Abstract
Background
Due to the low efficiency of a single clinical feature or laboratory variable in the diagnosis of tuberculous pleural effusion (TBPE), the diagnosis of TBPE is still challenging. This study aimed to build a scoring diagnostic model based on laboratory variables and clinical features to differentiate TBPE from non-tuberculous pleural effusion (non-TBPE).
Methods
A retrospective study of 125 patients (63 with TBPE; 62 with non-TBPE) was undertaken. Univariate analysis was used to select the laboratory and clinical variables relevant to the model composition. The statistically different variables were selected to undergo binary logistic regression. Variables B coefficients were used to define a numerical score to calculate a scoring model. A receiver operating characteristic (ROC) curve was used to calculate the best cut-off value and evaluate the performance of the model. Finally, we add a validation cohort to verify the model.
Results
Six variables were selected in the scoring model: Age ≤ 46 years old (4.96 points), Male (2.44 points), No cancer (3.19 points), Positive T-cell Spot (T-SPOT) results (4.69 points), Adenosine Deaminase (ADA) ≥ 24.5U/L (2.48 point), C-reactive Protein (CRP) ≥ 52.8 mg/L (1.84 points). With a cut-off value of a total score of 11.038 points, the scoring model’s sensitivity, specificity, and accuracy were 93.7%, 96.8%, and 99.2%, respectively. And the validation cohort confirms the model with the sensitivity, specificity, and accuracy of 92.9%, 93.3%, and 93.1%, respectively.
Conclusion
The scoring model can be used in differentiating TBPE from non-TBPE.
Similar content being viewed by others
Introduction
Tuberculosis (TB) affects about nine million people and causes 1.5 million deaths worldwide every year [1]. In 2016, tuberculosis claimed 1.3 million lives among HIV-negative people, exceeding the total number of deaths caused by HIV and becoming the first killer among infectious diseases [2]. On Sep 26, 2018, all UN Member States promised to end the global tuberculosis epidemic by 2030 in the UN General Assembly High Level meeting [3]. Currently, pleural tuberculosis (PT) is the most common type of extrapulmonary tuberculosis, and the frequency of all TB varies significantly in different countries. PT accounts for more than 20% of all TB cases in Africa [4, 5], 6.5 to 8.7% in China [6, 7], 8.7% in Brazil [8], and 3.7% in the United States [9]. PT appears to be the leading cause of pleural effusion (PE) in countries with a high prevalence of tuberculosis (e.g., in India) [10].
Tuberculous pleural effusion (TBPE) accounts for about 40% of PE cases in China [11, 12]. For patients with TBPE, untimely anti-tuberculosis treatment will affect its prognosis. The diagnostic criteria of TBPE is dependent on bacteria culture and histopathology, but the diagnostic sensitivity is limited and time-consuming. Thoracoscopic Pleural Biopsy is an effective method in diagnosing TBPE, but its application is limited because of its invasiveness, complexity, and technical difficulty [5, 13, 14]. Moreover, the diagnostic value of a single clinical biomarker of TBPE is limited, including erythrocyte sedimentation rate (ESR), blood T-cell spot (T-SPOT), adenosine deaminase (ADA), and lymphocyte ratio, so the diagnosis of TBPE is still challenging. Thus, our study aimed to build a scoring diagnostic model based on laboratory variables and clinical features to differentiate TBPE from non-TBPE.
Methods
Study subjects
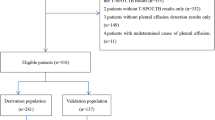
A retrospective study was conducted from 2016 to 2021 after approval by the Ethics Committee of Dongguan People's Hospital. All the enrolled patients met the indications of thoracic puncture or thoracoscopic pleural biopsy and signed the informed contents. The patients selected in the study should meet the following criteria: (1) Adult group; (2) Presence of PE on chest radiographs and ultrasonography; (3) Complete data in clinical were available for all patients. Finally, 125 PE patients were included in the retrospective study. In addition, we collected data from 29 new patients diagnosed with TBPE or non-TBPE to validate the diagnostic model. The data we collected mainly included six factors in the diagnostic model in calculating the model's score.
