Abstract
Background
Reduced forced vital capacity (FVC) is a risk factor of all-cause mortality; however, the prevalence and determinants of reduced FVC are not available for the Tunisian population. This study investigated the association of reduced FVC with risk factors and health variables in an urban population of subjects aged ≥ 40 years and living in the city of Sousse in Tunisia.
Methods
A cross-sectional survey was performed using data from the Tunisian Burden of Obstructive Lung Disease (BOLD) study. We defined reduced FVC as a post-bronchodilator FVC below the lower limit of normal using National Health and Nutrition Examination Survey (NHANES) values and Global Lung Function Initiative 2012 equations (GLI 2012) and determined the relation between this finding and the potential risk factors (demographic and socioeconomic factors and the presence of chronic diseases), using multivariable regression analysis.
Results
The prevalence of reduced FVC was 26.6% (176/661) when using NHANES values for white Americans and 14.2% (94/661) using the GLI 2012 equations. Compared to people with normal FVC, those with a reduced FVC were significantly older, taller, had a lower body mass index (BMI), more respiratory symptoms and a higher prevalence of heart disease and hypertension. Multivariable analysis showed that reduced FVC was essentially driven by exposure to biomass smoke for heating, a number of schooling years lower than or equal to 6 years, a childhood history of hunger for a lack of money, aging and height.
Conclusions
The prevalence of reduced FVC is associated with a poor socioeconomic status aging and height.
Similar content being viewed by others
Background
Accumulating findings indicate that reduced forced vital capacity (FVC) is significantly related to respiratory symptoms with various comorbid conditions, including cardiovascular diseases, metabolic syndrome, and obesity [1, 2]. Other studies have demonstrated a relation between survival and FVC [3] and have shown that reduced FVC may be a risk factor for higher mortality [3, 4] and can predict it better than systolic blood pressure or body mass index (BMI) [5].
The Atherosclerosis Risk in Communities (ARIC) study showed that African American individuals had a lower FVC than white individuals and that the higher mortality in African Americans could be explained by the lower FVC in this population [3]. Other studies showed a lower FVC in Africans compared to white individuals and explained it by differences in anthropometry, socioeconomic status, genetic background and environment between blacks and whites [6, 7]. In line with these findings, a high prevalence of reduced FVC has been found in a population of adults living in a West African city [8] and in asymptomatic Nigerians [9, 10].
However, in Tunisia, no studies are available on the prevalence and risk factors for reduced FVC. Because it may be a risk factor for higher mortality, reduced FVC deserves to be explored in the Tunisian population. Thus, in the present study, we aimed to evaluate the distribution of reduced FVC in a community-based study of a non-institutionalised sample of people aged more than 40 years living in the urban area of Sousse, using the Burden of Obstructive Lung Disease (BOLD) study methods. We also investigated potential risk factors leading to reduced FVC in the Tunisian population.
Methods
We followed the BOLD protocol as it has been described elsewhere [11]. Ethics approval was obtained from the University Farhat Hached Hospital of Sousse ethics’ committee and all participants provided written informed consent before taking part.
Study population
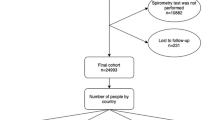
The survey was conducted on a representative gender-stratified random sample of non-institutionalized residents selected from the general population and living in Sousse (Tunisia). Home visits were scheduled for 807 adults aged ≥ 40 years to complete questionnaires and perform pre- and post-bronchodilator (BD) spirometry. Out of 730 eligible participants, 717 were eligible for spirometry and 661 completed the full protocol and met the quality control criteria. The exclusion criteria were mental illness, institutionalization, inability to conduct spirometry, and contraindications to salbutamol [11].
Spirometry and bronchodilator reversibility test
We measured ventilatory function based on the joint American Thoracic Society (ATS)/European Respiratory Society (ERS) guidelines criteria using the hand-held EasyOne spirometer (ndd Medizintechnik AG, Zurich, Switzerland). Participants were tested by trained and certified technicians before and 15 min after administering two puffs of Salbutamol (200 μg) (Ventolin; GlaxoSmithKline, Middlesex, UK) [11]. Spirometers were daily calibrated, using a 3.00 L syringe and all spirograms were checked locally and then sent via a secure Internet transfer to the BOLD Study operations centre located at Imperial College, London, UK, for further quality control checks [12].
