Abstract
Background
Acute exacerbation (AE) is the most lethal postoperative complication in idiopathic pulmonary fibrosis (IPF); however, prediction before surgery is difficult. We investigated the role of 18F-fluorodeoxyglucose positron emission tomography/computed tomography (18F-FDG PET/CT) in predicting postoperative AE in IPF.
Method
Clinical data of 48 IPF patients who underwent 18F-FDG PET/CT before thoracic surgery were retrospectively analyzed. Mean and maximal standardized uptake values (SUVmean and SUVmax, respectively) were measured in the fibrotic area. Additionally, adjusted values-SUV ratio (SUVR, defined as SUVmax-to-liver SUVmean ratio), tissue fraction-corrected SUVmean (SUVmeanTF), and SUVR (SUVRTF)-were calculated.
Results
The mean age of the subjects was 67.8 years and 91.7% were male. After thoracic surgery, 21 (43.8%) patients experienced postoperative complications including prolonged air leakage (29.2%), death (14.6%), and AE (12.5%) within 30 days. Patients who experienced AE showed higher SUVmax, SUVR, SUVmeanTF, and SUVRTF than those who did not, but other clinical parameters were not different between patients with and without AE. The SUV parameters did not differ for other complications. The SUVR (odds ratio [OR] 29.262; P = 0.030), SUVmeanTF (OR 3.709; P = 0.041) and SUVRTF (OR 20.592; P = 0.017) were significant predicting factors for postoperative AE following a multivariate logistic regression analysis. On receiver operating characteristics curve analysis, SUVRTF had the largest area under the curve (0.806, P = 0.007) for predicting postoperative AE among SUV parameters.
Conclusions
Our findings suggest that 18F-FDG PET/CT may be useful in predicting postoperative AE in IPF patients and among SUVs, SUVRTF is the best parameter for predicting postoperative AE in IPF patients.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Introduction
Idiopathic pulmonary fibrosis (IPF) is a disorder characterized by chronic progressive pulmonary fibrosis of unknown etiology [1], but shows variable course, including acute exacerbation (AE). AE of IPF could be provoked by viral infection, aspiration, and mechanical stress such as that from thoracic surgery. After thoracic surgery, IPF patients may experience more frequent postoperative complications than non-IPF patients [2,3,4,5,6]. AE occurs in 3–25% of IPF patients after thoracic surgery and is the most lethal postoperative complication, with a mortality rate between 7 and 23% [2, 7,8,9]. Therefore, it is important to identify the population at risk for postoperative AE among IPF patients before surgery. Previous studies reported several risk factors for postoperative AE, including low lung function, poor performance status, high composite physiologic index (CPI), and high lactate dehydrogenase (LDH) levels, in IPF patients [5, 7, 10,11,12]. However, in another study involving 56 IPF patients, no association between clinical parameters (lung function, levels of surfactant protein-D [SP-D] and Krebs von den Lungen-6 [KL-6], and operation type and time) and postoperative AE was observed [6]. Due to these conflicting results, predictors for postoperative AE in IPF are not well defined.
18F-fluorodeoxyglucose positron emission tomography with computed tomography (18F-FDG PET/CT) can assess the metabolic activity of lung tissue by detecting increased FDG uptake [13]. Fibrotic lung parenchyma shows an increased uptake of FDG due to increased numbers of erythrocytes and inflammatory cells with glucose transporter-1 expression resulting from neovascularization [14]. Previous studies reported that the standardized uptake value (SUV), a semi-quantitative index for FDG uptake in PET/CT, was associated with lung function, levels of C-reactive protein [CRP], LDH, SP-D, and KL-6, and clinical outcomes (decline in lung function, transplant-free survival, and death) in IPF patients [15,16,17,18]. These results suggest that 18F-FDG PET/CT could provide additional information on disease activity and prognosis in IPF patients before thoracic surgery. Therefore, we aimed to investigate the usefulness of 18F-FDG PET/CT in predicting postoperative complications, including AE, in IPF patients.