Diagnostic criteria
TBPE diagnosis was confirmed when PE met at least one of the following criteria: (1) pleural fluid/sputum/bronchial aspirate/bronchoscopic brushing specimen was positive for acid-fast bacilli or positive culture or positive polymerase chain reaction (PCR) for Mycobacterium tuberculosis. (2) Positive acid-fast staining or epithelioid caseous granuloma in pleural or lung tissue [15,16,17].
Malignant Pleural Effusion (MPE) was diagnosed when pleural histopathology demonstrated malignant lesions or when cytological examination of pleural effusion demonstrated malignant cells.
Empyema cases with negative M. tuberculosis culture were confirmed according to ATS guidelines [18]. Parapneumonic effusion (PPE) was caused by pneumonia which was approved based on the criteria of the American Thoracic Society (ATS) [19]. Thoracoscopic pleural biopsy was performed in patients with unknown etiology of PE. Except for the cases with TBPE, all the other cases were classified as a non-TBPE group.
All patients underwent a standard thoracocentesis procedure to collect pleural effusion samples, and blood was collected by venepuncture before intervention procedures. Record items include sex, age, clinical symptoms (cough, fever, chest pain, night sweats), T-SPOT, ESR, C-reactive protein (CRP), PE lymphocyte ratio, PE protein, PE lactate dehydrogenase (LDH), ADA, PE location, presence of cancer or not. Patients who had incomplete data were excluded from this study. The statistical analysis was performed using the first pleural fluid sample data before treatment. Hematological data were collected from the blood samples nearest to the first thoracentesis.
T-SPOT TB test was measured using Enzyme-Linked Immunospot (T-SPOT TB assay kit, Oxford Immunotec Co., Ltd., Abingdon, UK). Pleural effusion protein was measured using Colorimetric Determination (Dry tablets assay kit, Ortho-Clinical Diagnostics Co., Ltd., New York, USA). The activity of ADA was measured using a peroxidase assay (ADA assay kit, Beijing Leadman Biochemistry Co., Ltd., Beijing, China). LDH levels were measured using the lactic acid substrate method (LDH assay kit, Beckman Coulter Laboratory Systems Co., Ltd., Suzhou, China). CRP level was measured using the Quantitative Immunofluorescence method (Boditech Biotechnology Co., Ltd., Guangxi, China). ESR was measured using an Italian.
Microsed-System Automatic Blood Sedimentation Instrument. Differential cell counts in PE were counted manually.
Statistical analysis
The analyses were carried out using SPSS 22.0 software (SPSS Inc., Chicago, IL, USA). Continuous data are reported as median, with first and third quartiles. Mann–Whitney U test was used for comparisons between groups. The Chi-square test was used for the analysis of categorical variables. The results with p values less than 0.05 were considered statistical significance. Sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), accuracy, and the Youden index were calculated to estimate the diagnostic performance of the indicators. In addition, the receiver operating characteristic (ROC) curve was plotted to evaluate the diagnostic value of continuous data for TBPE. The continuous variable was converted into a binary variable according to the cut-off value corresponding to the maximum value of the Youden index. The factors with statistically significant differences between the two groups were selected in the binary logistic regression. A goodness-of-fit test of the binary logistic regression model was evaluated by Hosmer and Lemeshow test. Variables B coefficients were used to define a numerical score to calculate a scoring model. A receiver operating characteristic (ROC) curve was used to calculate the best cut-off value and evaluate the performance of the scoring model. Finally, a statistical evaluation of the diagnostic scoring model was performed in a validation cohort.
Results
Clinical and laboratory findings of the 125 patients with PE
In this study, 125 patients (63 with TBPE; 62 with non-TBPE) were enrolled according to the selection criteria. The demographic and clinical characteristics were collected from the patient's medical records and summarized in Table 1. There was no significant difference between the two groups in cough, chest pain, night sweats, pleural fluid protein, and LDH (p > 0.05). Still, there were significant differences among age, male, cancer diagnosis, pleural fluid location, fever, ESR, CRP, T-SPOT, pleural fluid lymphocyte ratio, and ADA (p < 0.05). The mean age of the TBPE group was lower than that of the non-TBPE group. TBPE predominated in men (46/63, 73.0%) who with unilateral pleural effusion (62/63, 98.4%). MPE accounts for most of the non-TBPE (43/62, 69.4%). The proportion of fever, positive T-SPOT, and the mean values of ESR, CRP, pleural fluid lymphocyte ratio, and ADA in patients with TBPE was higher than in patients with non-TBPE.