Acceptable spirograms had to consist of at least three trials, with two free from zero-flow errors, artifacts, termination prior to 6 s or before a plateau was evident on the volume-time curve, extra breaths, or coughing in the first second of the trial. For repeatability criteria, we accepted slightly greater variation in the highest and next-highest spirograms, if the difference was less than 200 ml, a slight relaxation of the ATS/ERS recommendation shown to have very little impact on quality while reducing missing data [13]. Analysis included only spirometry tests meeting these criteria.
Questionnaire data
Questionnaire data were collected through face-to-face interviews administered by trained and certified staff in the participants’ native language. The standardised BOLD questionnaires were translated from English into Arabic and then back translated to ensure validity.
All participants completed a core questionnaire that included information on sociodemographic characteristics, respiratory diagnoses, disease symptoms, comorbidities, activity limitation and risk factors for respiratory diseases. Participants were also expected to complete an occupational questionnaire and (for current cigarette smokers) a “stages of change” questionnaire that assessed readiness to quit smoking. There was also a questionnaire to assess exposure to biomass fuels used at home for either heating or cooking.
Definitions
FVC was considered reduced if it was less than the lower limit of normal (LLN) based on reference equations from participants in the Third National Health and Nutrition Examination Survey (NHANES) and in the Global Lung Function Initiative (GLI) 2012 using the GLI-2012 desktop software for large data sets (published on www.lungfunction.org) [14]. The diagnosis of COPD was considered in any patient who had a post-bronchodilator FEV1/FVC < LLN accompanied by symptoms of cough, sputum production, or dyspnea. COPD stages of severity were categorized according to GOLD guidelines (Stage I: if FEV1 ≥ 80% predicted; Stage II: if FEV1 ≥ 50 and < 80% predicted; Stage III: if FEV1 ≥ 30 and < 50%; and Stage IV: if FEV1 < 30% predicted) and using the proposed classification based on z-score of post-bronchodilator FEV1 (mild (z-score ≥ − 2), moderate (− 3 ≤ z-score < − 2), severe (− 4 ≤ z-score < − 3) and very severe (z-score < − 4)).
Response rate was defined as the proportion of eligible respondents who completed both the post-bronchodilator spirometry (regardless of the quality) and the core questionnaire.
Statistical analysis
All statistical tests were performed with IBM SPSS statistics software (version 23). The Kolmogorov–Smirnov test was used to test the continuous measures for normality. Normally distributed data were expressed as mean and standard deviation and were compared using an unpaired t-test and not normally distributed data were expressed as median and interquartile range (IQR) and compared using Mann Whitney U-test. Qualitative variables were compared using Chi squared or Fisher exact test. Calculations of odd ratios (ORs) and 95% CI values for reduced FVC in relation to potential risk factors were performed with multivariable logistic regression models. The predictors of reduced FVC that are associated with the greatest increase in R-squared value were introduced in the regression model. The final model included age category (40–49, 50–59, 60–69 and 70 + years), height, BMI, gender, smoking status, hunger for lack of money in childhood, education, occupational exposure to dusts/gases/fumes and exposure to biomass smoke for heating and to passive smoking during childhood. P value of < 0.05 was considered statistically significant.
Results
Sample description
Of a total sample of 807 subjects, 717 responders were interviewed, completed the core questionnaire and undertook spirometry (90% response rate). Among them, 661performed acceptable and reproducible post-BD spirometry.
No significant differences in age, sex and smoking status were found between responders and non-responders and the distribution pattern of these variables was similar in the two groups indicating that the study sample is highly representative of the general population.
Overall, the sample comprised subjects with secondary or higher level education (73.7%) who were overweight or obese (76.8%) and who were exposed to indoor air pollutants (50%).
40% of the participants gave a history of smoking, which was five times more prevalent in males than females. Among the smokers, 67% were current smokers.
About a quarter of the study subjects reported at least one of the three symptoms—cough, phlegm and wheeze in the past 12 months, however, only 9% of them reported a doctor diagnosed respiratory disease.
The most prevalent co-morbidities were hypertension (21%) and diabetes (11%). Gender differences in health status were noted in cough (p = 0.019), phlegm (p = 0.001) and doctor diagnosed respiratory diseases (p = 0.008). In each case, the rates were higher in men than in women.
Prevalence and clinical characteristics of subjects with reduced FVC
Overall, the prevalence of reduced FVC was 25.9% for men (80/309) and 27.3% for women (96/352) when using the NHANES values for white Americans and 14.6% for men (45/309) and 13.9% for women (49/352) when using the GLI 2012 reference equation.
