Abstract
Background
Medication adherence in chronic obstructive pulmonary disease (COPD) is low, though not enough is known about the factors that affect adherence in COPD. This study uses qualitative methods to understand the patient perspective on facilitators and barriers to medication adherence in COPD as well as patient-reported strategies for self-management of disease.
Methods
Semi-structured interviews were conducted with 30 individuals (n = 30). Transcripts were analyzed using iterative qualitative coding by 2 independent coders, and codes were categorized using thematic analysis.
Results
Challenges with adherence reported were gaps in understanding, forgetfulness of the patient, physician availability, cost navigation, and overcoming substance use. Most commonly, the financial burden of COPD medications caused patients to source other countries to obtain medications, rely on sample medications collected during doctors’ visits, and to alter medication dosage and frequency to extend the length of a prescription. Tools and resources reported by patients to support self-management of COPD included pharmacist assistance, physician office information, and community resources. Individuals further reported that the use of logs or diaries to track medication usage, visual or temporal cues to take medications, and support from family members were helpful in promoting adherence to their COPD medication regimen.
Conclusions
Medication adherence in individuals with COPD is affected by challenges with self-management of disease and financial burden of medications. However, patients reported multiple tools and resources to support adherence. Physician recognition of these factors impacting self-management, as well as awareness of strategies to promote adherence and manage disease, may improve patient outcomes.
Similar content being viewed by others
Introduction
Chronic obstructive pulmonary disease (COPD) is a highly prevalent disease worldwide and contributes to high mortality and cost within the medical system [1,2,3]. Adherence to medication regimens in COPD has been notoriously low, with reports of 15–30% adherence [4,5,6,7]. Low adherence in COPD is associated with higher healthcare costs, increased hospitalizations, and worse disease control [8]. In recent years, the European Union has made adherence practices a priority in health care, particularly in reference to COPD [3]. Low adherence stems from many factors, including suboptimal communication, financial burdens, time intensive regimens, comorbid diseases, and personal factors that affect the ability to self-manage disease [9,10,11].
Achieving good adherence involves a process including the initiation, implementation, and persistence of a treatment plan [12]. In COPD, this means obtaining and taking the first inhaled dose followed by obtaining and taking refills of the medication over time. To accomplish this, individuals must have the ability to understand, obtain and correctly administer medications on an appropriate schedule, which presents many challenges. One such challenge is that individuals with COPD often have comorbid diseases, resulting in a larger burden of care than managing an isolated disease [10, 13]. In addition, inhaled medications tend to be expensive and with varying insurance coverage making access to maintenance of therapy challenging [10, 13]. Patients taking medication for COPD are often forced to choose where to allocate financial resources each month among other medical and daily living needs [10].
The quantity and variety of different inhaler delivery devices for COPD pose unique challenges for self-management, particularly in an aging population with dexterity and visual limitations. There are at least seven inhaler delivery devices available, meaning individuals with more than one prescribed inhaler may have more than one device to learn [14]. Despite guidelines recommending inhaler teaching, the process of prescribing inhaled therapies and ensuring proper usage among affected patients is often inconsistent and unclear [15, 16]. This raises a technical challenge for patients and highlights the importance of good communication surrounding medication plans specifically with one’s care team. The strength of patient-doctor interactions is pivotal for supporting adherence to inhalers [17].
Though it is well-established that adherence in COPD is low, little is known regarding individual perspectives on these challenges and what resources and tools people with COPD view as most important to assist in adherence to COPD medication plans. Through the National Health Service in Great Britain, informative data at a population level documenting the presence of general patient experiences with COPD are available. However, exploration of such experiences and how they pertain to self-management of disease are lacking [18]. With so many factors contributing to adherence, it is important to understand what people with COPD value and experience. The goal of this study is to explore the patient perspective regarding medication adherence in COPD and gain insight to facilitators and barriers to medication adherence as well as patient-reported strategies for maintaining adherence. The patient perspective regarding medication adherence in COPD management will be informative to future efforts to target initiatives to improve adherence, a necessary step in improving COPD outcomes.
Methods
Participants
A convenience sample of individuals enrolled and followed in the ongoing Medication Adherence Research in COPD (MARC) Study was contacted to participate in semi-structured telephone interviews. The MARC study is a longitudinal observational study investigating adherence behaviors in individuals with COPD and COPD outcomes. All study procedures, including the need for verbal informed consent and the script for consent, were approved by the Johns Hopkins Institutional Review Board (IRB).
