Abstract
Background
Vascular Ehlers-Danlos syndrome (vEDS) is a rare autosomal dominant hereditary collagen disease caused by a defect or deficiency in the pro-α1 chain of type III procollagen encoded by the COL3A1 gene. Patients with vEDS rarely present with multiple pneumothoraces. The clinical features of this disease are not familiar to clinicians and are easily missed. We report a patient with a novel missense mutation in the COL3A1 gene (NM_000090.3: c.2977G > A) and hope to provide clinicians with valuable information.
Case presentation
We reported the case of a young man presenting with frequent episodes of pneumothorax and intrapulmonary cavities and nodular lesions without arterial or visceral complications. His skin was thin and transparent, and the joints were slightly hypermobile. Whole-exome sequencing (chip capture high-throughput sequencing) revealed a heterozygous missense mutation in exon 41 of the COL3A1 gene (NM_000090.3: c.2977G > A), confirming the diagnosis of vEDS. vEDS remains a very rare and difficult diagnosis to determine.
Conclusion
When a patient presents with recurrent pneumothorax, intrapulmonary cavities and nodular lesions, thin and transparent skin, and hypermobile joints, clinicians should consider the diagnosis of vEDS.
Similar content being viewed by others
Background
Vascular Ehlers-Danlos syndrome (vEDS, also known as type IV EDS) is characterized by thin, translucent skin, easy bruising, and a high risk of rupture of the arteries, bowel, and pregnant uterus [1]. The incidence of vEDS is approximately 1 in 150,000 [2]. In addition, it is often caused by mutations in the COL3A1 gene, which encodes the pro-α1 (III) chain of type III procollagen. Additionally, 90% of patients with vEDS present with external thoracic arterial dissection or rupture [1]. Patients with vEDS rarely present with multiple pneumothoraces.
In this report, we present a rare case of vEDS that manifested as recurrent pneumothorax and pulmonary lesions.
Case presentation
A 24-year-old Chinese man presented to the hospital with a 6-day history of haemoptysis, cough and dizziness. He had a history of intermittent cough and blood-tinged sputum for 2 years but denied a history of infectious diseases, occupational exposures or foreign travel. He had no history of smoking. A history of penicillin allergy was noted. No significant medical or drug history was recorded.
Physical examination revealed a temperature of 37.2 °C, a heart rate of 143 beats/min, a blood pressure of 132/64 mmHg, a respiratory rate of 18 breaths/min, and a pulse oximetry value of 98% in ambient air. The patient was tall and slender with a height of 175 cm and a weight of 65 kg (body mass index of 21.22 kg/m2). Chest auscultation revealed decreased lung sounds on the right hemithorax. The clinical examination was otherwise normal.
Laboratory tests showed a white blood cell count of 9.45 × 10^9/L (neutrophils 67%, lymphocytes 23%, and eosinophils 1%), a haemoglobin level of 153 g/L and a platelet count of 231 × 10^9/L. The serum creatinine level, liver function tests, erythrocyte sedimentation rate and C-reactive protein level were all within normal limits. Tests for connective tissue disease with auto-antibodies, including antinuclear, anti-neutrophil cytoplasmic, and anti-glomerular basement membrane antibodies, were negative. The test for human immunodeficiency virus was negative.
A subsequent computed tomography (CT) scan revealed a right-sided pneumothorax, several small cavitary lesions in the right lower lobe and nodules in the left lung (Fig. 1a, b and c). An intercostal chest drain was inserted in the patient, with complete resolution of the pneumothorax. Bronchoscopy showed bronchial inflammatory disease. Due to a history of penicillin allergy, the patient refused further bronchial artery computed tomography angiography (CTA) and an enhanced chest CT examination. On day 12 of the hospital stay, he was discharged after the removal of the chest tube.
