Abstract
Introduction
The maximum gain in quality of life after lung transplantation (LT) is expected between six months and one year after LT, as the occurrence of chronic lung allograft dysfunction may mask the beneficial effects beyond one year. Thus, the postoperative period could be the cornerstone of graft success. We sought to describe the factors present before postoperative admission to the ICU and associated with favorable, arduous or fatal pathway within 90 days of LT.
Materials and methods
We conducted a retrospective single-center study between January 2015 and December 2020. Using multinomial regression, we assessed the demographic, preoperative and intraoperative characteristics of patients associated with favorable (duration of postoperative mechanical ventilation < 3 days and alive at Day 90), arduous (duration of postoperative mechanical ventilation ≥ 3 days and alive at Day 90) or fatal (dead at Day 90) pathway within 90 days of LT.
Results
A total of 269 lung transplant patients were analyzed. Maximum graft cold ischemic time ≥ 6 h and intraoperative blood transfusion ≥ 3 packed red blood cells were associated with arduous and fatal pathway at Day 90, whereas intraoperative ECMO was strongly associated with fatal pathway.
Conclusion
No patient demographics influenced the postoperative pathway at Day 90. Only extrinsic factors involving graft ischemia time, intraoperative transfusion, and intraoperative ECMO determined early postoperative pathway.
Similar content being viewed by others
Introduction
Lung transplantation (LT) is currently recognized as a life-saving therapy for patients with end-stage lung disease. LT represents more than 4000 procedures per year in selected patients for whom optimal medical treatment is no longer sufficient to maintain quality of life or short-term survival [1, 2]. However, the overall median survival of 6.7 years is still lower than that of any of the other solid organ transplants and is impacted by early deaths mainly related to graft dysfunction, infections and cardiovascular complications [3].
The maximum gain in quality of life is expected between six months and one year after LT, as the occurrence of chronic lung allograft dysfunction may mask the beneficial effects beyond one year [4]. Thus, the postoperative period could be the cornerstone influencing patient outcome and transplant success. Unfortunately, it remains very difficult to predict the patient's early postoperative prognosis. Basically and arbitrarily, the early pathway of patients after LT could follow three trajectories: favorable, arduous and fatal.
Our study sought to identify factors present before postoperative admission to the intensive care unit (ICU) and associated with favorable, arduous and fatal pathway within 90 days of LT.
Materials and methods
Study design
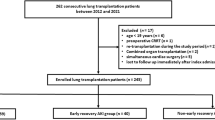
We conducted a retrospective single-center study that included all patients who underwent LT at our institution between January 2015 and December 2020.
Definition of favorable, arduous and fatal pathway within 90 days of LT
No definition exists to define favorable and arduous pathway within 90 days of LT. Therefore, we arbitrarily define favorable pathway as lung transplant patients with a duration of postoperative mechanical ventilation < 3 days and alive at Day 90, and arduous pathway as a duration of postoperative mechanical ventilation ≥ 3 days and alive at Day 90. Fatal pathway was defined as death within 90 days of LT. Three days of mechanical ventilation is the median duration in our cohort.
Assessment of factors present before postoperative admission to the ICU and associated with favorable, arduous or fatal pathway within 90 days of LT
Demographic, preoperative and intraoperative characteristics of patients were compared between favorable arduous and fatal pathway within 90 days of LT.
Ethics
All the experiment has been performed in accordance with the Declaration of Helsinki. The study was approved by the ethics committee CEERB Paris Nord, which waived the need for signed informed consent (Institutional Review Board -IRB 00,006,477- University of Paris, AP-HP.Nord. No organs/tissues were procured from prisoners and organs/tissues were procured exclusively from French hospitals.