Participants met the same inclusion criteria as the MARC study (≥ 40 years old, physician diagnosis of COPD, GOLD stage II-IV disease, and prescribed a long-term controller medication). Individuals also could not have a patient-provider relationship with any members of the research team. MARC participants were invited to be contacted to participate during study follow up phone calls or visits. Interested individuals were scheduled for an interview in the order in which they agreed to learn about this sub-study and oral consent was obtained prior to initiation of audio recording and other study procedures in accordance with IRB. Participants were reimbursed for their time.
Demographic information including educational history and cognitive impairment assessment data was collected as part of the MARC study. Cognitive impairment was assessed using the Montreal Cognitive Assessment (MoCA), a validated screening test for cognitive impairment with scores < 26 indicating mild cognitive impairment [19]. Additional items including working and independent living information were obtained prior to interview.
Interviews
Interviews were conducted using a semi-structured interview guide (Additional file 1). Following a literature review, the guide was discussed and developed with the help of experts in behavioral therapy and qualitative research (MNE, KR). Interviews were designed to explore strategies, barriers and processes related to self-management of COPD and communication during a doctor’s office visit. The semi-structured interview guide allowed for inclusion of follow-up questions based on individual interview responses. Ongoing review of interview transcripts allowed adaptation of subsequent interviews to explore themes as they emerged. The interview guide was updated by JO and MNE to include prompts for facilitation of more in-depth discussion of emerging themes. Questions were modified if necessary for clarity.
Individuals completed a semi-structured interview that was audio-recorded. All interviews were conducted by a female research pulmonary fellow (JO). She was identified to participants by first name as a research fellow with little emphasis or reference to her clinical practice, to avoid potential bias of interviewees’ responses based on their own experiences with physicians.
Audio recordings of interviews were transcribed by a confidential transcription company specializing in research transcripts and returned for review. Transcriptions were completed in batches of 4–6 to allow for review of interview content to ensure themes were being explored.
Analysis
Content analysis of interview responses were conducted using an inductive approach [20]. Following the completion of 10 interviews, MNE and JO independently reviewed transcripts for identification of themes and these were used to develop a code book. An additional five interviews were reviewed for further emerging themes to add to code book. Once no new themes emerged on three consecutive interviews, concept saturation was felt to be met [21]. Transcripts were subsequently coded with NVivo 12 software using the developed code book. Interviews were coded by two independent coders (JO and MK) with any major discrepancies adjudicated by a third party (MNE). An overall kappa was calculated to be 0.75 signifying good agreement [22]. Codes were then categorized into larger themes using thematic analysis.
Results
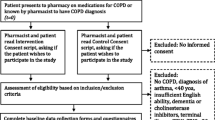
Of the 55 individuals enrolled in the MARC study between April 2017 and April 2019, 38 individuals were sequentially approached to participate in this sub-study with 37 of those agreeing to be contacted at the time the goal of 30 interviews was reached and recruitment stopped. Of the 37, two individuals were unable to be contacted after multiple attempts, 1 individual was excluded because of prior patient-provider relationship with the interviewer and 30 individuals completed interviews (Fig. 1). Once thirty interviews were completed, individuals were no longer approached to participate. Thirty individuals with COPD (mean age 70 ± 8.3 years, 43% men, 73% white, 93% high school degree or higher education) were interviewed by telephone. There were 11 (37%) living alone, 17 (57%) retired, 7 (23%) on disability, and 8 (27%) had been hospitalized within the 12 months prior to interview. At least mild cognitive impairment (< 26 MoCA) was seen in 53% of interviewees (Table 1). Interviews lasted a mean (SD) of 26.9 (7.6) minutes.
Three major concepts regarding self-management emerged from interviews: (1) Challenges with self-management of disease, (2) Financial burden of medications, and (3) Tools and resources utilized to support adherence.
Challenges with self-management of disease
Challenges reported with self-management included gaps in understanding, forgetfulness of the patient, physician availability, cost navigation, and overcoming substance use (Table 2). Many individuals reported forgetfulness as a barrier to medication administration, particularly medications due more than once a day. One individual reported he “may forget to take the evening medicines because I fell asleep.” (66M) Another referred to difficulty with dosing frequency, “for a while I was taking medications twice a day. And that did get a little confusing, especially when I started to feel better, and I would sometimes forget about taking my afternoon medication.” (67M).