Materials and methods
Subjects
Between April 2004 and March 2016, 1040 IPF patients were diagnosed at Asan Medical Center, Seoul, South Korea and 135 patients who underwent 18F-FDG PET/CT were screened for this study. Among them, 87 patients were excluded because they had not undergone surgery (n = 64), had factors that could affect measurement of SUV in fibrotic area including lung masses (> 3 cm, n = 13), multiple (> 3) lung nodules (n = 3), and undergoing PET/CT after surgery (n = 2), and had no baseline lung function data (n = 5). Finally, 48 IPF patients (biopsy proven cases = 26) who underwent 18F-FDG PET/CT before thoracic surgery for lung nodule were enrolled in the study (Fig. 1). All patients were confirmed with IPF according to the diagnostic criteria of the American Thoracic Society (ATS)/European Respiratory Society/Japanese Respiratory Society/Latin American Thoracic Association statement [1]. This study was approved by the Asan Medical Center Institutional Review Board (2017–0057), and the need to obtain informed consent was waived due to the retrospective nature of the study.
Clinical data
The clinical and survival data at the time of PET/CT were retrospectively obtained from medical records, telephone interviews, and/or the National Health Insurance of Korea. Spirometry, diffusing capacity of the lung for carbon monoxide (DLco), and total lung capacity (TLC) by plethysmography were measured according to recommendations [19,20,21]. The 6-min walk test (6MWT) was performed according to ATS guidelines [22]. All methods were performed in accordance with the relevant guidelines and regulations. The gender-age-physiology (GAP) index was calculated using the GAP model [23].
Postoperative complications were defined as the occurrence of the following events within 30 days after thoracic surgery: (1) AE of IPF; (2) prolonged air leakage via chest tube more than five days after thoracotomy; (3) any other event requiring treatment and extending hospitalization period; and (4) death. Based on an international working group report, we defined AE of IPF as an acute respiratory deterioration characterized by evidence of new widespread alveolar abnormality [24]. The Charlson comorbidity index (CCI) was used to evaluate the impact on of comorbidities on acute exacerbation [25].
PET/CT imaging protocol and analysis
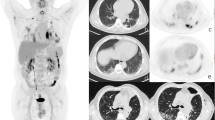
18F-FDG PET/CT and its imaging analysis were performed according to a previously documented protocol [26]. All patients underwent 18F-FDG PET/CT following fasting for at least six hours and blood glucose levels remained below 8.33 mmol/L (150 mg/dL) before PET/CT. PET/CT was conducted within 50–70 min after the injection of 5.18–7.4 MBq/kg (0.14–0.2 mCi/kg) of 18F-FDG. The following scanners were used for image acquisition: Biograph Sensation 16 (Siemens, Knoxville, TN, USA), Discovery STe 8 (GE Healthcare, Milwaukee, WI, USA), Biograph TruePoint 40 (Siemens), Discovery 690 Elite (GE Healthcare), Discovery 690 (GE Healthcare), and Discovery 710 (GE Healthcare). Depending on the PET/CT scanner used, three-dimensional PET images were obtained from the base of the skull to the mid-thigh with 5–8 beds for 2–3 min each. An iterative algorithm with attenuation correction in CT images was used to reconstruct PET images. The SUV, a common semi-quantification method for measuring FDG uptake in 18F-FDG PET/CT [27], was measured in the fibrosis area (red circle in Additional file 1: Fig. S1) by a certified physician (S.H.L., 8 years of experience in nuclear medicine). The SUV of the lung tissue was calculated according to the following equation: SUV = mean regional FDG activity (Bq/mL)/(injected activity [Bq]/body weight [g]).
The maximum SUV (SUVmax) and mean SUV (SUVmean, the mean of SUVs measured by drawing a circle with a diameter of 1 cm centered on the measurement point of SUVmax, Additional file 1: Fig. S1-D) of fibrotic areas were obtained except for a nodule on chest CT. To minimize the confounding effects of inhomogeneous density of fibrotic lung, different resolutions of various PET/CT machines, and measurement methods, adjusted values were calculated based on the SUVmax and SUVmean. To compensate for the SUV differences between individuals, the SUV ratio (SUVR) was calculated by dividing the SUVmax of the fibrotic area by the SUVmean of the liver (measured by drawing a 3 cm-sized circle in the right hepatic lobe) [28], and to compensate for the adjustment for air component in the lung tissue [29], the tissue fraction corrected mean SUV (SUVmeanTF) and SUVR (SUVRTF, defined as SUVmeanTF -to-liver SUVmean ratio) were calculated.