Data collection
We recorded the following data: (1) Demographic and preoperative characteristics of the patients (age, sex, body mass index (BMI), primary diagnosis of chronic pulmonary disease, Cytomegalovirus mismatch (Donor + /Recipient-), past medical history of diabetes and ischemic heart disease with angioplasty and/or coronary stent, high-emergency LT, extracorporeal membrane oxygenation (ECMO) as a bridge to transplant, mean pulmonary arterial pressure (mPAP) measured by a right heart catheterization at listing; (2) Intraoperative characteristics (type of LT, i.e., single or bilateral, maximum graft cold ischemia time, intraoperative blood transfusion, intraoperative ECMO and thoracic epidural analgesia); (3) specific lung transplant complications (grade 3 primary graft dysfunction (PGD) as defined by the ISHLT consensus [5], bronchial anastomosis dehiscence, acute cellular rejection confirmed by histopathological evidence after transbronchial lung biopsies performed only in cases of suspicion and not systematically [6], and definite, probable or possible antibody-mediated rejection, according to Levine et al. [7], with the need for plasmapheresis, (4) ICU stay characteristics (simplified acute physiology score II (SAPS II) and sequential organ failure assessment (SOFA) score at admission, acute kidney injury stage 3 of KDIGO, renal replacement therapy, duration of mechanical ventilation, duration of norepinephrine support, ECMO in ICU, tracheostomy, ICU length of stay); and (6) mortality rates during the postoperative ICU stay and at Day 90.
Perioperative management
Surgical transplantation procedures and perioperative care, including postoperative management, were standardized for all patients according to our local protocol that was published elsewhere [8, 8]. The immunosuppressive regimen included mycophenolate mofetil, corticosteroids and tacrolimus. Perioperative antibiotics were routinely administered for 48 h after LT. Cefazolin (or the antibiotic that was administered to the donor before LT) was the standard antibiotic prophylaxis. In the postoperative period, antibiotic therapy was adapted to the microbiological cultures obtained from the bronchoalveolar lavage performed systematically in postoperative admission to the ICU, and then performed according to clinical suspicion of pneumonia [10].
Statistical analysis
Baseline characteristics within each group were described with numbers and percentages for categorical variables and medians and interquartile ranges (IQR) for quantitative variables. We compared the characteristics of the three different pathways (favorable, arduous or fatal) using the Kruskal–Wallis test for quantitative variables and the χ2 or Fisher test for the categorical variables. We used multinomial regression as a multivariable analysis to assess the factors associated with those pathways (Results are represented in relative risks with their 95% confidence intervals (CI) [11]. Kaplan Meier survival curves were constructed for the 365-day period after lung the transplantation. Missing data were not replaced in the final dataset. The P values corresponded to the Wald statistic, and a threshold of 0.05 was used for statistical significance. Statistical analysis and data management were performed using Stata/IC®15.
Results
During the study period, 269 patients underwent LT. Most of the patients were male (64.3%), with a median (IQR) age of 57 (51–62) years. The main etiologies for LT were interstitial lung diseases (ILD) (48.7%) and chronic obstructive disease pulmonary disease (COPD) (36.4%). Patients had favorable (n = 109, 40.5%), arduous (n = 120, 44.6%) or fatal (n = 40, 14.9%) pathway within 90 days of LT (Table 1), with a median (IQR) hospital length of stay of 37 [31–50] days, 71 [49–112] days and 23 [6–55] days, respectively. The overall survival of the cohort was 91.8% at 30 days, 85.1% at 90 days and 76.2% at one year. Kaplan–Meier curve for survival at 365 days for patients with favorable, arduous or fatal pathway is displayed in the Fig. 1.
Patient characteristics before admission to the postoperative intensive care unit (ICU) and postoperative outcomes after LT are presented in Tables 1 and 2, respectively.
Factors present before postoperative admission to the ICU and associated with favorable, arduous or fatal pathway within 90 days of LT
In univariate analysis, age ≥ 60 years, high emergency LT, ECMO as a bridge to transplant, maximum graft cold ischemic time ≥ 6 h, intraoperative blood transfusion ≥ 3 PRBCs and intraoperative ECMO were factors associated with a non-favorable pathway within 90 days (Table 1).
After multinomial regression, a maximum graft cold ischemic time ≥ 6 h and intraoperative blood transfusion ≥ 3 PRBCs were independently associated with arduous or fatal pathway within 90 days, whereas intraoperative ECMO was strongly and independently associated with fatal pathway only (Table 3).