Subjects with reduced FVC were more likely to be older (p = 0.017), taller (p = 0.000) and to have a lower BMI (p = 0.007) compared to those with normal FVC (Table 1). However, there was no significant difference between the two groups in the number of years of schooling, smoking status and history of exposure to passive smoking during childhood, biomass smoke and occupational dusts/gases/fumes (Table 1).
Unlike those with normal FVC, subjects with a reduced FVC were more likely to have chronic cough, chronic wheeze and dyspnoea (Table 1). Moreover, reported diagnosis of chronic bronchitis, emphysema or COPD was significantly more frequent among subjects with reduced FVC versus those with normal FVC (p = 0.026).
Regarding comorbidities, hypertension and heart disease were significantly more prevalent in subjects with a reduced FVC compared to those with a normal FVC while there were no significant differences in the prevalence of diabetes and stroke between the two groups (Table 1).
Surprisingly, a large percentage of subjects with reduced FVC were never smokers (60.8%). This appears clearly especially in women with a reduced FVC since 89.6% of them had never smoked.
Lung function parameters
In general, the analysis suggested that the subjects with a reduced FVC had poorer lung function than the subjects with normal FVC (Table 1). Deficits in FEV1, FVC and FEV1/FVC were found in both men and women, across the age range. However, women with reduced FVC have less chronic cough and produced less sputum than men but suffered more frequently from dyspnoea (p < 0.05).
Risk factors for reduced FVC
We performed univariable and multivariable logistic regressions to assess the association of LLN-based reduced FVC with age, height, gender, BMI, smoking status, hunger for lack of money in childhood, education, occupational exposure to dusts/gases/fumes, and exposure to biomass smoke for heating and to passive smoking during childhood (Table 2). After mutual adjustment for all these potential factors in the model, we found that in our study population, the significant determinants of reduced FVC included, aging, height, a childhood history of hunger for a lack of money, having less than 7 years of schooling and a history of exposure to biomass smoke for heating.
Discussion
In this population of urban residents of Sousse, Tunisia, we found that more than one in four adults had a reduced FVC, which was essentially driven by aging, height, a childhood history of hunger for a lack of money, having less than 7 years of schooling and a history of exposure to biomass smoke for heating.
Since reduced FVC is an important determinant of mortality risk in the general population [3, 15,16,17], our findings indicate the importance of using the identified determinants to select from the general population subjects who are more likely to be diagnosed with a reduced FVC.
According to Burney et al. findings in a general population cohort of asymptomatic adults without chronic respiratory diagnoses or persistent respiratory symptoms, survival is associated with FVC and not with airway obstruction as measured by the FEV1/FVC ratio and FEV1 [3].
The prevalence of reduced FVC pattern in our study was higher than the 26.4% reported by de la Hoz et al., [18] in the workers and volunteers exposed to dust, gases and fumes at the World Trade Center disaster site in 2001–2002 and diagnosed in 2018. Obaseki et al., found a prevalence of 70.4% for reduced FVC among adults aged 40 years and older participating in the BOLD study in Ile-Ife, Nigeria, that was far higher than the 26.6% found in our sample [8]. However, in the study of Cooksley et al. [19] comprising Aboriginal and non-Indigenous residents of the Kimberley region of Western Australia aged 40 years or older and following the BOLD protocol, the prevalence of reduced FVC defined by a FVC < 80% varied from 9.7% in non-Indigenous to 74.0% in Aboriginal participants.
Global variations in the prevalence of reduced FVC pattern can be explained by regional differences in the distribution of a number of factors including participants’ demographics, socio-economic status, prevalence and type of comorbidities and prevalence of restrictive lung diseases. However, that restrictive diseases are rather rare in the general population suggests that they contribute less to the burden of reduced FVC prevalence across published studies [20].
In our population and according to the multivariable analysis, increasing age constitutes a statistically significant risk factor of reduced FVC (OR = 1.849; 95% CI = 1.031, 3.315; p = 0.039) (Table 2). This finding is in line with previous studies results [21] and is relatively not surprising since age has historically been one of the major factors in the evaluation of lung function [22]. Indeed, pulmonary maturity peaks around the age of 20–25 years, after which lung function progressively begins an accelerated decline in FEV1, FVC and FEV1/FVC ratio and an increase in residual volume and functional reserve capacity [22]. The lung function decline is likely explained by the combined effect of several phenomena occurring with aging which include deformation of the axial skeleton, deterioration of the elasticity and recoil capacity of the lung tissues and lung aging.