Statistical analysis
All values are presented as the mean ± standard deviation for continuous variables or as number (percentages) for categorical variables. For comparison between two groups, Mann–Whitney U test for continuous variables and Fisher's exact test for categorical variables were used. Logistic regression analysis was performed to determine risk factors for postoperative complications. In the multivariate analysis, the variables with a P value of < 0.05 in the unadjusted analysis or those considered to be clinically significant in previous studies [30, 31] such as GAP (gender, age, and physiology) index was included. Receiver operating characteristic (ROC) curve analysis was used to assess the performance of SUVs in predicting the development of postoperative complications. The discrimination power for postoperative complications was expressed using Area under the ROC curve (AUC). All statistical analyses were performed using SPSS 24.0 (IBM Corp.). A P < 0.05 was considered statistically significant (two-tailed).
Results
Study population
The mean age of all patients was 67.8 years, 91.7% of patients were males, and 93.7% were ever-smokers. Baseline lung function and exercise capacity were relatively preserved, and most patients had GAP stage I (68.8%) or II (29.2%) (Table 1). The median time from PET/CT to surgery was 11.5 days (interquartile range [IQR], 5.8–19.8 days). All patients underwent thoracic surgery due to suspected or confirmed malignant nodules, and 43 (89.6%) were finally diagnosed with malignant neoplasms. The most common type of surgery was lobectomy (56.3%), followed by wedge resection (35.4%) and segmentectomy (8.3%).
Twenty-one (43.8%) patients experienced postoperative complications within 30 days after surgery. The most common complication was prolonged air leakage (29.2%), followed by death (14.6%), and AE (12.5%). Total 7 (14.6%) patients experienced multiple postoperative complications and patients who underwent lobectomy showed a significant tendency for the high rate of multiple complications. Prolonged air leakage, AE, and death were more frequent among patients who underwent lobectomy compared to those who did not, but there was no statistical significance (Additional file 1: Table S1).
Baseline clinical characteristics and SUVs
Patients who experienced postoperative complications were older than those who did not. However, there were no significant differences in terms of gender, smoking history, lung function, exercise capacity, GAP index, and comorbidities between the two groups (Table 1). There were no differences in baseline clinical characteristics between patients with and without AE (Additional file 1: Table S2).
In terms of SUV parameters, there were no significant differences between patients with and without postoperative complications (Table 1, Fig. 2A). However, all SUV parameters, except the SUVmean, in patients with postoperative AE were significantly higher than those in patients without postoperative AE (Fig. 2B, Additional file 1: Table S2). The SUV parameters were not significantly different between patients with and without prolonged air leakage (Fig. 2C) and between survivors and non-survivors (Fig. 2D).
Comparison of baseline SUVs between IPF patients with and without postoperative complications. A All complications. B Acute exacerbation. C Prolonged air leakage. D Death. Each bar represents the mean and standard deviation. *p< 0.05, #p < 0.1. SUV, maximum standardized uptake value; IPF, idiopathic pulmonary fibrosis; Cx, complications; SUVmax, maximum standardized uptake value; SUVmean, mean standardized uptake value; SUVR, standardized uptake value ratio; SUVmeanTF, tissue fraction-corrected mean standardized uptake; SUVRTF, tissue fraction-corrected standardized uptake value ratio; AE, acute exacerbation; AL, air leakage
Predictors of postoperative acute exacerbation
Age was only significantly associated with the development of postoperative complications during univariate analysis (Additional file 1: Table S3). In the univariate logistic analysis, the SUVR, and SUVRTF were significantly associated with postoperative AE, while the SUVmax and resting saturation of oxygen (SpO2) showed marginal significance (Table 2). In the multivariate analysis including GAP index, the SUVR (odds ratio [OR] 29.262; 95% confidence interval [CI] 1.379–621.125; P = 0.030), SUVmeanTF (OR 3.709; 95% CI 1.052–13.080; P = 0.041), and SUVRTF (OR 20.592; 95% CI 1.725–245.841; P = 0.017) were independent risk factors for postoperative AE, while other SUVs showed marginal significance in predicting postoperative AE (Table 3).