Factors associated with intraoperative ECMO, graft cold ischemia time ≥ 6 h and intraoperative blood transfusion ≥ 3 PRBCs
Intraoperative ECMO was associated with ILD, high emergency LT, ECMO as a bridge to transplant, pretransplant mPAP ≥ 25 mmHg and intraoperative blood transfusion ≥ 3 PRBCs. Interstitial lung disease and pretransplant mPAP ≥ 25 mmHg remained independently associated with intraoperative ECMO after multivariate analysis (Additional file 1: Table S1).
Graft cold ischemia time ≥ 6 h was associated with age ≥ 60 years (protective factor), other primary diagnoses than COPD or ILD, single LT (protective factor, as expected) and intraoperative blood transfusion ≥ 3 PRBCs. After multivariate analysis, age ≥ 60 years was as an independent protective factor for graft cold ischemia time ≥ 6 h (Additional file 1: Table S2).
Intraoperative blood transfusion ≥ 3 PRBCs was associated with age ≥ 60 years (protective factor), other primary diagnoses than COPD or ILD, cytomegalovirus mismatch (protective factor), high emergency LT, ECMO as a bridge to transplant, double LT, graft cold ischemia time ≥ 6 h and thoracic epidural analgesia (protective factor). After multivariate analysis, cytomegalovirus mismatch and thoracic epidural analgesia were independent protective factors for intraoperative blood transfusion ≥ 3 PRBCs, whereas high emergency LT and bilateral LT were independent risk factors (Additional file 1: Table S3: Factors associated with intraoperativr ECMO, graft cold ischemia time ≥ 6 hours and intraoperative blood transfusion ≥ 3 PRBCs ).
Discussion
We explored for the first time, to our knowledge, the risk factors present before postoperative ICU admission and associated with three trajectories of pathway, favorable, arduous or fatal, within 90 days after LT. Intraoperative ECMO, graft cold ischemic time ≥ 6 h and intraoperative blood transfusion ≥ 3 PRBCs were independent risk factors for arduous or fatal pathway at Day 90.
Intraoperative ECMO was strongly associated with a comorbid postoperative stay in the ICU, grade 3 PGD and 90-day mortality. The impact of intraoperative ECMO on 90-day survival has been poorly studied. Zhang et al. recently showed that patients with intraoperative ECMO were more likely to have an increased 3-month mortality rate compared with those without ECMO, although it did not reach statistical significance (27.3 vs. 17.2%, p = 0.25) [12]. The largest studies of perioperative ECMO use have primarily evaluated patient survival at one year and beyond, with contradictory results. Ius et al. showed higher in-hospital mortality rates [13] and higher 3-, 5- and 8-year mortality rates for patients requiring intraoperative ECMO [14], although ECMO was not selected as an independent risk factor. In contrast, Hoetzenecker et al. observed improved 1-, 3-, and 5-year survival rates compared with non-ECMO patients [15]. It should be noted that central cannulation was predominant, except for patients using peripheral ECMO as a bridge to transplant.
The high rate of intraoperative ECMO (70%) in our cohort and the poor prognosis of patients under ECMO could be explained by their significant comorbidities. Forty-nine patients required preoperative ECMO and all were transplanted with intraoperative ECMO using the high-emergency procedure. Therefore, candidates for high-emergency transplantation requiring preoperative ECMO should be carefully selected. The median (IQR) and mean ± SD age were 56 (50–62) years and 54 ± 11 years, respectively, compared to 48 (31–55) years in the study of Ius et al. [13] and 45.2 ± 16.2 years in the study of Hoetzenecker et al. [15]. In a retrospective analysis of 8363 patients from the UNOS database, Weiss et al. showed that older patients had an increased risk of death after LT [16]. Pulmonary fibrosis, a well-known risk factor for intraoperative ECMO [12, 12] and for higher posttransplant mortality [3], represented 58% of our transplant recipients, compared to 30% in the two studies mentioned above [13, 13]. Single LT accounted for 31.5% of the procedures, whereas it was only 3% for the study of Ius et al. [13] and the study of Hoetzenecker et al. exclusively focused on bilateral LT [15]. Single LT has previously been identified as a risk factor for intraoperative ECMO [13] and overall poorer survival rates compared with double LT [17]. In addition, 66.8% of patients with intraoperative ECMO were male, compared to 54% [13] and 48% [15] in the other studies. Male sex was consistently associated with a higher mortality rate after LT [18,19,20,21].