Computed tomography images of the chest. a, b, and c A CT scan of the lungs showed right-sided pneumothorax, small cavitary lesions in the right lower lobe and nodules in the left lung. d, e and f Chest CT images showing a large amount of gas in the left thoracic cavity and the left lung tissue compressed by approximately 60%. Both lungs displayed scattered, patchy and nodular high-density shadows, and some exhibited a ground-glass density. g, h, and i Chest CT images showing bilateral pneumothorax with the mediastinum deviated to the right, patch-like high-density shadows scattered in both lungs, and an ambiguous boundary. j, k, and l A CT scan of lungs showed gas on both sides of the chest cavity, and both lungs presented scattered nodules and patchy high-density shadows with unclear boundaries
However, after 3 months, he visited our clinic again and complained of dyspnoea with a duration of 2 days. A high-resolution computed tomography (HRCT) scan of the chest was performed, which showed a large amount of gas in the left thoracic cavity, and the left lung tissue was compressed by approximately 60%. Both lungs had scattered patchy and nodular high-density shadows, and some shadows exhibited ground-glass density (Fig. 1d, e and f). The left-sided pneumothorax was managed with an intercostal chest drain. Both occurrences were diagnosed as primary spontaneous pneumothorax.
After 1 month, the pneumothorax relapsed, and the patient was readmitted to the hospital. Chest CT scans revealed bilateral pneumothorax with the mediastinum deviated to the right, and patch-like high-density shadows with an ambiguous boundary scattered in the two lungs (Fig. 1g, h and i). He was treated conventionally with an intercostal drain. Laboratory tests for connective tissue disease were performed again, and auto-antibodies were still negative. Bronchoscopy was performed again, and visible endobronchial lesions were not detected. An analysis of the bronchoalveolar lavage fluid (BALF) revealed a red blood cell count of 1270 cells/μL and a white blood cell count of 590 cells/μL (neutrophils 36%, lymphocytes 7%, monocytes 3% and other cell types 54%), prompting suspicion of occult intrapulmonary haemorrhage. Because of repeated relapses of pneumothorax and the presence of intrapulmonary nodules, the patient was referred for CT-guided lung puncture. A histopathological investigation of the right lower lung nodules revealed mild atelectasis and hyalinization of the alveolar tissue, and haemosiderin-containing macrophages were observed (Fig. 2). No specific indication of lung puncture pathology was found; he was discharged from the hospital and followed.
In the next month, the pneumothorax relapsed again, and chest CT scans revealed gas on both sides of the chest cavity, scattered nodules in both lungs, and patchy high-density shadows with unclear boundaries (Fig. 1j, k and l). We performed wedge resection of the pulmonary parenchyma using video-assisted thoracoscopic surgery for his recurrent pneumothorax and obtained a specimen of the right lower lung lesion for pathology. The histopathological investigation revealed fresh and old haemorrhages and fibrous nodules. The wall of the cavity showed granulation tissue and calcification. Intra-alveolar haemosiderin-containing macrophages were indicative of a previous haemorrhage (Fig. 3).
Pathological findings of the lung lesions resected through video-assisted thoracotomy. a The right arrow indicates the cavity, and the left arrow indicates the wall of the cavity containing granulation tissue. HE staining: X100 magnification. b The box indicates granulation tissue. HE staining: X200 magnification. c The arrows indicate fresh and old haemorrhages and intra-alveolar haemosiderin-containing macrophages, which indicated a previous haemorrhage. HE staining: X200 magnification. d Note the accumulation of haemosiderin-containing macrophages. HE staining: X400 magnification
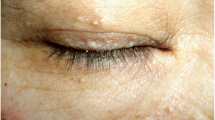
An in-depth physical examination was performed to determine the underlying cause of these atypical clinical manifestations. His skin was thin and translucent with visible venous patterns on the dorsal side of his feet. The clinical examination revealed subtle skin hyperextensibility on the face and forearm and hypermobility of the elbow and metacarpophalangeal joints (Fig. 4). No evidence of scoliosis and no history of joint dislocations were observed. These findings led us to suspect a diagnosis of vEDS.
Genomic deoxyribonucleic acid (DNA) was extracted from a blood sample collected from the patient. Whole-exome sequencing analysis was performed on the DNA sample using chip capture high-throughput sequencing at Shenzhen Huada Clinical Laboratory Center, Shenzhen, China. Chip capture high-throughput sequencing was performed to sequence exons and adjacent exon sequences of approximately 20,000 genes in the human genome. A variant analysis was performed on 3583 genes in the Online Mendelian Inheritance in Man (OMIM) database that had a clear correlation with a single genetic disease. The process of analysis involved first filtering the sequences by 1% of the population frequency (variants present in greater than 1% of the population frequency were evaluated as sites with a low probability of aetiology). According to the patient’s clinical complaint, we next assessed whether mutation sites were likely to fit the symptoms in the filtered mutation dataset. The pathogenicity of the variants was interpreted and classified according to the American College of Medical Genetics (ACMG) Guidelines published in 2015 [3].