Performance of SUV parameters
In the ROC curve analysis, the SUVRTF showed significance in predicting postoperative AE (AUC = 0.806; 95% CI 0.666–0.905; P = 0.007) and the best cut-off value was 1.84 (sensitivity: 66.7%, specificity: 95.2%, positive predictive value [PPV]: 66.7%, negative predictive value [NPV]: 75.2%) (Fig. 3A). Patients with high SUVRTF showed lower 30-day AE free survival rate than those with low SUVRTF (33.3% [> 1.84] vs. 95.2% [≤ 1.84]; P < 0.001; Fig. 3B). The SUVmeanTF also significantly predicted postoperative AE with best cut-off value of 2.44 (AUC = 0.754; 95% CI 0.608–0.867; P = 0.010; sensitivity: 66.7% specificity: 71.4%, PPV: 23.8%, NPV: 76.3%; Fig. 3C), and patients with high SUVmeanTF had lower 30-day AE free survival rate than those with low SUVmeanTF (76.2% [> 2.44] vs. 96.3% [≤ 2.44]; P = 0.049; Fig. 3D). There was no difference in discrimination power between SUVRTF and SUVmeanTF (P = 0.339). However, baseline SUVmean (AUC = 0.655; 95% CI 0.396–0.913; P = 0.224), SUVR (AUC = 0.702; 95% CI 0.452–0.953; P = 0.112) and SUVmax (AUC = 0.700; 95% CI 0.485–0.915; P = 0.115) could not predict postoperative AE in IPF patients.
The receiver operating characteristic and Kaplan Meier’s survival curves of SUVs for acute exacerbation in patients with IPF. A Receiver operating characteristic curve of SUVRTF for AE. B Comparison of AE free survival curves between patients with values above and below the best cut-off value of the SUVRTF. C Receiver operating characteristic curve of SUVmeanTF for predicting AE. D Comparison of AE free survival curves between patients with values above and below the best cut-off value of the SUVmeanTF. Differences between the two groups were assessed using the log rank test. IPF, idiopathic pulmonary fibrosis; AE, acute exacerbation; SUVmeanTF, tissue fraction-corrected mean standardized uptake; SUVRTF, tissue fraction-corrected standardized uptake value ratio
Discussion
In this study, increased SUV was associated with postoperative AE in IPF patients. Among SUV parameters, the SUVR and SUVRTF were independent predictors for postoperative AE in IPF patients, and the SUVRTF was the best parameter for predicting postoperative AE in IPF patients.
Although lung function of the subjects was relatively preserved in our study, 43.8% of subjects experienced postoperative complications including AE (12.5%), which were similar to those of previous studies [6, 32, 33]. Saito et al. reported that 10.7% and 40.7% of IPF patients (n = 28, mean vital capacity: 87.1%) with stage IA non-small cell lung cancer (NSCLC) developed postoperative AE and complications, respectively [32]. Watanabe et al., in IPF patients with lung cancer (n = 56, vital capacity: 103.8%, DLco: 61.4%), also reported that 7.1% experienced postoperative AE [6]. Moreover, Otsuka et al., in IPF patients with lung cancer (n = 9), reported that 44.4% experienced AE after thoracic surgery although lung function of the subjects was not impaired (mean vital capacity: 89% predicted, DLco: 73% predicted) [33]. These results suggest that occurrence of AE is not uncommon after thoracic surgery even in IPF patients with relatively preserved lung function.
Patient demographics and baseline lung function were not associated with postoperative AE in IPF patients in our study. Our findings are consistent with those of previous studies [6, 34]. Watanabe et al. reported that clinical parameters (vital capacity, DLco, white blood cell count, CRP, LDH, SP-D. KL-6, operation time and type, and histopathologic cancer type) were not different between IPF patients suffering from lung cancer with (n = 4) and without (n = 52) postoperative AE after lung resection [6]. However, other studies showed different results [5, 7, 10,11,12, 35]. Sato et al. reported that in 1763 patients with interstitial lung disease (ILD, including 1235 IPF) who underwent thoracic surgery for lung cancer, the male gender, history of previous acute exacerbation, preoperative steroid use, serum KL-6 levels, low vital capacity, usual interstitial pneumonia pattern on chest CT scan, and type of surgery were independent predicting factors for postoperative AE [35]. Kumar et al., in 22 IPF patients with NSCLC, also showed that postoperative acute respiratory distress syndrome (ARDS) was associated with low baseline DLco and high CPI [7]. In addition, Kusibe et al. reported that baseline FVC in 33 IPF patients with lung cancer was lower in patients who developed acute lung injury or ARDS (n = 9) after lung resection (74.0 vs. 103.7% predicted, P < 0.001) compared to those without acute lung injury or ARDS [5]. These inconsistent results suggest that clinical variables might be insufficient to predict the occurrence of postoperative AE in IPF patients.