Frustration with inhalers and lack of guidance contributed to challenges at home. This was exemplified by one individual expressing that his provider “didn’t give me any instructions how to use it [inhaler]” (65M). Other individuals expressed lack of physician follow-up on inhaler technique despite experiencing difficulties learning or remembering how to properly administer their inhaled medications. One individual reported she does not use a spacer when in public because “I find it very embarrassing… but I just won't take that chamber out of my purse and use it.” (62F) Drug use was also reported as a barrier to self-management of disease both in regards to associated stigma when seeking care and in regards to taking medication appropriately, as substance use caused some to report they “didn't care whether I was doing the right thing or not with my medications.” (56F) (Table 2).
Managing the financial burden of medications
Navigating costs of medication and insurance rules as a challenge was reported, in particular starting a medication because of a coupon or free sample only later to find out “my insurance company wouldn’t pay for that.” (70M) Individuals also reported different techniques to deal with the burden of cost of inhaled therapies (Table 3). Some individuals reported they circumvented costs by going “over the border into Canada” (60F) to obtain affordable medications. Others had success with self-advocacy and writing to pharmaceutical companies to obtain waivers of cost for medications. Still, some reported adjusting medication dosing and frequency to extend the length of a prescription in an attempt to save costs, resulting in medication underuse. While others reported overuse of medications in an attempt to recoup costs already spent, reporting “He had told me that I can stop taking the XXX, but actually, I still take the XXX because I got three canisters of it, and I paid my money for it, so I just take both of them… it's so expensive. I don't want to just throw that away.” (70M) when a new inhaler was prescribed soon after he had refilled his prior inhaler. Several individuals reported the use of sample medications from doctor appointments in order to avoid buying prescriptions at the pharmacy. As one patient describes, “Every time I go, I ask for samples because they're so expensive, even with my co-pay.” (60F) An alternative strategy for addressing this issue included making it a point to discuss cost with doctors to request medicine changes “because it’s cheaper” (72F) for certain inhalers as compared others.
Tools and resources to support adherence
Pharmacist assistance, physician office information, and community resources were among the tools and resources reported to aid in adherence to a patient’s COPD medication regimen (Table 4). One individual reported that her “pharmacy helps me a lot with my medicine.” (54F) Another said that summaries from her physician’s office are something she uses for reference of her disease control and she “keep[s] a copy of my results from year to year” marking stability of disease. (71F) Community resources included support groups through the American Lung Association and local health fairs.
Tools for ensuring medications were taken included the use of logs or diaries to track when medications were administered. Incorporating medication into the morning routine helped some, one individual saying “checking your email and taking your meds, that’s what I do [each morning].” (60F) Others rely on visual cues, choosing to store inhalers in specific locations as they are used during the day. As one patient expressed, “if it’s at 10 o’clock in the morning, if it’s still on the dining room table, I know I haven’t taken it.” (74M).
Additionally, many patients cited social support as a facilitator to adherence to their prescribed medication regimen. When speaking about the importance of having support during an office visit, one individual remarked “if someone were to go with me, that person could take notes…Because sometimes what we're hearing and how we interpret it are two different things.” (62F).
Discussion
This study highlights the perspectives and experiences from people with COPD as it relates to their disease management. Individuals identified facing many barriers to medication adherence, including inhaler visibility, disease stigma, medication costs, and challenges with patient-provider communication. Participants also provided a number of strategies they used to help manage medications including maintaining a daily routine, engaging in patient empowerment by providers and pharmacists, and family accompaniment to doctor visits.
One of the primary strategies for medication adherence was the maintenance of a daily routine with an established time and location for taking medications. As such, disruption of this daily routine contributed to failure to take medications as prescribed. Individuals also reported the second dose of medication each day was challenging to successfully take. These observations suggest that people with COPD who are unable to establish a regular daily routine may struggle to take their medications on time until these external factors have stabilized. Incorporating medication schedules into a daily routine with anticipatory guidance for how to handle late doses and doses due when away from the home is an area for intervention.
Outside of the home, participants reported that the visibility of inhalers and inability to hide their taking of medication affected their willingness to take medication in public. Stigma associated with disease has been reported previously to impact quality of life and medication adherence in COPD [23]. Prior data on people with COPD perspectives and oxygen utilization also demonstrated hesitancy to use a treatment modality so visible to others [9]. Providers have an opportunity to discuss openly these concerns and work with each patient, potentially identifying support to improve upon stigma perceived by patients with COPD.