Heterozygosity was observed for a novel variant, c.2977G > A (NM_000090.3), which represented a missense mutation that changes a glycine to serine at amino acid 993 (p. Gly993Ser) in the collagen type III alpha 1 protein. The mutation is named according to the Human Genome Variation Society (HGVS) recommendations [4]. This variation was not detected in gnomAD. The variant (COL3A1; NM_000090.3; c.2977G > A; p. Gly993Ser) was validated by Sanger sequencing in the proband and his parents. It was not detected in either of his parents, indicating that this variant was a de novo mutation. Other genes that caused overlapping features (such as FLCN) were excluded. Finally, a definitive diagnosis of vEDS was established.
Considering the deadly arterial complications of this disease, we then recommended further imaging tests to evaluate for possible arterial complications. The patient was referred for a magnetic resonance imaging (MRI) angiography of the intracerebral, thoracic and abdominal arteries, which showed normal calibres of all arteries.
However, 1 month after surgery, the patient experienced a left-sided pneumothorax that was treated with a chest tube again, and he was closely monitored in the clinic. Fortunately, as of 8 months after discharge, the patient remains symptom-free and lives well, without a relapse of pneumothorax or new pulmonary lesions.
Discussion and conclusions
Primary spontaneous pneumothorax is a very common disease of the respiratory system that usually occurs in young and thin males. When pneumothorax develops, oxygen inhalation and thoracic puncture drainage often achieve satisfying results. However, the frequent occurrence of pneumothorax in a short time is rare. The list of differential diagnoses for pneumothorax is extensive (Table 1, data from reference [5]). When this young patient developed pneumothorax, unusual lesions in the lungs captured our attention. In fact, we considered the differential diagnoses for pneumothorax and suspected a variety of rare pulmonary diseases, including eosinophilic pneumonia, Langerhans cell histiocytosis, idiopathic pulmonary haemosiderosis, vasculitis or other interstitial lung diseases. Few eosinophils were detected in the patient’s blood and bronchoalveolar lavage fluid, and no eosinophils were observed in the lung tissue biopsy. Auto-antibodies were not detected in the blood. The histopathological investigation of the right lower lung biopsy specimen revealed fresh and old haemorrhage and did not show any evidence of vasculitis. The patient did not experience anaemia. A physical examination revealed that his skin was thin, transparent and hyperextensible, and the joints were hypermobile. All of these findings led us to suspect the diagnosis of vEDS. We waited 2 months for the results of the genetic report, and it revealed a mutation in the COL3A1 gene, thus confirming the diagnosis of vEDS. A detailed physical examination was very important, and the lengthy wait for the results of the genetic report was worthwhile.
vEDS results from structural defects or a deficiency in the pro-alpha 1 chain of type III procollagen encoded by the COL3A1 gene, which is a key component of many hollow organ tissues. Thus, this abnormal type III collagen synthesis is associated with hyperextensibility of the skin, joint hypermobility, and increased tissue fragility [6]. As shown in Table 2 (data from reference [7]), the international EDS consortium proposed a set of major and minor clinical criteria that are suggestive of vEDS diagnosis in 2017 [7]. A formal diagnosis of vEDS relies on molecular confirmation with the identification of a causative genetic variant. According to the 2017 diagnostic criteria for vEDS, this patient met three minor criteria: thin, translucent skin with increased venous visibility, spontaneous pneumothorax and hypermobility of small joints.