In our study, SUV parameters such as SUVR, SUVmeanTF, and SUVRTF were only significant predictors for postoperative AE in IPF patients. No study has demonstrated the role of PET/CT in predicting postoperative complications in IPF patients. However, some studies suggested that FDG uptake was associated with severity and prognosis in IPF patients [15,16,17,18]. Lee et al. reported significant correlation between SUV and baseline lung function (FVC: r = -0.6, P = 0.024; DLco: r = -0.7, P = 0.001) in 8 IPF patients [15]. Low baseline lung function was reported to be associated with postoperative AE in IPF patients [5, 7, 10]. Nobashi et al. reported that SUV parameters (SUVmax, SUVmean, SUVmeanTF) in 90 patients with ILD (including 24 IPF) were correlated with baseline CRP and LDH, which are risk factors for postoperative AE in ILD patients [10]. These findings support the role of SUV parameters in predicting postoperative AE in IPF. Justet et al. also reported that in 27 IPF patients, lung volume adjusted SUV metrics, were significantly associated with disease progression including AE, using the multivariate Cox analysis adjusted by age, FVC, and DLco [18]. Therefore, these results suggest that patients with high SUV levels in the fibrotic area need measures to prevent acute exacerbation such as preoperative antifibrotic treatment, and careful observation after surgery.
In our study, SUVRTF, which was corrected for both individual variation and air component of lung tissue, was an independent predictor and was the best predictor of postoperative AE among SUV parameters. Although SUV parameters such as SUVmax and SUVmean are useful in assessing disease activity, they can be affected by multiple factors such as individual variations [27] and distribution of air component without SUV activity [29]. Other studies also suggested that adjusted SUV parameters have higher correlation with disease severity [17] and prognosis [18] compared to the SUVmax and SUVmean, similar to our results.
This study has some limitations. First, this was a retrospective observational study in a single center. However, the baseline characteristics of patients were similar to those in previous reports [6, 32, 33]. Secondly, PET/CT images were acquired from various PET/CT scanners. Thus, our analysis was also conducted using adjusted SUV parameters, such as the SUVR, which adjusts each individual’s 18F-FDG uptake [28]. Third, most subjects had malignant lung nodules. This may have affected SUV measurement in the fibrotic area. However, we attempted to minimize these effects by excluding patients with clinical findings that could affect the results (e.g. lung mass, and multiple lung nodules) and measured SUV of fibrosis area except a nodule. Lastly, some known risk factors for AE, such as treatment (home oxygen, antifibrotic agents, or steroids) before thoracic surgery or the time of surgery, were not addressed in this study, and this might affect the results. However, we could not include home oxygen and antifibrotic use in our analysis, because all patients did not use them due to the relatively preserved lung function or limited access (in South Korea, pirfenidone was covered by insurance after 2016, and most patients in this study underwent surgery before 2016). Also, most of the patients except for one did not use steroids before surgery (only one patient used steroids for a short time before surgery), and data on operation time were not available.
Conclusion
In conclusion, our results suggest that SUV parameters may be useful in predicting postoperative AE in IPF patients. Among them, SUVRTF was the best parameter and postoperative AE was more frequent in patients with high SUVRTF than those with low SUVRTF. These findings suggest that PET/CT could provide additional information on postoperative AE in IPF patients before surgery.