The diagnostic performance of a single indicator for TBPE
ROC curve was used to evaluate the diagnostic value of continuous data (Fig. 1A, B). The continuous variable was converted into a binary variable according to the cut-off value corresponding to the maximum value of the Youden Index (Table 2). The diagnostic performance, including Sensitivity, Specificity, Positive predictive value (PPV), Negative predictive value (NPV), and Accuracy, were calculated and summarized in Table 3.
The multivariate logistic regression of binary variables and the establishment of the scoring model
The factors with statistically significant differences between the two groups were selected in the logistic regression, using the forward selection method to select indicators to enter the final model. At last, ESR ≥ 27.5 mm/h, fever, lymphocyte ratio ≥ 91.5%, unilateral pleural effusion was eliminated by the model, And age ≤ 46.5 years, male, no cancer, positive T-SPOT, CRP ≥ 52.8 mg/L, ADA ≥ 24.5 U/L were accepted into the final binary logistic regression model. Hosmer and Lemeshow test Confirmed a good goodness-of-fit test of the binary logistic regression model (p = 0.499). Variables B coefficients were used to define a numerical score to calculate a scoring model (Table 4).
The diagnostic performance of the scoring model
The total score is equal to the sum Variables score in Table 4 when matching the scoring criteria. The calculation equation as follow: Y (total score) = Age score + Gender score + Cancer score + T-SPOT score + CRP score + ADA score. ROC curve was used to calculate the best cut-off value. When the total score was ≥ 11.038, The area under the curve (AUC) value was 0.992 (95%CI 0.982–1.000) (Fig. 2). The scoring model's performance for diagnosing TBPE was summarized in Table 5.
Validation of the diagnostic scoring model
Twenty-nine new patients with PE in the validation cohort were from the same retrospective database from 2016–2021 and approved by the Ethics Committee of Dongguan People’s Hospital, of which 14 were diagnosed with TBPE; among the remaining 15 patients were non-TBPE; 13 of 14 patients with TBPE had a score of ≥ 11.038; 1 of 15 patients with non-TBPE had a score of ≥ 11.038. The baseline characteristics with six indicators included in the model in the validation cohort were summarized in Table 6. And the validation results of the scoring model are shown in Table 7.
Discussion
There are many causes of pleural effusion. Since the high prevalence of TB occurred in China, TBPE accounts for about 40% of pleural effusion [11, 12]. Currently, the diagnostic performance of clinical features or laboratory variables in diagnosing TBPE is poor; therefore, it’s an urgent need to find a new method to diagnose TBPE. Our study built a scoring diagnostic model by using logistic regression based on laboratory variables and clinical features to differentiate TBPE from non-TBPE.
A total of 15 indicators were included in this study. No symptoms were introduced into the final binary logistic regression model, which shows that clinical symptoms had no significant diagnosis value to the TBPE. And the results were similar to the previously reported data [20]. In terms of pleural effusion tests, unilateral pleural effusion, pleural effusion protein, LDH, and pleural effusion lymphocytes ratio ultimately failed to enter the final model, which may be due to TBPE and MPE being dominated by unilateral lymphocytic exudates [21]. Most of the non-TBPE in this study were MPE. As expected, ESR, a non-specific indicator for the diagnosis of TBPE, was eventually eliminated by the regression model, even though there was a difference in univariate analysis between the two groups.
Finally, six indicators of age, sex, cancer, CRP, T-SPOT, and ADA were included in the diagnostic scoring model by multivariate binary logistics regression, which showed that those six indicators significantly contributed significantly to the diagnosis. In terms of age, the non-TBPE group was significantly older than the TBPE group, which may attribute to the elderly-onset of cancer that was the leading cause of non-TBPE. Our result showed that TBPE predominated in men (46/63; 73.0%), and it was similar to the previously reported data of Roberta et al. [21]. Consistent with the previous studies [22], we also demonstrated the potential diagnostic value of CRP for TBPE. The sensitivity and specificity of ADA, which had an excellent diagnostic performance in diagnosing TBPE, were above 90% in diagnosing TBPE. T-SPOT also showed an excellent diagnostic value for the diagnosis of TBPE that the sensitivity and specificity were 93.7% and 77.4%, respectively. The above results were similar to the previous studies [20, 23, 24].