The finding that reduced FVC prevalence increases with age does not minimize the fact that, in the present study, height constitutes a potential risk factor of reduced FVC (OR = 1.026; 95% CI = 1.005, 1.047; p = 0.014). An extensive bibliography is available on how anthropometric parameters affects lung morphology and function. FVC is affected by height, since it is proportional to body size [23]. This means that in tall individuals, who accordingly have greater lung capacity, lung volume will decrease at a greater rate to shorter individuals as they grow older [24, 25]. This process is favoured by smoking and / or exposure to air pollution such as exposure to biomass smoke, which constitutes an important risk factor for reduced FVC [26,27,28]. Indeed, our study demonstrated that exposure to indoor biomass is an influential risk factor of reduced FVC (OR = 2.743; 95% CI = 1.338, 5.626; p = 0.006). In the present study, 40.3% of the subjects with reduced FVC were exposed to biomass for cooking and 39.2% have been exposed to coal smoke for heating for 17 and 20 years, respectively. Moreover, 87% of our participants had poor indoor ventilation conditions and were then exposed to a more concentrated smoke. These results are consistent with the findings of other studies from developing countries, such as sub-Saharian African city, rural Nepal and Turkey showing elevated prevalence of respiratory symptoms and reduced lung function in association with solid fuel utilisation [26, 27, 29]. According to Balcan et al. [30], intensive exposure of household members to biomass fuel smoke and particles could accelerate the reduction of FVC especially in case of long-term exposition. Moreover, Cooksley et al., reported that, in Aboriginal Australians, exposure to domestic combustion could accelerate age-related loss of lung function and partially account for a reduced FVC [19].
The use of coal for heating and cooking is very widespread in Tunisia and is related to the poor socioeconomic status of the Tunisians. The participants to this study have certainly been exposed to biomass fumes since their childhood increasing, then, their exposure at the critical times of lung development. A reduction of FVC was also demonstrated in patients who were exposed to biomass smoke based on radio-imaging findings consistent with restrictive pathology and histologic problems, such as septal enlargements, metaplasia of goblet cells, glandular hyperplasia, and fibrosis [31, 32].
In line with these findings, a positive association was found between reduced FVC and a participants’ number of schooling years lower than or equal to 6 years (OR = 1.625; 95%CI = 1.087, 2.430; p = 0.018). There is a significant body of literature supporting these findings and showing that the level of education is a risk factor for reduced maximal ventilatory function and chronic lung disease in adults [33,34,35]. The association we found between reduced FVC and a childhood history of hunger for a lack of money (OR = 1.466; 95%CI = 1.000, 2.151; p = 0.050) supports these findings. Inequalities in lifelong respiratory health originate in childhood when adequate nutrition is essential [34, 36, 37]. Indeed, from conception until adolescence, the respiratory system develops rapidly especially during the first 3 years of life. Disruption to this development in childhood is one of the several consequences of living in poverty, including malnutrition [37]. These early-life disruptions influence anthropometry and constitute risk factors for a lung function decline [37]. We used this last factor and the education level as indicators of a poor socioeconomic status [37] a risk factor that encapsulates exposure to several agents affecting foetal lung development and postnatal lung growth [8]. The very high prevalence of reduced FVC seen in Sousse, Tunisia, is consistent with the low socioeconomic status of the Tunisians and had been previously reported by other BOLD Study sites with similar economies such as Nigeria [8], Malawi [38] and South Asia [39].
Interestingly, in the present study, a large percentage of subjects with reduced FVC were never smokers (60.8%). Moreover, 89.6% of the women with a reduced FVC had never smoked. In line with these findings, no association was found between a reduced FVC and smoking habits. These results support the role of risk factors, other than tobacco smoking, that may contribute to a reduced FVC and include childhood respiratory tract infection, low social status, nutrition and environmental pollution exposure [40].
Although cardiovascular diseases had been reported to be associated with a reduced FVC [41], this was not found in the present study. The cardiovascular diseases are known to be highly under-diagnosed and the information collected on the status of the disease in our sample was based on subjects’ self-report. It is very likely that our sample includes a high number of undiagnosed cardiovascular diseases and the latter could obliterate any real association.