Availability of data and materials
Any data generated and/or analysed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- 6MWD:
-
6-Minute walk distance
- 6MWT:
-
Six-minute walk test
- 18F-FDG:
-
18F-fluorodeoxyglucose
- PET:
-
Positron emission tomography
- AE:
-
Acute exacerbation
- ATS:
-
American Thoracic Society
- AUC:
-
Area under the curve
- CI:
-
Confidence interval
- CPI:
-
Composite physiologic index
- CRP:
-
C-reactive protein
- DLco:
-
Diffusing capacity for carbon monoxide
- FVC:
-
Forced vital capacity
- Glut-1:
-
Glucose transporter-1
- ILD:
-
Interstitial lung disease
- IPF:
-
Idiopathic pulmonary fibrosis
- KL-6:
-
Sialylated carbohydrate antigen, Krebs von den Lungen-6
- LDH:
-
Lactate dehydrogenase
- OR:
-
Odds ratio
- ROC:
-
Receiver operating characteristics
- SpO2 :
-
Saturation of oxygen
- SP-D:
-
Surfactant protein-D
- SUVmax :
-
Maximum standardized uptake value
- SUVmean :
-
Mean standardized uptake value
- SUVmeanTF :
-
Tissue fraction-corrected mean standardized uptake value
- SUVR:
-
Standardized uptake value ratio
- SUVRTF :
-
Tissue fraction-corrected mean standardized uptake value ratio
- TLC:
-
Total lung capacity
References
Raghu G, Collard HR, Egan JJ, Martinez FJ, Behr J, Brown KK, Colby TV, Cordier J-F, Flaherty KR, Lasky JA. An official ATS/ERS/JRS/ALAT statement: idiopathic pulmonary fibrosis: evidence-based guidelines for diagnosis and management. Am J Respir Crit Care Med. 2011;183(6):788–824.
Choi SM, Lee J, Park YS, Cho YJ, Lee CH, Lee SM, Yoon HI, Yim JJ, Lee JH, Yoo CG, et al. Postoperative pulmonary complications after surgery in patients with interstitial lung disease. Respiration Int Rev Thoracic Dis. 2014;87(4):287–93.
Kawasaki H, Nagai K, Yoshida J, Nishimura M, Nishiwaki Y. Postoperative morbidity, mortality, and survival in lung cancer associated with idiopathic pulmonary fibrosis. J Surg Oncol. 2002;81(1):33–7.
Park JH, Kim DK, Kim DS, Koh Y, Lee SD, Kim WS, Kim WD, Park SI. Mortality and risk factors for surgical lung biopsy in patients with idiopathic interstitial pneumonia. Eur J Cardio-Thoracic Surg. 2007;31(6):1115–9.
Kushibe K, Kawaguchi T, Takahama M, Kimura M, Tojo T, Taniguchi S. Operative indications for lung cancer with idiopathic pulmonary fibrosis. Thorac Cardiovasc Surg. 2007;55(8):505–8.
Watanabe A, Higami T, Ohori S, Koyanagi T, Nakashima S, Mawatari T. Is lung cancer resection indicated in patients with idiopathic pulmonary fibrosis? J Thorac Cardiovasc Surg. 2008;136(5):1357-1363.e1352.
Kumar P, Goldstraw P, Yamada K, Nicholson AG, Wells AU, Hansell DM, Dubois RM, Ladas G. Pulmonary fibrosis and lung cancer: risk and benefit analysis of pulmonary resection. J Thorac Cardiovasc Surg. 2003;125(6):1321–7.
Chiyo M, Sekine Y, Iwata T, Tatsumi K, Yasufuku K, Iyoda A, Otsuji M, Yoshida S, Shibuya K, Iizasa T, et al. Impact of interstitial lung disease on surgical morbidity and mortality for lung cancer: analyses of short-term and long-term outcomes. J Thorac Cardiovasc Surg. 2003;126(4):1141–6.
Minegishi Y, Takenaka K, Mizutani H, Sudoh J, Noro R, Okano T, Azuma A, Yoshimura A, Ando M, Tsuboi E, et al. Exacerbation of idiopathic interstitial pneumonias associated with lung cancer therapy. Internal Med (Tokyo, Japan). 2009;48(9):665–72.
Koizumi K, Hirata T, Hirai K, Mikami I, Okada D, Yamagishi S, Kawashima T, Nakajima Y, Shimizu K. Surgical treatment of lung cancer combined with interstitial pneumonia: the effect of surgical approach on postoperative acute exacerbation. Ann Thoracic Cardiovasc Surg. 2004;10(6):340–6.
Mizuno Y, Iwata H, Shirahashi K, Takamochi K, Oh S, Suzuki K, Takemura H. The importance of intraoperative fluid balance for the prevention of postoperative acute exacerbation of idiopathic pulmonary fibrosis after pulmonary resection for primary lung cancer. Eur J Cardio-Thoracic Surg. 2012;41(6):e161-165.