In our case, the patient exhibited a missense mutation c.2977G > A in the COL3A1 gene, which, to our knowledge, had never been reported. Missense mutations in the COL3A1 gene are more likely to be classified as pathogenic rather than benign in the ClinVar database, and the variant c.2977G > A detected in the proband was also a missense mutation. It changed a glycine to serine at amino acid 993 (p. Gly993Ser), which was predicted to be damaging by many computational algorithms, such as DANN, DEOGEN2, EIGEN, FATHMM-MKL, M-CAP, MVP, MutationAssessor, MutationTaster, PrimateAI, REVEL and SIFT. The results from the computational algorithms were obtained from a public interpretation platform named varsome [https://varsome.com/]. The variant is located in exon 41, the region encoding chains of the triple helical domain of type III procollagen [8], and two other missense variants in the same codon, Gly993Cys and Gly993Asp, have been reported to be pathogenic [9], indicating that this site is essential for the function of the COL3A1 gene. In addition, this variant was a de novo mutation in the proband and was consistent with his symptoms, suggesting that it provided a moderate support for the pathogenicity. Finally, we classified this variant as likely pathogenic, based on the evidence described above.
Mutations that cause a glycine substitution in the triple helical protein domain might damage the structural integrity of collagen molecules [10]. As a result, the production of mature type III collagen is substantially reduced and subsequently reduced the mechanical strength of arteries and other hollow organs [11, 12].
Up to 80% of patients with vEDS may have a life-threatening vascular or viscus rupture before the age of 40 years [1]. Although lethal complications had not yet occurred in this patient, the risk of developing dissection, aneurysms, and rupture of arteries later in life was significant. As reported in the study by Kumagaya et al., a patient diagnosed with vEDS initially presented with recurrent pneumothorax and intrapulmonary lesions without vascular complications; however, an aneurysmal formation of the left ulnar artery developed 7 years later, and an aneurysmal formation or arterial dilation of the right ulnar artery, celiac artery, and left iliac artery were observed 12 years later [13]. Our patient was informed about the risk of bleeding in the lungs and other organs later in life.
This patient was treated with the placement of a chest tube and wedge resection of the lungs before a clear diagnosis was established. However, the placement of chest tube, pleurodesis or wedge resection may be complicated by lethal haemothorax in patients with vEDS, and thus a conservative approach should always be used if at all possible.
Celiprolol is a unique β-blocker with β1-adrenergic receptor antagonism and partial β2-agonist activity that results in a lower pulse pressure and heart rate. As shown in the study by Frank et al., untreated patients displayed a significantly worse outcome than patients treated with celiprolol (survival rate of 72.4% vs. 52.2%; p < 0.001) [14]. In terms of the drug dosage, 400 mg/day should be considered the optimal treatment dose [14].
Patients with vEDS rarely present with multiple pneumothoraces. In this case, we observed recurrent pneumothoraces, pulmonary consolidation and cavities, which probably resulted from the rupture of blebs and lung lesions. The lungs of patients with vEDS are vulnerable to laceration.
The reasons why we present this case are listed below. 1. vEDS is a rare disease and patients rarely present with multiple pneumothoraces. 2. We report a patient with a novel missense mutation in the COL3A1 gene (NM_000090.3: c.2977G > A).
When a patient initially presents with pulmonary complications, vEDS is usually not suspected. However, when a patient presents with recurrent pneumothorax, intrapulmonary cavities and nodular lesions, and a physical examination reveals thin and transparent skin, hypermobility of joints, vEDS should be considered. Considering the severity and rapid progression of vEDS, a molecular diagnosis is crucial.
Availability of data and materials
All the data regarding the findings are available within the manuscript.
Abbreviations
- vEDS:
-
Vascular Ehlers-Danlos syndrome
- CT:
-
Computed tomography
- CTA:
-
Computed tomography angiography
- HRCT:
-
High-resolution computed tomography
- BALF:
-
Bronchoalveolar lavage fluid
- DNA:
-
Deoxyribonucleic acid
- OMIM:
-
Online Mendelian Inheritance in Man
- ACMG:
-
American College of Medical Genetics
- HGVS:
-
Human Genome Variation Society
- MRI:
-
Magnetic resonance imaging
References
Pepin M, Schwarze U, Superti-Furga A, Byers PH. Clinical and genetic features of Ehlers-Danlos syndrome type IV, the vascular type. N Engl J Med. 2000;342(10):673–80.
Cortini F, Marinelli B, Seia M, De Giorgio B, Pesatori AC, Montano N, Bassotti A. Next-generation sequencing and a novel COL3A1 mutation associated with vascular Ehlers-Danlos syndrome with severe intestinal involvement: a case report. J Med Case Rep. 2016;10(1):303.