In our cohort, the vast majority of intraoperative ECMO was unplanned, except for patients on ECMO bridging before LT. Our practice is to implant ECMO "on demand” when irreversible hemodynamic and/or respiratory instability is deemed irreversible by the practitioners in charge of the patient occurs during surgery, especially when clamping the pulmonary artery branch. However, the impact of intraoperative ECMO on survival does not appear to be related to implantation strategies, as Ius et al. observed no difference in survival between a priori and on-demand strategies [13]. The rate of prolonged ECMO during the postoperative course in the ICU was 40% and was similar to their rates of 41% [13] and 36% [15].
We identified a primary diagnosis of ILD and pretransplant mPAP ≥ 25 mmHg as risk factors for intraoperative ECMO, as previously reported [12, 12]. The association of intraoperative ECMO with a greater risk of grade 3 PGD confirmed what had previously been observed [14].
The impact of graft cold ischemic time on graft failure and survival remains under debate. Prolonged ischemia ≥ 6 h was an independent risk factor for arduous and fatal pathway within 90 days and was likely to be associated with more reports of grade 3 PGD, although the difference did not reach statistical significance. Some previous studies have reported similar results [22, 22], while others have found no association [24,25,26]. However, it was suggested that the cold ischemia time could have a greater impact for the most fragile patients [26]. We identified recipient age ≥ 60 years, a marker of frailty, as an independent factor associated with prolonged ischemia ≥ 6 h.
Intraoperative blood transfusion ≥ 3 PRBCs was independently associated with arduous and fatal pathway, with more reports of comorbidities during postoperative ICU stay and more reports of grade 3 PGD. Blood loss during surgery has been consistently associated with more postoperative complications, but the impact on mortality remains unclear [27, 27]. Factors associated with high PRBC transfusion during surgery were high-emergency LT and double LT. High-emergency LT is a national prioritization system for the most severe patients with fibrosis, cystic fibrosis or pulmonary hypertension that was introduced in France in 2007. As a different method than the Lung Allocation Score [29], the allocation rules in France are developed by the Agence de la Biomédecine in collaboration with the transplant community, and this is the responsibility of a lung transplant team that selects the recipient it believes will benefit most from the allograft [30]. The Agence de la Biomédecine develops the allocation rules in France in collaboration with the transplant community. Before September 2020, the lung transplant allocation system was based on national allocation for patients with high-emergency status and local, regional, and then national allocation for elective patients. However, this transplant allocation model has generated significant geographic disparities across transplant centers, with an average of grafts offered per candidate ranging from 1.4 to 5.2. Thus, as of 8 September 2020, a new system was implemented that restricted the local allocation according to the supply/demand ratio, eliminating regional sharing and increasing national sharing. The supply/demand ratio was defined as the ratio of the number of lungs recovered in the local allocation unit to transplants performed in the center [31]. Patients can be candidates for the high-emergency waiting list if they need mechanical ventilation or if they are at risk of undergoing mechanical ventilation with an oxygen dependency greater than 12 L/min associated with an SpO2 < 90% despite maximal treatment and in the absence of a reversible cause. The presence of severe organ failure or uncontrolled sepsis contraindicates access to this emergency procedure [32]. Overall, this indication is assessed by independent and anonymous experts designed by the French Biomedicine agency. Patients on a high emergency waiting list often require ECMO as a bridge to transplant, a condition that has been associated with blood loss during LT [28]. In contrast, patients who received thoracic epidural analgesia were less likely to receive a massive transfusion. We hypothesized that epidural anesthesia, by providing sympathetic blockade, may reduce peripheral venous bleeding mediated by venous hypotension resulting from decreased peripheral resistance, while vital organ perfusion is maintained by sustaining cardiac output through norepinephrine and fluid management. Hypotensive epidural anesthesia has been shown to reduce blood loss in urological surgery [33].
This study has several major limitations, which are its single-center and retrospective nature. The external validity of our results must be interpreted with caution, as these observations depend on the etiologies of LT, the selection of candidate patients, and their management, all of which may vary from one center to another.