Yano M, Sasaki H, Moriyama S, Hikosaka Y, Yokota K, Kobayashi S, Hara M, Fujii Y. Post-operative acute exacerbation of pulmonary fibrosis in lung cancer patients undergoing lung resection. Interact Cardiovasc Thorac Surg. 2012;14(2):146–50.
Dewan NA, Gupta NC, Redepenning LS, Phalen JJ, Frick MP. Diagnostic efficacy of PET-FDG imaging in solitary pulmonary nodules: potential role in evaluation and management. Chest. 1993;104(4):997–1002.
El-Chemaly S, Malide D, Yao J, Nathan SD, Rosas IO, Gahl WA, Moss J, Gochuico BR. Glucose transporter-1 distribution in fibrotic lung disease: association with [(1)(8)F]-2-fluoro-2-deoxyglucose-PET scan uptake, inflammation, and neovascularization. Chest. 2013;143(6):1685–91.
Lee EY, Wong CS, Fung SL, Yan PK, Ho JC. SUV as an adjunct in evaluating disease activity in idiopathic pulmonary fibrosis - a pilot study. Nucl Med Commun. 2014;35(6):631–7.
Nobashi T, Kubo T, Nakamoto Y, Handa T, Koyasu S, Ishimori T, Mishima M, Togashi K. 18F-FDG uptake in less affected lung field provides prognostic stratification in patients with interstitial lung disease. J Nucl Med. 2016;57(12):1899–904.
Castiaux A, Van Simaeys G, Goldman S, Bondue B. Assessment of 18F-FDG uptake in idiopathic pulmonary fibrosis: influence of lung density changes. Eur J Hybrid Imaging. 2018;2(1):27.
Justet A, Laurent-Bellue A, Thabut G, Dieudonné A, Debray M-P, Borie R, Aubier M, Lebtahi R, Crestani B. [(18)F]FDG PET/CT predicts progression-free survival in patients with idiopathic pulmonary fibrosis. Respir Res. 2017;18(1):74–74.
Wanger J, Clausen JL, Coates A, Pedersen OF, Brusasco V, Burgos F, Casaburi R, Crapo R, Enright P, van der Grinten CP, et al. Standardisation of the measurement of lung volumes. Eur Respir J. 2005;26(3):511–22.
Miller MR, Hankinson J, Brusasco V, Burgos F, Casaburi R, Coates A, Crapo R, Enright P, van der Grinten CP, Gustafsson P, et al. Standardisation of spirometry. Eur Respir J. 2005;26(2):319–38.
Macintyre N, Crapo RO, Viegi G, Johnson DC, van der Grinten CP, Brusasco V, Burgos F, Casaburi R, Coates A, Enright P, et al. Standardisation of the single-breath determination of carbon monoxide uptake in the lung. Eur Respir J. 2005;26(4):720–35.
ATS statement: guidelines for the six-minute walk test. Am J Respir Crit Care Med 2002, 166(1):111–117.
Ley B, Ryerson CJ, Vittinghoff E, Ryu JH, Tomassetti S, Lee JS, Poletti V, Buccioli M, Elicker BM, Jones KD, et al. A multidimensional index and staging system for idiopathic pulmonary fibrosis. Ann Intern Med. 2012;156(10):684–91.
Collard HR, Ryerson CJ, Corte TJ, Jenkins G, Kondoh Y, Lederer DJ, Lee JS, Maher TM, Wells AU, Antoniou KM, et al. Acute exacerbation of idiopathic pulmonary fibrosis an international working group report. Am J Respir Crit Care Med. 2016;194(3):265–75.
Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–83.
Lee SH, Sung C, Lee HS, Yoon HY, Kim SJ, Oh JS, Song JW, Kim MY, Ryu JS. Is (18)F-FDG PET/CT useful for the differential diagnosis of solitary pulmonary nodules in patients with idiopathic pulmonary fibrosis? Ann Nucl Med. 2018;32(7):492–8.
Thie JA. Understanding the standardized uptake value, its methods, and implications for usage. J Nucl Med. 2004;45(9):1431–4.