Addressing challenges with cost of medication was noted by the participants as one of the most salient themes. Most commonly, discussion of cost directly related to underuse of medication. However, on more than one occasion, cost was a causative factor in promoting overuse of medication or use of duplicate medications. Personal strategies to address the high cost of medications, including using sample medications from doctors’ visits and traveling to other countries to obtain medications, were commonly reported by people with COPD. Given the number of inhalers in each drug class available, providers may be able to address this challenge by working with patients and pharmacies to prescribe the most financially accessible medication with appropriate efficacy for an individual. Working with people with COPD to find inhalers that are not cost-prohibitive can help improve the likelihood a person is able to obtain the medication [10]. Challenges with medication adherence due to cost can further be addressed by utilization of combination inhalers where appropriate and streamlined dosages to once daily. Although individuals with COPD who live outside of the US may not have as many challenges related to cost of medications, the EU congress report still reported that medications can be switched to appease insurance requirements which can be very burdensome for patients [3].
Furthermore, challenges with patient-provider communication were reported frequently as barriers to medication adherence. Such challenges included confusion regarding what the provider is asking of the patient, insufficient or confusing instructions regarding inhaler use and techniques, and an inability to communicate with providers outside of scheduled appointments. Lack of clear and consistent patient-provider communication may also pose a barrier to discussion of cost concerns. These challenges contributed to gaps in understanding of the disease process and indications for therapies, which in turn may have affected adherence, according to some individual’s reports.
A common sentiment expressed relating to personal motivation for COPD management was the importance of feeling heard during doctor visits, which contributed to confidence in providers and prescribed care regimen. Communication with community pharmacists has previously been noted to improve self-confidence in use and may increase adherence [24], as does physician follow-up regarding technique and usage of inhaled devices [25, 26]. Pharmacy involvement is particularly important for inhaler usage given previous data showing physicians lack nuanced understanding themselves of appropriate use of all inhalers [27, 28].
Accompaniment by family at physician visits was cited by participants to have a positive impact on information retention by patients which in turn may improve adherence to prescribed medications. Specifically, patients felt that the presence of a family member at visits who could make comments, ask questions, or take notes enhanced their ability to communicate with their provider during the visit and allowed for more detailed recall of provider instructions after the visit when taking their medications. This is particularly notable in the context of the burden of cognitive impairment in individuals with COPD, both previously demonstrated and demonstrated in this cohort of people with COPD with 53% having at least mild cognitive impairment [7, 29]. Interestingly, similarities to previously documented factors affecting asthma management were observed, including that individuals valued clear communication with physicians as facilitators to disease control and behaviors were influenced by the cost of inhalers [30]. This work will be informative for the development of interventions aimed at improving self-management in COPD.
A strength of the semi-structured interview format used for data collection in this study is the ability to capture experiences and priorities that multiple choice or quantitative measures cannot access or may not properly represent. However, one limitation to phone interviews is the inability of the interviewer to observe non-verbal cues, which may contribute to understanding of interview responses.
Finally, when interviewing and subsequently coding qualitative data, there is an inherent bias of the interviewer that requires framing and acknowledgement. The practice of utilizing memos and reflection during the coding process served to mitigate some of these biases [31]. Although efforts were made to include a diverse sample (e.g., approximately 25% non-white), our results may not generalize to all individuals with COPD given that we were restricted to one geographic location. Qualitative analysis itself is not generalizable as it represents specific experiences by individuals, though by interviewing to the point saturation of themes is reached one can make inferences into thematic trends with confidence [31, 32].
Conclusion
Many factors impact medication adherence in people with COPD. The significance of such factors from a patient perspective is imperative to understand when working to improve adherence. The results of this study shed light on challenges commonly faced in medication adherence, strategies to reconcile with the financial implications of COPD medication regimens, and tools to assist with medication regimens, as reported by people with COPD. The recognition of such factors effecting adherence is prerequisite to the delivery of collaborative care and improvement of self-management of disease in COPD. By acknowledging and discussing tools commonly used by people with COPD for disease self-management and promoting open communication between patients and their care team, a patient-centered approach for adherence to a COPD medication plan can be enacted.
Availability of data and materials
The datasets generated during and analyzed during the current study are not publicly available due to the possibility that containing that could compromise the privacy of research participants as well as occasions of institutional and pharmacologic references within interview transcripts. Data are available from the corresponding author on reasonable request.