A link between diabetes and reduced FVC has also been reported from many studies including ARIC [42], and has more often been ascribed to an adverse effect of diabetic pathology on the ventilatory function [43], a view that is supported by a more rapid decline of lung function in subjects with diabetes [44]. However, in our study, the correlation between diabetes and reduced FVC did not reach the statistical significance level.
Conclusions
In a population-based study of adults living in a Tunisian city, we found a high prevalence of reduced FVC similar to the prevalence found in other sites where the economic conditions are similarly poor. Reduced FVC was independently associated with exposure to biomass fumes for heating, a number of schooling years lower than or equal to 6 years, a childhood history of hunger, aging and height,. These findings support the view that the potential contribution of environmental factors to reduced FVC should be considered rather than assuming that having a small lung volume is accounted for solely by genetic factors. Our findings also indicate the importance of using the identified risk factors to select from the general population, subjects who are more likely to be diagnosed with reduced FVC, and to benefit from targeted risk factors control to mitigate the adverse outcomes.
Availability of data and materials
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- ATS:
-
American thoracic society
- BD:
-
Bronchodilator
- BMI:
-
Body mass index
- BOLD:
-
Burden of obstructive lung disease
- CI:
-
Confidence interval
- COPD:
-
Chronic obstructive pulmonary disease
- ERS:
-
European respiratory society
- FEV1 :
-
Forced expiratory volume in 1 s
- FVC:
-
Forced vital capacity
- GLI 2012:
-
Global lung function initiative 2012
- IQR:
-
Interquartile range
- LLN:
-
Lower limit of normal
- NHANES:
-
National Health and Nutrition Examination Survey
- OR:
-
Odds ratio
- SD:
-
Standard deviation
References
Godfrey MS, Jankowich MD. The vital capacity is vital: epidemiology and clinical significance of the restrictive spirometry pattern. Chest. 2016;149:238–51.
Leone N, Courbon D, Thomas F, Bean K, Jégo B, Leynaert B, et al. Lung function impairment and metabolic syndrome: the critical role of abdominal obesity. Am J Respir Crit Care Med. 2009;179:509–16.
Burney PG, Hooper R. Forced vital capacity, airway obstruction and survival in a general population sample from the USA. Thorax. 2011;66(Suppl 1):49–54.
Magnussen C, Ojeda FM, Rzayeva N, Zeller T, Sinning CR, Pfeiffer N, et al. FEV1 and FVC predict all-cause mortality independent of cardiac function—results from the population based Gutenberg health study. Int J Cardiol. 2017;234:64–8.
Gupta RP, Strachan DP. Ventilatory function as a predictor of mortality in lifelong non-smokers: evidence from large British cohort studies. BMJ Open. 2017;7(Suppl 7): e015381.
Harik-Khan RI, Muller DC, Wise RA. Racial difference in lung function in African-American and white children: effect of anthropometric, socioeconomic, nutritional, and environmental factors. Am J Epidemiol. 2004;160:893–900.
Whitrow MJ, Harding S. Ethnic differences in adolescent lung function: anthropometric, socioeconomic, and psychosocial factors. Am J Respir Crit Care Med. 2008;177:1262–7.
Obaseki DO, Erhabor GE, Awopeju OF, Adewole OO, Adeniyi BO, Buist EAS, et al. Reduced forced vital capacity in an African population prevalence and risk factors. Ann ATS. 2017;14:5.
Femi-Pearse D, Elebute EA. Ventilatory function in healthy adult Nigerians. Clin Sci. 1971;41:203–11.
VanderJagt DJ, Mcclung KD, Kassam HA, Harkins MS, Glew RH. Pulmonary function of herdsmen. J Natl Med Assoc. 2004;96:550–5.
Daldoul H, Denguezli M, Jithoo A, Gnatiuc L, Buist S, Burney P, et al. Prevalence of COPD and tobacco smoking in Tunisia—results from the BOLD study. Int J Environ Res Public Health. 2013;10:7257–727.
Miller MR, Hankinson J, Brusasco V, Burgos F, Casaburi R, Coates A, et al. ATS/ ERS task force. Standardisation of spirometry. Eur Respir J. 2005;26:319–38.
Hankinson JL, Eschenbacher B, Townsend M, Stocks J, Quanjer PH. Use of forced vital capacity and forced expiratory volume in 1 second quality criteria for determining a valid test. Eur Respir J. 2015;45:1283–92.
