LN visualized (ie. N3) and proceed more proximally, guided by ROSE results. Where ROSE was unavailable, PET-positive LN should be sampled a minimum of three times by TBNA. Assessment of contralateral hilar LN is not planned due to the impact on procedure time & complexity, given the exceedingly low expected rate of disease at this anatomic site [27]
-
Samples per nodal station:
-
For PET-negative LN (low pre-test probability) where ROSE demonstrates adequate samples, no further sampling from that site will occur. We would plan to perform as few as 1 TBNA per LN station
-
For PET-positive LN (where pre-test probability of malignant involvement is higher), Three TBNA needle passes will be performed when ROSE indicates benign tissue, to ensure highest NPV at these high risk sites.
-
-
At centres where expertise & experience allows, endoscopic staging will comprise both EBUS and EUS-B mediastinal examination & sampling. Remaining centres will undertake systematic sampling of mediastinal LN via EBUS-TBNA.
Dosimetry endpoints
This component is an in silico study that will examine dosimetric consequences of PET-EBUS-TBNA discrepancies observed. Clinical treatment plans will be constructed after active involvement in the study has been completed. Dosimetry of radiation treatment plans are routinely performed in all patients using standardised computer-based dosimetric calculations, which include routine dose/volume constraints such as the volume of lung that receives 5 Gray (Gy) (V5Gy), the V20Gy, V30Gy and mean lung dose. Clinical treatment plans are routinely constructed on the basis of PET-identified disease extent. Where Endosonographic findings suggest a different extent of mediastinal involvement, the dosimetric differences in lymph node and critical organ dose between treatment plans based on FDG-PET/CT only vs FDG-PET/CT & EBUS-TBNA staging will be calculated.
For study endpoints, two different scenarios will occur where dosimetric consequences of systematic endosonographic staging will be calculated. These will be theoretical in nature, comparing the radiation plan based upon PET based target volumes to a radiation plan based upon PET- and EBUS- based target volumes.
-
(1)
EBUS-TBNA identifies PET-occult disease (PET-/EBUS +): The clinical treatment plan will be constructed to include in the treatment field both the PET + and EBUS + mediastinal LN sites. The hypothetical plan will be based solely on PET + sites. The difference in radiation dose at PET-/EBUS + sites between the clinical and hypothetical plans will be determined. From this difference the risk of recurrence will be estimated according to published studies relating radiation dose to tumour control probability.
-
(2)
EBUS-TBNA demonstrates benign lymph node tissue in PET-positive lymph nodes (PET + /EBUS-): The clinical treatment plan will be constructed to include all PET + sites (due to the imperfect negative predictive value of endosonographic staging—approx 95%). The hypothetical plan will be based on the endosonographic-determined disease extent. The difference in radiation dose to critical organs including the lung, heart, oesophagus between the clinical and hypothetical treatment plans will be calculated. Estimates of toxicity probability will be computed based on existing relationships between organ dose and toxicity risk.
Sample size calculation
Study sample size has been calculated at 190. This is based on the view of investigators regarding the expected primary outcome (proportion of participants with PET-occult disease detected by EBUS-TBNA) of 9–11 percent, based on previously published studies [6]
95% CI for this outcome with n = 190 is 7.3–16.4%.
Data analysis
Primary study outcome will be reported using observed prevalence (%) with 95% confidence intervals.
Identification of clinicoradiologic risk factors for presence of PET-occult disease will be completed using multinomial or ordinal regression.
Application of established risk prediction tools for prediction of nodal disease in patients with NSCLC as determined by EBUS-TBNA [28] will also be performed for identification of patients with PET-EBUS discrepant findings.
Radiation dosimetry endpoints will be based on in silico radiation plans developed according to staging based on PET findings alone, versus PET + EBUS staging findings.