Watanabe H, Kanematsu M, Goshima S, Kondo H, Kawada H, Noda Y, Moriyama N. Adrenal-to-liver SUV ratio is the best parameter for differentiation of adrenal metastases from adenomas using 18F-FDG PET/CT. Ann Nucl Med. 2013;27(7):648–53.
Lambrou T, Groves AM, Erlandsson K, Screaton N, Endozo R, Win T, Porter JC, Hutton BF. The importance of correction for tissue fraction effects in lung PET: preliminary findings. Eur J Nucl Med Mol Imaging. 2011;38(12):2238–46.
Kakugawa T, Sakamoto N, Sato S, Yura H, Harada T, Nakashima S, Hara A, Oda K, Ishimoto H, Yatera K, et al. Risk factors for an acute exacerbation of idiopathic pulmonary fibrosis. Respir Res. 2016;17(1):79.
Kobayashi H, Omori S, Nakashima K, Wakuda K, Ono A, Kenmotsu H, Naito T, Murakami H, Endo M, Takahashi T. Modified GAP index for prediction of acute exacerbation of idiopathic pulmonary fibrosis in non-small cell lung cancer. Respirology (Carlton, Vic). 2017;22(7):1379–85.
Saito Y, Kawai Y, Takahashi N, Ikeya T, Murai K, Kawabata Y, Hoshi E. Survival after surgery for pathologic stage IA non-small cell lung cancer associated with idiopathic pulmonary fibrosis. Ann Thorac Surg. 2011;92(5):1812–7.
Otsuka H, Sugino K, Hata Y, Makino T, Koezuka S, Isobe K, Tochigi N, Shibuya K, Homma S, Iyoda A. Clinical features and outcomes of patients with lung cancer as well as combined pulmonary fibrosis and emphysema. Mol Clin Oncol. 2016;5(3):273–8.
Suzuki H, Sekine Y, Yoshida S, Suzuki M, Shibuya K, Yonemori Y, Hiroshima K, Nakatani Y, Mizuno S, Takiguchi Y, et al. Risk of acute exacerbation of interstitial pneumonia after pulmonary resection for lung cancer in patients with idiopathic pulmonary fibrosis based on preoperative high-resolution computed tomography. Surg Today. 2011;41(7):914–21.
Sato T, Teramukai S, Kondo H, Watanabe A, Ebina M, Kishi K, Fujii Y, Mitsudomi T, Yoshimura M, Maniwa T, et al. Impact and predictors of acute exacerbation of interstitial lung diseases after pulmonary resection for lung cancer. J Thorac Cardiovasc Surg. 2014;147(5):1604-1611.e1603.
Acknowledgements
We would like to express our deep gratitude to Mynkyu Han (statistician, Asan Medical Center) for his valuable advice on the statistical analysis.
Funding
This work was supported by a grant from the Basic Science Research Program through the National Research Foundation of Korea (NRF), which is funded by the Ministry of Science and Technology, Republic of Korea (NRF-2016R1A2B4016318, NRF-2019R1A2C2008541).
Author information
Authors and Affiliations
Contributions
J.W.S takes full responsibility for the content of this manuscript, including its data and analysis. J.W.S. made substantial contributions to the conception and design of the study. H.Y.Y and J.W.S. made substantial contributions to the analysis and interpretation of the data. S.H.L., S.J.H., and J.S.R. interpreted and measured the nuclear medicine images. H.Y.Y and J.W.S. drafted the initial manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study was approved by the Institutional Review Board of Asan Medical Centre (2017-0057). Informed consent was waived because of the retrospective nature of the study.
Consent for publication
Not applicable.
Competing interests
The authors have no conflicts of interest to declare.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Table S1.
Comparison of postoperative complications according to the type of surgery. Table S2. Comparison of baseline characteristics between IPF patients with and without acute exacerbation. Table S3. Risk factors for postoperative complications in IPF patients assessed using univariate logistic regression analysis. Figure S1. Measurement of the standardized uptake value in the fibrotic area in 18F-FDG PET/CT.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Yoon, HY., Lee, S.H., Ha, S. et al. 18F-FDG PET/CT predicts acute exacerbation in idiopathic pulmonary fibrosis after thoracic surgery. BMC Pulm Med 21, 294 (2021). https://doi.org/10.1186/s12890-021-01659-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12890-021-01659-4