Quanjer PH, Stanojevic S, Cole TJ, Baur X, Hall GL, Culver BH, et al. Multi-ethnic reference values for spirometry for the 3–95-yr age range: the global lung function 2012 equations. Eur Respir J. 2012;40(Suppl 6):1324–43.
Mannino DM, Buist AS, Petty TL, Enright PL, Redd SC. Lung function and mortality in the United States: data from the First National Health and Nutrition Examination Survey follow up study. Thorax. 2003;58(Suppl 5):388–93.
Mannino DM, Ford ES, Redd SC. Obstructive and restrictive lung disease and functional limitation: data from the Third National Health and Nutrition Examination. J Intern Med. 2003;254(Suppl 6):540–7.
Guerra S, Sherrill DL, Venker C, Ceccato CM, Halonen M, Martinez FD. Morbidity and mortality associated with the restrictive spirometric pattern: a longitudinal study. Thorax. 2010;65(Suppl 6):499–504.
de la Hoz RE, Shapiro M, Nolan A, Celedón JC, Szeinuk J, Lucchini RG. Association of low FVC spirometric pattern with WTC occupational exposures. Respir Med. 2020. https://doi.org/10.1016/j.rmed.2020.106058.
Cooksley NAJB, Atkinson D, Marks GB, Toelle BG, Reeve D, Johns DP, et al. Prevalence of airflow obstruction and reduced forced vital capacity in an Aboriginal Australian population: the cross-sectional BOLD study. Respirology. 2015;20(Suppl 5):766–74.
Ford ES, Mannino DM, Wheaton AG, Giles WH, Presley-Cantrell L, Croft JB. Trends in the prevalence of obstructive and restrictive lung function among adults in the United States: findings from the National Health and Nutrition Examination Surveys from 1988–1994 to 2007–2010. Chest. 2013;143(Suppl 5):1395–406.
Mannino DM, Holguin F, Pavlin BI, Ferdinands JM. Risk factors for prevalence of and mortality related to restriction on spirometry: findings from the First National Health and Nutrition Examination Survey and follow-up. Int J Tuberc Lung Dis. 2005;9(Suppl 6):613–21.
Barroso AT, Márquez ME, Romero RLM, Ruiz OF. Factors affecting lung function: a review of the literature. Arch Bronconeumol. 2018;54(Suppl 6):327–32.
Quanjer PH, Capderou A, Mazicioglu MM, Aggarwal AN, Banik SD, Popovic S, et al. All-age relationship between arm span and height in different ethnic groups. Eur Respir J. 2014;44:905–12.
Rufino R, Costa CH, Lopes AJ, Maiworm AI, Maynard K, Silva LM, et al. Spirometry reference values in the Brazilian population. Braz J Med Biol Res. 2017;50: e5700.
Talaminos Barroso A, Márquez Martín E, Roa Romero LM, Ortega RF. Factores que afectan a la función pulmonar: una revisión bibliográfica. Arch Bronconeumol. 2018;54:327–32.
Ocakli B, Acarturk E, Aksoy E, Gungor S, Ciyiltepe F, Oztas S, et al. The impact of exposure to biomass smoke versus cigarette smoke on inflammatory markers and pulmonary function parameters in patients with chronic respiratory failure. Int J Chron Obstruct Pulmon Dis. 2018;13:1261–7.
Kurmi OP, Devereux GS, Smith WCS, Semple S, Steiner MFC, Simkhada P, et al. Reduced lung function due to biomass smoke exposure in young adults in rural Nepal. Eur Respir J. 2013;41:25–30.
Torzillo P, Waterford J, Hollows F, Jones D. Respiratory disease among Aborigines in the Pilbara. Int J Epidemiol. 1983;12:105–6.
Walter EPY, Balkissou AD, Kengne AP. Determinants of restrictive spirometric pattern in a sub-saharan urban setting: a cross-sectional population-based study. Open Respir Med J. 2016;10:86–95.
Balcan B, Akan S, Ugurlu AO, Handemir BO, Ceyhan BB, Ozkaya S. Effects of biomass smoke on pulmonary functions: a case control study. Int J Chron Obstruct Pulmon Dis. 2016;11:1615–22.
Kara M, Bulut S, Tas F, Akkurt I, Seyfikli Z. Evaluation of pulmonary changes due to biomass fuels using high-resolution computed tomography. Eur Radiol. 2003;13:2372–7.