Radiomics analysis
Individual lymph node stations will be manually contoured on the diagnostic contrast enhanced CT and PET scans of all patients by a radiation oncologist and used as the volume of interest (VOI). Radiomic features will be extracted from the VOI using a specialized, validated, open source software (PyRadiomics) available on the python platform. Radiomics features extracted will include first order features, shape features and second order texture features, including grey scale co-occurrence matrix, neighbourhood grey tone difference matrix, grey level run-length matrix, grey level size zone matrix, and level dependence matrix. The association between lymph node status and radiomics features will be modelled using a variety of binary classifiers (such as logistic regression, random forests and support vector machines). Each of the classifiers will be employed within a nested ten-fold cross-validation framework and classifier accuracy will be reported with sensitivity, specificity, and area under the receiver operating characteristic curve (AUC).
Discussion
Non-invasive staging is a critical step in assessment of patients with NSCLC. However the imperfect sensitivity of PET in mediastinal staging of NSCLC means (minimally) invasive confirmation of findings is frequently sought. With specificity of PET of 90%, guidelines recommend tissue confirmation of positive mediastinal LN to ensure potentially early stage patients are not erroneously denied potentially curative resection. However, while confirmation of pathologic LN is routinely sought, the extent of mediastinal evaluation during staging EBUS-TBNA varies considerably [29, 30], and the exact extent of mediastinal LN involvement in NSCLC in patient with Stage III NSCLC is rarely established. Consequently, a proportion of patients receiving radical radiotherapy may be at risk of geographic miss (in the event of PET-occult LN metastases) and higher rates of disease recurrence, or receive radiation to a larger field than is needed (in the event of false-positive PET findings) with consequent elevated toxicity risk in OAR. We believe this variance in care presents an important opportunity to improve clinical outcomes in NSCLC.
Systematic mediastinal LN staging with EBUS-TBNA in patients with early stage NSCLC has demonstrated a significant rate of detection of PET-occult disease [15] as well as down-staging [31, 32] Thus it is well established that a significant rate of discrepancy in mediastinal assessment of patients with NSCLC between PET/CT and EBUS-TBNA exists. Less established is the rate of discrepancy in patients with locally advanced/cN2 NSCLC.
We hypothesize that systematic mediastinal LN staging in patients with Stage III NSCLC will provide more accurate information on the extent of mediastinal LN involvement. The SEISMIC study will examine both the outcomes following systematic LN staging, as well as the impact on radiation treatment planning.
On the other hand, systematic mediastinal assessment with EBUS-TBNA as compared to selective sampling is a non-trivial extension of current practice. Additional procedural time and potential for complications must be outweighed by the clinical utility of the approach. It may be that the low-intermediate dose wash of current radiotherapy treatments may cover subclinical radiological disease sufficiently to achieve cancer control. The purpose of this study is to evaluate these possibilities in a rigorously conducted prospective trial.
Patterns of lymph node metastases, based on primary tumour position have been reported previously in surgical cohorts [33] as have patterns of locoregional relapse following definitive chemoradiation [34]. Depending on findings from this study, a more focused approach to LN sampling by EBUS-TBNA prior to definitive chemoradiation may be identified, based either on radiologic factors, or linear EBUS imaging findings [35].
Study limitations
This study is not intended as an examination of diagnostic accuracy of EBUS-TBNA. Given the previously reported specificity of EBUS-TBNA for detection of NSCLC of 100% [36], findings of PET-occult disease will not be performed surgically. Outcomes following systematic LN staging will be reported as a proportion of patients in whom PET-occult metastases are identified by systematic EBUS-TBNA. As surgical confirmation will not be performed, we will not be able to report a true prevalence of PET-occult LN metastases in this group, nor the sensitivity of EBUS-TBNA for detection of PET-occult LN metastases.
While EUS-B-FNA may be performed by pulmonologists, this is dependent on local expertise and institutional credentialing practice. Thus we expect only a minority of patients to have undergone “complete” endoscopic mediastinal staging. Furthermore, PET staging will include assessment of all LN stations, while endoscopic staging will be limited only to LN stations accessible by the technique. EBUS is unable to assess Stations 5, 6, 8 and 9.