Abbreviations
- COPD:
-
Chronic Obstructive Pulmonary Disease
- MARC:
-
Medication Adherence Research in COPD
- IRB:
-
Institutional Review Board
- GOLD:
-
Global Initiative for Chronic Obstructive Lung Disease
- MoCA:
-
Montreal Cognitive Assessment
References
Chartbook on long-term trends in health [Internet]. 2017. www.cdc.gov/nchs/data/hus/hus16.pdf#019. Accessed 19 Sept 2017.
Kim J-A, Lim MK, Kim K, Park J, Rhee CK. Adherence to inhaled medications and its effect on healthcare utilization and costs among high-grade chronic obstructive pulmonary disease patients. Clin Drug Investig. 2018;38(4):333–40.
van Boven JFM, Lavorini F, Dekhuijzen PNR, Blasi F, Price DB, Viegi G. Urging Europe to put non-adherence to inhaled respiratory medication higher on the policy agenda: a report from the First European Congress on Adherence to Therapy. Eur Respir J. 2017;49(5):1700076.
Bogart M, Stanford RH, Laliberté F, Germain G, Wu JW, Duh MS. Medication adherence and persistence in chronic obstructive pulmonary disease patients receiving triple therapy in a USA commercially insured population. Int J Chron Obstruct Pulmon Dis. 2019;19(14):343–52.
Duarte-de-Araújo A, Teixeira P, Hespanhol V, Correia-de-Sousa J. COPD: understanding patients’ adherence to inhaled medications. Int J Chron Obstruct Pulmon Dis. 2018;6(13):2767–73.
Huetsch JC, Uman JE, Udris EM, Au DH. Predictors of adherence to inhaled medications among Veterans with COPD. J GenInternMed. 2012;27(11):1506–12.
Sulaiman I, Seheult J, MacHale E, D’Arcy S, Boland F, McCrory K, et al. Irregular and ineffective: a quantitative observational study of the time and technique of inhaler use. J Allergy Clin Immunol Pract. 2016;4(5):900-909.e2.
Davis JR, Wu B, Kern DM, Tunceli O, Fox KM, Horton J, et al. Impact of nonadherence to inhaled corticosteroid/LABA therapy on COPD exacerbation rates and healthcare costs in a commercially insured US population. Am Health Drug Benefits. 2017;10(2):92–102.
Arnold E, Bruton A, Donovan-Hall M, Fenwick A, Dibb B, Walker E. Ambulatory oxygen: why do COPD patients not use their portable systems as prescribed? A qualitative study. BMC Pulm Med. 2011;11(1):9.
Castaldi PJ, Rogers WH, Safran DG, Wilson IB. Inhaler costs and medication nonadherence among seniors with chronic pulmonary disease. Chest. 2010;138(3):614–20.
Mäkelä MJ, Backer V, Hedegaard M, Larsson K. Adherence to inhaled therapies, health outcomes and costs in patients with asthma and COPD. Respir Med. 2013;107(10):1481–90.
Vrijens B, De Geest S, Hughes DA, Przemyslaw K, Demonceau J, Ruppar T, et al. A new taxonomy for describing and defining adherence to medications. Br J Clin Pharmacol. 2012;73(5):691–705.
Doward L, Svedsater H, Whalley D, Crawford R, Leather D, Lay-Flurrie J, et al. Salford Lung Study in chronic obstructive pulmonary disease (SLS COPD): follow-up interviews on patient-centered outcomes. Npj Prim Care Respir Med. 2017;27(1):1–9.
National Jewish Health: Inhaled Medicines [Internet]. National Jewish; 2018. https://njhealth.org/.
Global strategy for the diagnosis, management, and prevention of chronic obstructive lung disease: the GOLD science committee report 2019 | European Respiratory Society [Internet]. [cited 2020 Feb 11]. https://erj.ersjournals.com/content/53/5/1900164.long.
Maricoto T, Marques-Gomes J, Correia-de-Sousa J, Taborda-Barata L. Inhaler review in older adults with asthma or COPD: a cost-effectiveness study and a perspective in Portugal. J Am Geriatr Soc. 2019;67(7):1430–6.
Fischer W, Brandstetter S, Brandl M, Finger T, Bohmer MM, Pfeifer M, et al. Specific, but not general beliefs about medicines are associated with medication adherence in patients with COPD, but not asthma: cohort study in a population of people with chronic pulmonary disease. J Psychosom Res. 2018;107:46–52.