Hosmer and Lemeshow test confirmed a good goodness-of-fit test of the binary logistic regression model (p = 0.499). The performance of the scoring model for diagnosis of TBPE was evaluated by the ROC curve; when the total score was ≥ 11.038, the AUC value was 0.992 (95%CI 0.982–1.000), the sensitivity, specificity, PPV, NPV were 93.7%, 96.8%, 100%, and 93.9% respectively, the diagnostic performance of the scoring model was better than the reported data of Petborom et al. [20, 25]. Our scoring model can aidin diagnosing TBPE and provide more evidence for anti-tuberculosis treatment. Still, we need to track the effectiveness of treatment to verify the accuracy of the diagnostic model follow-up. When anti-tuberculosis treatment is ineffective, a thoracoscopic pleural biopsy should be used to further confirm the diagnosis. For medical units with limited sanitary conditions, the diagnostic model can help to find TBPE, so that patients can be referred to specialist hospitals for earlier treatment.
The retrospective study has a selection bias; for example, patients with incomplete data were excluded from the study, leading to a reduction in the number of cases. Some test indicators, such as cytokines, CD4, and CD8, were not included in our study because of the small number of people tested. Therefore, establishing a better TBPE diagnostic scoring model requires a prospective, multi-center study to achieve.
Conclusions
The diagnostic score model established by logistic regression combined with multiple indicators improves diagnostic performance. It is better than a single index of laboratory variables or clinical features in diagnosing TBPE. In brief, establishing a scoring model for diagnosing TBPE provides a good diagnostic method based on routine clinical data to assist clinicians in making better clinical decisions.
Availability of data and materials
Most of the data were included in the submission. More data details were available from the corresponding authors on reasonable request.
Abbreviations
- TB:
-
Tuberculosis
- TBPE:
-
Tuberculous pleural effusion
- ROC:
-
Receiver operating characteristic
- T-SPOT:
-
T-cell spot
- ADA:
-
Adenosine deaminase
- CRP:
-
C-reactive protein
- PT:
-
Pleural tuberculosis
- PE:
-
Pleural effusion
- ESR:
-
Erythrocyte sedimentation rate
- LDH:
-
Lactate dehydrogenase
- PPV:
-
Positive predictive value
- NPV:
-
Negative predictive value
References
Macias A, Sanchez-Montalva A, Salvador F, et al. Epidemiology and diagnosis of pleural tuberculosis in a low incidence country with high rate of immigrant population: a retrospective study. Int J Infect Dis. 2019;78:34–8.
Glaziou P, Floyd K, Raviglione MC. Global epidemiology of tuberculosis. Semin Respir Crit Care Med. 2018;39(3):271–85.
Harding E. WHO global progress report on tuberculosis elimination. Lancet Respir Med. 2020;8(1):19.
Saks AM, Posner R. Tuberculosis in HIV positive patients in South Africa: a comparative radiological study with HIV negative patients. Clin Radiol. 1992;46(6):387–90.
Zhai K, Lu Y, Shi HZ. Tuberculous pleural effusion. J Thorac Dis. 2016;8(7):E486-494.
Wang X, Yang Z, Fu Y, et al. Insight to the epidemiology and risk factors of extrapulmonary tuberculosis in Tianjin, China during 2006–2011. PLoS ONE. 2014;9(12): e112213.
Pang Y, An J, Shu W, et al. Epidemiology of extrapulmonary tuberculosis among inpatients, China, 2008–2017. Emerg Infect Dis. 2019;25(3):457–64.
Seiscento M, Vargas FS, Rujula MJ, et al. Epidemiological aspects of pleural tuberculosis in the state of Sao Paulo, Brazil (1998–2005). J Bras Pneumol. 2009;35(6):548–54.
Peto HM, Pratt RH, Harrington TA, LoBue PA, Armstrong LR. Epidemiology of extrapulmonary tuberculosis in the United States, 1993–2006. Clin Infect Dis. 2009;49(9):1350–7.
Udwadia ZF, Sen T. Pleural tuberculosis: an update. Curr Opin Pulm Med. 2010;16(4):399–406.