Prediction models estimating the probability of nodal disease have been validated [28] and may be valuable in identifying a subset of patients with higher probability of downstaging to early stage (resectable) NSCLC. However, in these models patients with N2 and N3 are grouped together. This study will examine outcomes on a per-lymph node basis and therefore discrimination between N2 and N3 status is not expected from this model it is unlikely.
While in silico modeling will examine the dosimetric consequences of PET-EBUS discrepancies, this study will not directly compare survival outcomes. Delivery of radiotherapy will be determined by local tumour control board assessments, incorporating both PET and EBUS-TBNA findings.
Conclusion
While the importance of accurate LN staging, both for prognostication and treatment decision-making, for patients with early stage NSCLC is emphasized in the literature as well as international society guidelines, the same importance is not attached to LN staging in patients with stage III disease undergoing curative-intent therapy, despite the evidence supporting the critical impact this may have on treatment planning and outcomes. We intend that this study provide evidence to support increasing use of this simple and established technique of EBUS-TBNA in this patient group.
Availability of data and materials
This is a study protocol article and no datasets were generated or analysed for this protocol manuscript. To obtain access to the raw data, you may contact A/Prof Daniel Steinfort at Daniel.steinfort@mh.org.au.
Abbreviations
- EBUS:
-
Endobronchial ultrasound
- EUS:
-
Endoscopic ultrasound
- EUS-B:
-
Endoscopic ultrasound staging
- EUS-B-FNA:
-
Endoscopic ultrasound with bronchoscope-guided fine needle aspiration
- TBNA:
-
Transbronchial needle aspiration
- FNA:
-
Fine needle aspiration
- LN:
-
Lymph node
- PET:
-
Positron emission tomography
- FDG-PET:
-
Fluorodeoxyglucose positron emission tomography
- NSCLC:
-
Non-small cell lung cancer
- ROSE:
-
Rapid on-site cytology examination
References
Silvestri GA, Gonzalez AV, Jantz MA, Margolis ML, Gould MK, Tanoue LT, Harris LJ, Detterbeck FC. Methods for staging non-small cell lung cancer: diagnosis and management of lung cancer, 3rd ed: American college of chest physicians evidence-based clinical practice guidelines. Chest. 2013;143(5 Suppl):e211S-250S.
Schmidt-Hansen M, Baldwin DR, Hasler E, Zamora J, Abraira V, Roque IFM. PET-CT for assessing mediastinal lymph node involvement in patients with suspected resectable non-small cell lung cancer. Cochrane Database Syst Rev. 2014. https://doi.org/10.1002/14651858.CD009519.pub2.
Vilmann P, Clementsen PF, Colella S, Siemsen M, De Leyn P, Dumonceau JM, Herth FJ, Larghi A, Vazquez-Sequeiros E, Hassan C, Crombag L, Korevaar DA, Konge L, Annema JT. Combined endobronchial and oesophageal endosonography for the diagnosis and staging of lung cancer. Eur Resp J. 2015;46(1):40–60. https://doi.org/10.1183/09031936.00064515.
De Leyn P, Dooms C, Kuzdzal J, Lardinois D, Passlick B, Rami-Porta R, Turna A, Van Schil P, Venuta F, Waller D, Weder W, Zielinski M. Revised ESTS guidelines for preoperative mediastinal lymph node staging for non-small-cell lung cancer. Eur J Cardio-Thoracic Surg. 2014;45(5):787–98.
Nestle U, De Ruysscher D, Ricardi U, Geets X, Belderbos J, Pottgen C, Dziadiuszko R, Peeters S, Lievens Y, Hurkmans C, Slotman B, Ramella S, Faivre-Finn C, McDonald F, Manapov F, Putora PM, LePechoux C, Van Houtte P. ESTRO ACROP guidelines for target volume definition in the treatment of locally advanced non-small cell lung cancer. Radiother Oncol. 2018;127(1):1–5.