Conclusion
We identified and deciphered three independent risk factors present prior to ICU admission after LT and associated with arduous or fatal pathway within 90 days. Patient candidates aged ≥ 60 years with a primary diagnosis of ILD, preoperative pulmonary hypertension, undergoing a high emergency bilateral LT or LT, should be warned of an expected hemorrhagic surgery requiring ECMO, with an increased risk of unfavorable pathway.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- BMI:
-
Body mass index
- COPD:
-
Chronic obstructive pulmonary disease
- ECMO:
-
Extracorporeal membrane oxygenation
- ICU:
-
Intensive care unit
- ILD:
-
Interstitial lung diseases
- LT:
-
Lung transplantation
- SAPS II:
-
Simplified acute physiology score II
- SOFA:
-
Sequential organ failure assessment
References
Rodrigue JR, Baz MA, Kanasky WF, MacNaughton KL. Does lung transplantation improve health-related quality of life? The university of Florida experience. J Heart Lung Transp Off Publ Int Soc Heart Transp. 2005;24:755–63.
Vasiliadis H-M, Collet J-P, Poirier C. Health-related quality-of-life determinants in lung transplantation. J Heart Lung Transpl. 2006;25:226–33.
Khush KK, Cherikh WS, Chambers DC, Harhay MO, Hayes D, Hsich E, et al. The international thoracic organ transplant registry of the international society for heart and lung transplantation: thirty-sixth adult heart transplantation report - 2019; focus theme: donor and recipient size match. J Heart Lung Transp Off Publ Int Soc Heart Transp. 2019;38:1056–66.
Singer JP, Singer LG. Quality of life in lung transplantation. Semin Respir Crit Care Med. 2013;34:421–30.
Snell GI, Yusen RD, Weill D, Strueber M, Garrity E, Reed A, et al. Report of the ISHLT working group on primary lung graft dysfunction, part I: definition and grading—a 2016 consensus group statement of the international society for heart and lung transplantation. J Heart Lung Transp. 2017;36:1097–103.
Stewart S, Fishbein MC, Snell GI, Berry GJ, Boehler A, Burke MM, et al. Revision of the 1996 working formulation for the standardization of nomenclature in the diagnosis of lung rejection. J Heart Lung Transp. 2007;26:1229–42.
Levine DJ, Glanville AR, Aboyoun C, Belperio J, Benden C, Berry GJ, et al. Antibody-mediated rejection of the lung: a consensus report of the international society for heart and lung transplantation. J Heart Lung Transp. 2016;35:397–406.
Atchade E, Younsi M, Elmaleh Y, Tran-Dinh A, Jean-Baptiste S, Tanaka S, et al. Intensive care readmissions in the first year after lung transplantation: Incidence, early risk factors and outcome. Anaesth Crit Care Pain Med. 2021;40(6):100948.
Tanaka S, Geneve C, Tebano G, Grall N, Piednoir P, Bronchard R, et al. Morbidity and mortality related to pneumonia and tracheobronchitis in ICU after lung transplantation. BMC Pulm Med. 2018;18:43.
Husain S, Mooney ML, Danziger-Isakov L, Mattner F, Singh N, Avery R, et al. A 2010 working formulation for the standardization of definitions of infections in cardiothoracic transplant recipients. J Heart Lung Transp Off Publ Int Soc Heart Transp. 2011;30:361–74.
Hosmer D, Lemeshow S. Applied Logistic Regression. Hoboke: Wiley; 2000.
Zhang R, Xu Y, Sang L, Chen S, Huang Y, Nong L, et al. Factors associated with intraoperative extracorporeal membrane oxygenation support during lung transplantation. Respir Res. 2020;21:85.
Ius F, Sommer W, Tudorache I, Avsar M, Siemeni T, Salman J, et al. Five-year experience with intraoperative extracorporeal membrane oxygenation in lung transplantation: indications and midterm results. J Heart Lung Transp Off Publ Int Soc Heart Transp. 2016;35:49–58.