Villena Garrido V, Cases Viedma E, Fernandez Villar A, et al. Recommendations of diagnosis and treatment of pleural effusion. Update. Arch Bronconeumol. 2014;50(6):235–49.
Wang XJ, Yang Y, Wang Z, et al. Efficacy and safety of diagnostic thoracoscopy in undiagnosed pleural effusions. Respiration. 2015;90(3):251–5.
Steingart KR, Flores LL, Dendukuri N, et al. Commercial serological tests for the diagnosis of active pulmonary and extrapulmonary tuberculosis: an updated systematic review and meta-analysis. PLoS Med. 2011;8(8): e1001062.
Wong CF. Early diagnosis of tuberculous pleural effusion: apart from pleural fluid adenosine deaminase, pleural biopsy still has a role. Hong Kong Med J. 2018;24(3):316–7.
Flores-Ibarra AA, Ochoa-Vazquez MD, Sanchez-Tec GA. Diagnostic strategies in the tuberculosis clinic of the hospital general La Raza National Medical Center. Rev Med Inst Mex Seguro Soc. 2016;54(1):122–7.
Klimiuk J, Krenke R, Safianowska A, Korczynski P, Chazan R. Diagnostic performance of different pleural fluid biomarkers in tuberculous pleurisy. Adv Exp Med Biol. 2015;852:21–30.
Abrao FC, de Abreu IR, Miyake DH, Busico MA, Younes RN. Role of adenosine deaminase and the influence of age on the diagnosis of pleural tuberculosis. Int J Tuberc Lung Dis. 2014;18(11):1363–9.
American Thoracic S. Infectious Diseases Society of A: guidelines for the management of adults with hospital-acquired, ventilator-associated, and healthcare-associated pneumonia. Am J Respir Crit Care Med. 2005;171(4):388–416.
Niederman MS, Mandell LA, Anzueto A, et al. Guidelines for the management of adults with community-acquired pneumonia. Diagnosis, assessment of severity, antimicrobial therapy, and prevention. Am J Respir Crit Care Med. 2001;163(7):1730–54.
Petborom P, Dechates B, Muangnoi P. Differentiating tuberculous pleuritis from other exudative lymphocytic pleural effusions. Ann Palliat Med. 2020;9(5):2508–15.
Sales RK, Vargas FS, Capelozzi VL, et al. Predictive models for diagnosis of pleural effusions secondary to tuberculosis or cancer. Respirology. 2009;14(8):1128–33.
Porcel JM, Vives M, Cao G, et al. Biomarkers of infection for the differential diagnosis of pleural effusions. Eur Respir J. 2009;34(6):1383–9.
Zhang M, Li D, Hu ZD, Huang YL. The diagnostic utility of pleural markers for tuberculosis pleural effusion. Ann Transl Med. 2020;8(9):607.
Luo Y, Yan F, Xue Y, et al. Diagnostic utility of pleural fluid T-SPOT and interferon-gamma for tuberculous pleurisy: a two-center prospective cohort study in China. Int J Infect Dis. 2020;99:515–21.
Ren Z, Hu Y, Xu L. Identifying tuberculous pleural effusion using artificial intelligence machine learning algorithms. Respir Res. 2019;20(1):220.
Acknowledgements
Not applicable.
Funding
This work was supported by Grants from the Dongguan Social Science and Technology Development Project [No. 202050715001774].
Author information
Authors and Affiliations
Contributions
All listed authors meet the requirements for authorship. SW, HW, and PZ conceived and designed the study. SL, NF, and WM collected clinical information. SW, SL, and NF analyzed the data. SW wrote the manuscript. All authors critically reviewed and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
This study was conducted in accordance with the amended Declaration of Helsinki. The study was approved by the Ethics Committee of Dongguan People's Hospital, and the Institutional Review Board of Dongguan People’s Hospital approved a waiver of patient informed consent due to its retrospective design posing minimal risk to the study participants (IRB NO.KYKT2020-35).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Wu, S., Li, S., Fang, N. et al. A scoring model for diagnosis of tuberculous pleural effusion. BMC Pulm Med 22, 332 (2022). https://doi.org/10.1186/s12890-022-02131-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12890-022-02131-7