Steinfort DP, Siva S, Leong TL, Rose M, Herath D, Antippa P, Ball DL, Irving LB. Systematic endobronchial ultrasound-guided mediastinal staging versus positron emission tomography for comprehensive mediastinal staging in NSCLC before radical radiotherapy of non-small cell lung cancer: a pilot study. Medicine (Baltimore). 2016;95(8): e2488.
Cole AJ, Hardcastle N, Turgeon GA, Thomas R, Irving LB, Jennings BR, Ball D, Kron T, Steinfort DP, Siva S. Systematic endobronchial ultrasound-guided transbronchial needle aspiration improves radiotherapy planning in non-small cell lung cancer. ERJ Open Res. 2019;5(3):00004–2019. https://doi.org/10.1183/23120541.00004-2019.
Rami-Porta R, Wittekind C, Goldstraw P. Complete resection in lung cancer surgery: proposed definition. Lung Cancer. 2005;49(1):25–33. https://doi.org/10.1016/j.lungcan.2005.01.001.
Choe G, Schipper P. Quality of lymph node assessment and survival among patients with non-small cell lung cancer. JAMA Oncol. 2018;4(1):1–2.
Gagliasso M, Migliaretti G, Ardissone F. Assessing the prognostic impact of the international association for the study of lung cancer proposed definitions of complete, uncertain, and incomplete resection in non-small cell lung cancer surgery. Lung Cancer. 2017;111:124–30.
Smeltzer MP, Faris NR, Ray MA, Osarogiagbon RU. Association of pathologic nodal staging quality with survival among patients with non-small cell lung cancer after resection with curative intent. JAMA Oncol. 2018;4(1):80–7.
Edwards JG, Chansky K, Van Schil P, Nicholson AG, Boubia S, Brambilla E, Donington J, Galateau-Sallé F, Hoffmann H, Infante M, Marino M. The IASLC lung cancer staging project: analysis of resection margin status and proposals for residual tumor descriptors for non–small cell lung cancer. J Thor Oncol. 2020;15(3):344–59.
Leong TL, Loveland PM, Gorelik A, Irving L, Steinfort DP. Preoperative staging by EBUS in cN0/N1 Lung cancer: systematic review and meta-analysis. J Bronchol Interv Pulmonol. 2019;26(3):155–65.
Yasufuku K, Nakajima T, Waddell T, Keshavjee S, Yoshino I. Endobronchial ultrasound-guided transbronchial needle aspiration for differentiating N0 versus N1 lung cancer. Ann Thorac Surg. 2013;96(5):1756–60.
Vial MR, O’Connell OJ, Grosu HB, Hernandez M, Noor L, Casal RF, Stewart J, Sarkiss M, Jimenez CA, Rice D, Mehran R, Ost DE, Eapen GA. Diagnostic performance of endobronchial ultrasound-guided mediastinal lymph node sampling in early stage non-small cell lung cancer: a prospective study. Respirology. 2018;23(1):76–81.
Serra P, Centeno C, Sanz-Santos J, Torky M, Baeza S, Mendiluce L, Martínez-Barenys C, de Castro PL, Abad J, Rosell A, Andreo F. Is it necessary to sample the contralateral nodal stations by EBUS-TBNA in patients with lung cancer and clinical N0/N1 on PET-CT? Lung Cancer. 2020;142:9–12.
Lehman M. Clinical_question:What_are_the_principles_of_radiation_therapy_in_the_definitive_management_of_stage_III_inoperable_NSCLC. Clinical practice guidelines for the treatment of lung cancer 2015 [cited 2015 Apr 16]; Available from: http://wiki.cancer.org.au/australia/Clinical_question:What_are_the_principles_of_radiation_therapy_in_the_definitive_management_of_stage_III_inoperable_NSCLC%3F.
Nestle U, Schimek-Jasch T, Kremp S, Schaefer-Schuler A, Mix M, Kusters A, Tosch M, Hehr T, Eschmann SM, Bultel YP, Hass P, Fleckenstein J, Thieme A, Stockinger M, Dieckmann K, Miederer M, Holl G, Rischke HC, Gkika E, Adebahr S, Konig J, Grosu AL. Imaging-based target volume reduction in chemoradiotherapy for locally advanced non-small-cell lung cancer (PET-Plan): a multicentre, open-label, randomised, controlled trial. Lancet Oncol. 2020;21(4):581–92.