Ius F, Aburahma K, Boethig D, Salman J, Sommer W, Draeger H, et al. Long-term outcomes after intraoperative extracorporeal membrane oxygenation during lung transplantation. J Heart Lung Transp Off Publ Int Soc Heart Transp. 2020;39:915–25.
Hoetzenecker K, Schwarz S, Muckenhuber M, Benazzo A, Frommlet F, Schweiger T, et al. Intraoperative extracorporeal membrane oxygenation and the possibility of postoperative prolongation improve survival in bilateral lung transplantation. J Thorac Cardiovasc Surg. 2018;155:2193-2206.e3.
Weiss ES, Merlo CA, Shah AS. Impact of advanced age in lung transplantation: an analysis of united network for organ sharing data. J Am Coll Surg. 2009;208:400–9.
Schaffer JM, Singh SK, Reitz BA, Zamanian RT, Mallidi HR. Single- vs double-lung transplantation in patients with chronic obstructive pulmonary disease and idiopathic pulmonary fibrosis since the implementation of lung allocation based on medical need. JAMA. 2015;313:936.
Loor G, Brown R, Kelly RF, Rudser KD, Shumway SJ, Cich I, et al. Gender differences in long-term survival post-transplant: A single-institution analysis in the lung allocation score era. Clin Transpl. 2017;31(3):e12889. https://doi.org/10.1111/ctr.12889.
Roberts DH, Wain JC, Chang Y, Ginns LC. Donor-recipient gender mismatch in lung transplantation: impact on obliterative bronchiolitis and survival. J Heart Lung Transp Off Publ Int Soc Heart Transp. 2004;23:1252–9.
Sato M, Gutierrez C, Waddell TK, Liu M, Keshavjee S. Donor-recipient gender mismatch in lung transplantation: impact on obliterative bronchiolitis and survival. J Heart Lung Transp Off Publ Int Soc Heart Transp. 2005;24:2000–1.
Alvarez A, Moreno P, Illana J, Espinosa D, Baamonde C, Arango E, et al. Influence of donor-recipient gender mismatch on graft function and survival following lung transplantation. Interact Cardiovasc Thorac Surg. 2013;16:426–35.
Thabut G, Mal H, Cerrina J, Dartevelle P, Dromer C, Velly J-F, et al. Graft ischemic time and outcome of lung transplantation: a multicenter analysis. Am J Respir Crit Care Med. 2005;171:786–91.
Ghaidan H, Fakhro M, Lindstedt S. Impact of allograft ischemic time on long-term survival in lung transplantation: a Swedish monocentric study. Scand Cardiovasc J. 2020;54:322–9.
Gammie JS, Stukus DR, Pham SM, Hattler BG, McGrath MF, McCurry KR, et al. Effect of ischemic time on survival in clinical lung transplantation. Ann Thorac Surg. 1999;68:2015–9.
Fiser SM, Kron IL, Long SM, Kaza AK, Kern JA, Cassada DC, et al. Influence of graft ischemic time on outcomes following lung transplantation. J Heart Lung Transp Off Publ Int Soc Heart Transp. 2001;20:1291–6.
Grimm JC, Valero V, Kilic A, Magruder JT, Merlo CA, Shah PD, et al. Association between prolonged graft ischemia and primary graft failure or survival following lung transplantation. JAMA Surg. 2015;150:547.
Weber D, Cottini SR, Locher P, Wenger U, Stehberger PA, Fasshauer M, et al. Association of intraoperative transfusion of blood products with mortality in lung transplant recipients. Perioper Med. 2013;2:20.
Grande B, Oechslin P, Schlaepfer M, Seifert B, Inci I, Opitz I, et al. Predictors of blood loss in lung transplant surgery: a single center retrospective cohort analysis. J Thorac Dis. 2019;11:4755–61.
Egan TM, Murray S, Bustami RT, Shearon TH, McCullough KP, Edwards LB, et al. Development of the new lung allocation system in the United States. Am J Transp Off J Am Soc Transp Am Surg. 2006;6(5 Pt 2):1212–27.
Boussaud V, Mal H, Trinquart L, Thabut G, Danner-Boucher I, Dromer C, et al. One-year experience with high-emergency lung transplantation in France. Transplantation. 2012;93:1058–63.