Mena MA, Woll F, Cok J, Ferrufino JC, Accinelli RA. Histopathological lung changes in children due to biomass fuel. Am J Respir Crit Care Med. 2012;185:687–8.
Jackson B, Kubzansky LD, Cohen S, Weiss S, Wright RJ. A matter of life and breath: childhood socioeconomic status is related to young adult pulmonary function in the CARDIA study. Int J Epidemiol. 2004;33:271–8.
Pei L, Chen G, Mi J, Zhang T, Song X, Chen J, Ji Y, Li C, Zheng X. Low birth weight and lung function in adulthood: retrospective cohort study in China, 1948–1996. Pediatrics. 2010;125:e899–905.
Slachtova H, Gehring U, Hoek G, Tomaskova H, Luttmann-Gibson H, Moshammer H, et al. Parental education and lung function of children in the PATY study. Eur J Epidemiol. 2011;26:45–54.
Sinha IP, Lee AR, Bennett D, McGeehan L, Abrams EM, Mayell SJ, et al. Child poverty, food insecurity, and respiratory health during the COVID-19 pandemic. Lancet Respir Med. 2020;8(Suppl 8):762–3.
Burney P, Jithoo A, Kato B, Janson C, Mannino D, Nizankowska-Mogilnicka E, et al. Burden of Obstructive Lung Disease (BOLD) Study. Chronic obstructive pulmonary disease mortality and prevalence: the associations with smoking and poverty—a BOLD analysis. Thorax. 2014;69:465–73.
Meghji J, Nadeau G, Davis KJ, Wang D, Nyirenda MJ, Gordon SB, et al. Non communicable lung disease in sub-Saharan Africa: a community-based cross-sectional study of adults in urban Malawi. Am J Respir Crit Care Med. 2016;194:67–76.
Burney P, Jarvis D, Perez-Padilla R. The global burden of chronic respiratory disease in adults. Int J Tuberc Lung Dis. 2015;19:10–20.
Wang C, Qu Y, Niu H, Pan Y, He Y, Liu J, et al. The effect of residential environment on respiratory diseases and pulmonary function in Children from a community in Jilin Province of China. Risk Manag Healthc Policy. 2021;14:1287–97.
Soriano JB, Miravitlles M, García-Río F, Muñoz L, Sánchez G, Sobradillo V, et al. Spirometrically-defined restrictive ventilatory defect: population variability and individual determinants. Prim Care Respir J. 2012;21(Suppl 2):187–93.
Yeh H, Punjabi N, Wang N, Pankow JS, Duncan BB, Brancati FL. Vital capacity as a predictor of incident type 2 diabetes: the atherosclerosis risk in communities study. Diabetes Care. 2005;28:1472–9.
Yeh H, Punjabi N, Wang N, Pankow JS, Duncan BB, Cox CE, et al. Cross-sectional and prospective study of lung function in adults with type 2 diabetes: the atherosclerosis risk in communities (ARIC) study. Diabetes Care. 2008;31:741–6.
Kaminsky D. Spirometry and diabetes: implications of reduced lung function. Diabetes Care. 2004;27:837–8.
Acknowledgements
We sincerely thank the BOLD central office, Imperial College, London for guidance and spirometry quality control and the BOLD Sousse committee for the technical support.
Funding
The BOLD Study was funded by a grant from the Wellcome Trust (085790/Z/08/Z). Additional local support for BOLD sites was provided by Boerhringer. The funding bodies played no role in study design and data collection, interpretation of data or writing of the manuscript.
Author information
Authors and Affiliations
Contributions
The study was designed by PB. IH provided population-sampling support. The study was coordinated by PB, IH, MD and MZ and data was collected by SH, NL and MD. The data was analysed by SH and MD. The findings were interpreted and manuscript was drafted by SH and MD. All authors critically revised the report and approved the final version.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Ethics approval was obtained from the University Farhat Hached Hospital of Sousse ethics’ committee and all participants provided written informed consent before taking part in the study. Institution: University Farhat Hached Hospital Sousse. IRB provided by OHRP: IRB 00008931. All methods were carried out in accordance with relevant guidelines and regulations (declarations of helsinki).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Hsan, S., Lakhdar, N., Harrabi, I. et al. Reduced forced vital capacity is independently associated with, aging, height and a poor socioeconomic status: a report from the Tunisian population-based BOLD study. BMC Pulm Med 22, 267 (2022). https://doi.org/10.1186/s12890-022-02062-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12890-022-02062-3