Peeters ST, Dooms C, Van Baardwijk A, Dingemans AM, Martinussen H, Vansteenkiste J, Decaluwe H, De Leyn P, Yserbyt J, Nackaerts K, De Wever W, Deroose CM, De Ruysscher D. Selective mediastinal node irradiation in non-small cell lung cancer in the IMRT/VMAT era: how to use E(B)US-NA information in addition to PET-CT for delineation? Radiother Oncol. 2016;120(2):273–8.
Sanz-Santos J, Serra P, Torky M, Andreo F, Centeno C, Mendiluce L, Martínez-Barenys C, de Castro PL, Ruiz-Manzano J. Systematic Compared With Targeted Staging With Endobronchial Ultrasound in Patients With Lung Cancer. The Annals of Thoracic Surgery. 2018;106(2):398–403.
Leong P, Deshpande S, Irving LB, Bardin PG, Farmer MW, Jennings BR, Steinfort DP. Endoscopic ultrasound fine-needle aspiration by experienced pulmonologists: a cusum analysis. Eur Resp J. 2017;50(5):1701102. https://doi.org/10.1183/13993003.01102-2017.
Steinfort DP, Farmer MW, Irving LB, Jennings BR. Pulmonologist-performed per-esophageal needle aspiration of parenchymal lung lesions using an ebus bronchoscope: diagnostic utility and safety. J Bronchol Interv Pulmonol. 2017;24(2):117–24.
Korevaar DA, Crombag LM, Cohen JF, Spijker R, Bossuyt PM, Annema JT. Added value of combined endobronchial and oesophageal endosonography for mediastinal nodal staging in lung cancer: a systematic review and meta-analysis. Lancet Respir Med. 2016;4(12):960–8.
Casal RF, Sepesi B, Sagar AS, Tschirren J, Chen M, Li L, Sunny J, Williams J, Grosu HB, Eapen GA, Jimenez CA, Ost DE. Centrally located lung cancer and risk of occult nodal disease: an objective evaluation of multiple definitions of tumour centrality with dedicated imaging software. Eur Resp J. 2019;53(5):1802220. https://doi.org/10.1183/13993003.02220-2018.
Rami-Porta R, Eberhardt WEE. Clinical implications of the innovations in the primary tumour and metastasis of the 8(th) edition of the TNM classification for lung cancer. J Thorac Dis. 2018;10(Suppl 22):S2682–5.
Rami-Porta R, Asamura H, Travis WD, Rusch VW. Lung cancer major changes in the American joint committee on cancer eighth edition cancer staging manual. CA Cancer J Clin. 2017;67(2):138–55.
Sainz Zuniga PV, Martinez-Zayas G, Molina S, Grosu HB, Arain MH, Ost DE. Is biopsy of contralateral hilar N3 lymph nodes with negative PET-CT scan findings necessary when performing endobronchial ultrasound staging? Chest. 2021;159(4):1642–51.
Martinez-Zayas G, Almeida FA, Yarmus L, Steinfort D, Lazarus DR, Simoff MJ, Saettele T, Murgu S, Dammad T, Duong DK, Mudambi L, Filner JJ, Molina S, Aravena C, Thiboutot J, Bonney A, Rueda AM, Debiane LG, Hogarth DK, Bedi H, Deffebach M, Sagar AS, Cicenia J, Yu DH, Cohen A, Frye L, Grosu HB, Gildea T, Feller-Kopman D, Casal RF, Machuzak M, Arain MH, Sethi S, Eapen GA, Lam L, Jimenez CA, Ribeiro M, Noor LZ, Mehta A, Song J, Choi H, Ma J, Li L, Ost DE. predicting lymph node metastasis in non-small cell lung cancer: prospective external and temporal validation of the HAL AND HOMER models. Chest. 2021;160(3):1108–20.