Bayer F, Dorent R, Cantrelle C, Legeai C, Kerbaul F, Jacquelinet C. France’s new lung transplant allocation system: combining equity with proximity by optimizing geographic boundaries through the supply/demand ratio. Transp Int Off J Eur Soc Organ Transp. 2022;35:10049.
Loor G, Simpson L, Parulekar A. Bridging to lung transplantation with extracorporeal circulatory support: when or when not? J Thorac Dis. 2017;9:3352–61.
Freeman AK, Thorne CJ, Gaston CL, Shellard R, Neal T, Parry MC, et al. Hypotensive epidural anesthesia reduces blood loss in pelvic and sacral bone tumor resections. Clin Orthop. 2017;475:634–40.
Acknowledgements
We would like to thank the list of investigators involved in the management of lung transplant patients at Bichat Hospital: Service d'Anesthésie-Réanimation: Dan Longrois, Alexandre Mignon, Aurélie Snauwaert, Parvine Tashk, Maksud Assadi, Jules Stern, Sacha Rozencwajg, Adnan El Kalai, Christian de Tymowski, Ali Jendoubi, Aurélie Gouel, Fabien Lion, Laura Soldan, Adela Harpan, Marie-Pierre Dilly, Yassine Rkik, Atanas Sabahov, Claire Depont, Elie Kantor, Laetitia Desplanque, Nils Carrara, Sonia Yung, Morgan Roue, Sophie Provenchère, Julia Voulgaropoulos, Alexandra Younes, Charles Moulin, Bozena Wachoswka, Corentin Gouezel, Elie Succar, Mohamed Foufa, Laila Guezouli, Lea Copelovici, Iulia Balcan, Jose Luis Carrasco, Julien Do vale, Lucie Mariani, Hadrien Portefaix. Service de Pneumologie B et Transplantation Pulmonaire: Cendrine Godet, Vincent Bunel, Gaelle Weisenburger, Tiphaine Goletto, Chahine Medraoui, Gilles Jebrak, Armelle Marceau, Mathilde Salpin, Domitille Mouren, Charlotte Thibaut de Menonville, Alice Savary, Malika Hammouda, Lucie Genet, Gwenn Frère, Laurie Torus, Agnès Abadie, Diego Ferreira, Sandrine Tissot, Linda Hajouji-Idrissi, Zohra Brouk.
Service de chirurgie vasculaire, thoracique et transplantation pulmonaire: Arnaud Roussel, Quentin Pellenc, Jean Senemaud, Jules Iquille, Pierre Cerceau, Regis Renard, Paul Labed.
Funding
None.
Author information
Authors and Affiliations
Contributions
ATD: Participated in the research design, the writing of the paper, the performance of the research and the data analysis. DB: Participated in the statistical analysis. AEK: Participated in the writing of the paper, the performance of the research and data analysis. EA: Participated in the performance of the research and data analysis. ST: Participated in the performance of the research and data analysis. BLJ: Participated in the performance of the research and data analysis. SJB: Participated in the performance of the research and data analysis. NZ: Participated in the performance of the research and data analysis. SB: Participated in the performance of the research and data analysis. YC: Participated in the performance of the research and data analysis. HM: Participated in the performance of the research and data analysis. PM: Participated in the writing of the paper, the performance of the research and data analysis. JM: Participated in the writing of the paper, the performance of the research and data analysis. PM: Participated in the research design, the writing of the paper, the performance of the research and the data analysis. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
All the experiment has been performed in accordance with the Declaration of Helsinki. The study was approved by the ethics committee CEERB Paris Nord, which waived the need for signed informed consent (Institutional Review Board -IRB 00006477- University of Paris, AP-HP.Nord).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1
. Table S1. Factors associated with intraoperative ECMO. Table S2. Factors associated with graft cold ischemia time ≥ 6 hours. Table S3. Factors associated with intraoperative blood transfusion ≥ 3 PRBCs.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Tran-Dinh, A., Bouzid, D., El Kalai, A. et al. Favorable, arduous or fatal postoperative pathway within 90 days of lung transplantation. BMC Pulm Med 22, 326 (2022). https://doi.org/10.1186/s12890-022-02120-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12890-022-02120-w