Richards S, Aziz N, Bale S, Bick D, Das S, Gastier-Foster J, Grody WW, Hegde M, Lyon E, Spector E. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med. 2015;17(5):405–23.
den Dunnen JT, Dalgleish R, Maglott DR, Hart RK, Greenblatt MS, McGowan-Jordan J, Roux A-F, Smith T, Antonarakis SE, Taschner PEM. HGVS recommendations for the description of sequence variants: 2016 update. Hum Mutat. 2016;37(6):564–9.
Lim R, Marciniak SJ, Marcadier J, Rassl D, Mitchell PD. Time is of the essence: a young man with recurrent pneumothorax and cavitating lung lesions. Ann Am Thorac Soc. 2018;15(8):988–91.
Abel MD, Carrasco LR. Ehlers-Danlos syndrome: classifications, oral manifestations, and dental considerations. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006;102(5):582–90.
Malfait F, Francomano C, Byers P, Belmont J, Berglund B, Black J, Bloom L, Bowen JM, Brady AF, Burrows NP, et al. The 2017 international classification of the Ehlers-Danlos syndromes. Am J Med Genet C: Semin Med Genet. 2017;175(1):8–26.
Frank M, Albuisson J, Ranque B, Golmard L, Mazzella J-M, Bal-Theoleyre L, Fauret A-L, Mirault T, Denarié N, Mousseaux E. The type of variants at the COL3A1 gene associates with the phenotype and severity of vascular Ehlers–Danlos syndrome. Eur J Hum Genet. 2015;23(12):1657–64.
Pepin MG, Schwarze U, Rice KM, Liu M, Leistritz D, Byers PH. Survival is affected by mutation type and molecular mechanism in vascular Ehlers–Danlos syndrome (EDS type IV). Genet Med. 2014;16(12):881–8.
Mizuno K, Boudko S, Engel J, Bächinger HP. Vascular Ehlers-Danlos syndrome mutations in type III collagen differently stall the triple helical folding. J Biol Chem. 2013;288(26):19166–76.
Schwarze U, Schievink WI, Petty E, Jaff MR, Babovic-Vuksanovic D, Cherry KJ, Pepin M, Byers PH. Haploinsufficiency for one COL3A1 allele of type III procollagen results in a phenotype similar to the vascular form of Ehlers-Danlos syndrome, Ehlers-Danlos syndrome type IV. Am J Hum Genet. 2001;69(5):989–1001.
Beridze N, Frishman WH. Vascular Ehlers-Danlos syndrome: pathophysiology, diagnosis, and prevention and treatment of its complications. Cardiol Rev. 2012;20(1):004–7.
Ishiguro T, Takayanagi N, Kawabata Y, Matsushima H, Yoshii Y, Harasawa K, Yamaguchi S, Yoneda K, Miyahara Y, Kagiyama N, et al. Ehlers-Danlos syndrome with recurrent spontaneous pneumothoraces and cavitary lesion on chest X-ray as the initial complications. Intern Med. 2009;48(9):717–22.
Frank M, Adham S, Seigle S, Legrand A, Mirault T, Henneton P, Albuisson J, Denarie N, Mazzella JM, Mousseaux E, et al. Vascular Ehlers-Danlos syndrome: long-term observational study. J Am Coll Cardiol. 2019;73(15):1948–57.
Acknowledgements
We thank the patient for providing consent to publish. We appreciate Qinmei Fu for the interpretation of the genetic results.
Funding
No form of funding to report.
Author information
Authors and Affiliations
Contributions
TTW and JYY are co-first authors. XYH and LXW are co-corresponding authors. XYH, LXW, TTW and JYY collected the data and participated in writing the manuscript, and they contributed equally to this study. PLW conceived this study and participated in writing the manuscript. MSC, BHJ, and HLW participated in patient management. JML and JM participated in the pathological analysis. All authors have read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Written informed consent for the publication of this case report and any accompanying images was obtained from the patient. A copy of the consent form is available for review by the Editor of this journal.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Wan, T., Ye, J., Wu, P. et al. Recurrent pneumothorax and intrapulmonary cavitary lesions in a male patient with vascular Ehlers-Danlos syndrome and a novel missense mutation in the COL3A1 gene: a case report. BMC Pulm Med 20, 149 (2020). https://doi.org/10.1186/s12890-020-1164-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12890-020-1164-4