Miller RJ, Mudambi L, Vial MR, Hernandez M, Eapen GA. Evaluation of appropriate mediastinal staging among endobronchial ultrasound bronchoscopists. Ann Am Thorac Soc. 2017;14(7):1162–8.
Guinde J, Roy P, Dutau H, Musani A, Quadrelli S, Stratakos G, Vergnon JM, Tremblay A, Fortin M. An international survey of mediastinal staging practices amongst interventional bronchoscopists. Resp Inter Rev Thor Dis. 2020;99(6):508–15.
Vial MR, Khan KA, O’Connell O, Peng SA, Gomez DR, Chang JY, Rice DC, Mehran R, Jimenez CJ, Grosu HB, Ost DE, Eapen GA. Endobronchial ultrasound-guided transbronchial needle aspiration in the nodal staging of stereotactic ablative body radiotherapy patients. Ann Thorac Surg. 2017;103(5):1600–5.
Guberina M, Darwiche K, Hautzel H, Ploenes T, Pottgen C, Guberina N, Herrmann K, Umutlu L, Wetter A, Theegarten D, Aigner C, Eberhardt WEE, Schuler M, Karpf-Wissel R, Stuschke M. Impact of EBUS-TBNA in addition to [(18)F]FDG-PET/CT imaging on target volume definition for radiochemotherapy in stage III NSCLC. Eur J Nucl Med Mol Imaging. 2021;48(9):2894–903.
Watanabe S, Asamura H, Suzuki K, Tsuchiya R. The new strategy of selective nodal dissection for lung cancer based on segment-specific patterns of nodal spread. Interact Cardiovasc Thorac Surg. 2005;4(2):106–9.
Garg S, Gielda BT, Kiel K, Turian JV, Fidler MJ, Batus M, Bonomi P, Sher DJ. Patterns of locoregional failure in stage III non-small cell lung cancer treated with definitive chemoradiation therapy. Pract Radiat Oncol. 2014;4(5):342–8.
Sullivan KA, Farrokhyar F, Leontiadis GI, Patel YS, Churchill IF, Hylton DA, Xie F, Seely AJE, Spicer J, Kidane B, Turner SR, Yasufuku K, Hanna WC. Routine systematic sampling versus targeted sampling during endobronchial ultrasound: A randomized feasibility trial. J Thor Cardiovasc Surg. 2022;164(1):254-261.e1. https://doi.org/10.1016/j.jtcvs.2021.11.062.
Gu P, Zhao YZ, Jiang LY, Zhang W, Xin Y, Han BH. Endobronchial ultrasound-guided transbronchial needle aspiration for staging of lung cancer: a systematic review and meta-analysis. Eur J Cancer. 2009;45(8):1389–96.
Acknowledgements
Not applicable.
Funding
DPS received a part-time research fellowship from National Health and Medical Council of Australia. The funding body has had no role in the design nor conduct of the study.
Author information
Authors and Affiliations
Contributions
Study conception and protocol development: DPS, SS, LBI, DEO, KY, GK, NH. Project administration: DPS, SS, LBI, DEO, KY, DF, PN, BJ. Investigation including performance of procedures, data collection/management: DPS, LBI, DEO, KR, JA, LC, KY, BJ, SY, PL, PN. Manuscript preparation: DPS, SS, GK, NH. Manuscript revision: All authors. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
SEISMIC was initially approved by the Melbourne Health Human Research Ethics Committee on 13th October 2016 (Ref: HREC/16/MH/227) and has received IRB approval at individual international centers. All participants will provide written informed consent.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Steinfort, D.P., Siva, S., Rangamuwa, K. et al. Systematic endoscopic staging of mediastinum to determine impact on radiotherapy for locally advanced lung cancer (SEISMIC): protocol for a prospective single arm multicentre interventional study. BMC Pulm Med 22, 364 (2022). https://doi.org/10.1186/s12890-022-02159-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12890-022-02159-9