Philip K, Gaduzo S, Rogers J, Laffan M, Hopkinson NS. Patient experience of COPD care: outcomes from the British Lung Foundation Patient Passport. BMJ Open Respir Res. 2019;6(1): e000478.
Petersen RC, Lopez O, Armstrong MJ, Getchius TSD, Ganguli M, Gloss D, et al. Practice guideline update summary: mild cognitive impairment: report of the guideline development, dissemination, and implementation subcommittee of the American Academy of Neurology. Neurology. 2018;90(3):126–35.
Hsieh H, Shannon S. Three approaches to qualitative content analysis. Qual Health Res. 2005;15(9):1277–88.
Francis JJ, Johnston M, Robertson C, Glidewell L, Entwistle V, Eccles MP, et al. What is an adequate sample size? Operationalising data saturation for theory-based interview studies. PsycholHealth. 2010;25(1476-8321 (Electronic)):1229–45.
Marks DF, Yardley L. Research methods for clinical and health psychology. London: SAGE; 2004.
Svedsater H, Roberts J, Patel C, Macey J, Hilton E, Bradshaw L. Life impact and treatment preferences of individuals with asthma and chronic obstructive pulmonary disease: results from qualitative interviews and focus groups. Adv Ther. 2017;34(6):1466–81.
Hesso I, Gebara SN, Kayyali R. Impact of community pharmacists in COPD management: inhalation technique and medication adherence. Respir Med. 2016;1(118):22–30.
Newman S. Improving inhaler technique, adherence to therapy and the precision of dosing: major challenges for pulmonary drug delivery. Expert Opin Drug Deliv. 2014;11(3):365–78.
Price D, Keininger DL, Viswanad B, Gasser M, Walda S, Gutzwiller FS. Factors associated with appropriate inhaler use in patients with COPD—lessons from the REAL survey. Int J Chron Obstruct Pulmon Dis. 2018;26(13):695–702.
Alismail A, Song CA, Terry MH, Daher N, Almutairi WA, Lo T. Diverse inhaler devices: a big challenge for health-care professionals. Respir Care. 2016;61(5):593–9.
Ruud K, Ronningen S, Faksvag P, Ariansen H, Hovland R. Evaluation of a structured pharmacist-led inhalation technique assessment service for patients with asthma and COPD in Norwegian pharmacies. Patient Educ Couns. 2018;101(10):1828–37.
Baird C, Lovell J, Johnson M, Shiell K, Ibrahim JE. The impact of cognitive impairment on self-management in chronic obstructive pulmonary disease: a systematic review. Respir Med. 2017;129:130–9.
Davis SA, Carpenter D, Lee C, Garcia N, Reuland DS, Tudor G, et al. Effect of an asthma question prompt list and video intervention on adolescents’ medication adherence 12 months later. Ann Pharmacother. 2019;53(7):683–9.
Saldaña J. The coding manual for qualitative researchers. 3rd ed. Los Angeles: SAGE; 2016.
Richards L. Handling qualitative data: a practical approach. 3rd ed. Los Angeles: SAGE; 2014. p. 85–101.
Acknowledgements
Not applicable.
Funding
Funding for this study was provided through the following grants from the National Heart, Lung, and Blood Institute: T32 HL 007534 and F32 HL143864-0, NIH R01 HL128620-02.
Author information
Authors and Affiliations
Contributions
JO led data collection, data analyses and writing the manuscript. MK conducted data analysis and contributed to writing the manuscript. KR contributed to study design and provided edits on the manuscript. MNE contributed to study design, supervised data collection, analysis, and provided edits on the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study was approved by the Johns Hopkins IRB (IRB00160444). The IRB provides oversight and ethical approval for human subjects research within Johns Hopkins University. Verbal informed consent was obtained and script for consent was approved by the IRB. All methods were carried out in accordance with relevant guidelines and regulations (eg. Helsinki declaration).
Consent for publication
Participants provided informed consent for the use and disclosure of their data for this study.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1.
Patient participant semi-structured phone interview guide.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
O’Toole, J., Krishnan, M., Riekert, K. et al. Understanding barriers to and strategies for medication adherence in COPD: a qualitative study. BMC Pulm Med 22, 98 (2022). https://doi.org/10.1186/s12890-022-01892-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12890-022-01892-5