Abstract
Background
Epidemiologic studies have shown inconsistent conclusions about the effect of ulinastain treatment for acute respiratory distress syndrome (ARDS). It is necessary to perform a meta-analysis of ulinastatin’s randomized controlled trials (RCTS) to evaluate its efficacy for treating ARDS.
Methods
We searched the published RCTs of ulinastatin treatment for ARDS from nine databases (the latest search on April 30th, 2017). Two authors independently screened citations and extracted data. The meta-analysis was performed using Rev. Man 5.3 software.
Results
A total of 33 RCTs involving 2344 patients satisfied the selection criteria and were included in meta-analysis. The meta-analysis showed that, compared to conventional therapy, ulinastatin has a significant benefit for ARDS patients by reducing mortality (RR = 0.51, 95% CI:0.43~0.61) and ventilator associated pneumonia rate (RR = 0.50, 95% CI: 0.36~0.69), and shortening duration of mechanical ventilation (SMD = -1.29, 95% CI: -1.76~-0.83), length of intensive care unit stay (SMD = -1.38, 95% CI: -1.95~-0.80), and hospital stay (SMD = -1.70, 95% CI:-2.63~−0.77). Meanwhile, ulinastatin significantly increased the patients’ oxygenation index (SMD = 2.04, 95% CI: 1.62~2.46) and decreased respiratory rate (SMD = -1.08, 95% CI: -1.29~-0.88) and serum inflammatory factors (tumor necrosis factor-α: SMD = -3.06, 95% CI:-4.34~-1.78; interleukin-1β: SMD = -3.49, 95% CI: -4.64~-2.34; interleukin-6: SMD = -2.39, 95% CI: -3.34~-1.45; interleukin-8: SMD = -2.43, 95% CI: -3.86~-1.00).
Conclusions
Ulinastatin seemly showed a beneficial effect for ARDS patients treatment and larger sample sized RCTs are needed to confirm our findings.
Similar content being viewed by others
Background
Acute respiratory distress syndrome (ARDS) is a multifactorial lung injury that continues to be associated with high levels of morbidity and mortality [1]. Although the risk of ARDS was significantly reduced over the past 20 years, its incidence is still as high as 20%~ 30% [2]. Low tidal volume ventilation, timely resuscitation and antimicrobial administration, restrictive transfusion practices, and primary prevention of aspiration and nosocomial pneumonia have likely contributed to this reduction [3]. Drug research of ARDS has been involved in all the currently recognized stages of disease pathogenesis, which included anti-inflammatory therapy of glucocorticoid, selective expansion of pulmonary vascular therapy of nitric oxide and prostacyclin, and alternative treatment of exogenous surfactant. However, no drugs are utilized in clinical practice for ARDS due to their contradictory findings across studies. Due to the lack of capable drugs for ARDS treatment, the mortality remains high, up to 45% [4]. Neutrophil and neutrophil elastase are important components of the inflammatory response that characterizes ARDS [5,6,7]. Current notion holds that activated neutrophil releases a large number of neutrophil elastase when it serves as a powerful host defense [5]. Neutrophil elastase is able to escape from regulation by multiple protease inhibitors at inflammatory sites. Excessive neutrophil elastase, beyond levels controlled by endogenous proteinase inhibitors, disturbs the function of the lung permeability barrier and induces the release of pro-inflammatory cytokines. Then, these actions will cause a series of symptoms that are typical in the pathophysiology of ARDS [5, 6, 8, 9]. Further supporting this proposed pathogenesis, animal model studies have shown that supplements of proteinase inhibitors reduce symptoms of acute lung injury [10]. Currently, ulinastatin is a glycoprotein that acts as a protease inhibitor and has been used to treat ARDS [11, 12], pancreatitis [13], multi-organ failure [14] and sepsis [15] in Asia for several years. A summary of the potential mechanisms accounting for the effects of ulinatistin in ARDS is shown in Table 1. For example, ulinatistin can inhibit the serine proteases (such as trypsin and α-chymotrypsin) and different enzymes (such as granulocyte elastase and hyaluronidase) as well as stabilizing lysosomal membranes to prevent the release of lysosomal enzymes [20,21,22]. Moreover, ulinastatin possesses anti-inflammatory properties by reducing the elevation of tumor necrosis factor-α (TNF-α), interleukin-6 (IL-6), and interleukin-8 (IL-8), which are produced by neutrophil elastase [17,18,19]. Currently, several animal studies [23, 24], clinical trials [19, 25] and systematic reviews [11, 12] have confirmed its beneficial effect in lung protection. However, whether ulinastatin can be recommended as a standard medication for ARDS remains uncertain, since its clinical benefit has not been fully understood [26]. Therefore, a meta-analysis to quantitatively evaluate the efficacy of ulinastatin for ARDS is essential.
Methods
Search strategy
We searched all published randomized controlled trials (RCTs) assessing the efficacy of ulinastatin for ARDS from 9 databases: Pubmed, Ovid, the Cochrane Library, ClinicalTrials.gov, Elsevier, Web of Science, Wanfang database, China Knowledge Resource Integrated database, and VIP database. We used the following search terms: “ulinastatin” and “acute respiratory distress syndrome” or “ARDS”. The search deadline was April 30, 2017. No other restrictions were placed on the search criteria. All potentially relevant papers based on titles and abstracts were retrieved for full text screening. We also collected relevant articles by checking the references of the retrieved papers.
Study selection
Patients, 18 years of age or older, diagnosed with ARDS were eligible for inclusion. ARDS was defined as acute onset, pulmonary artery wedge pressure ≤ 18 mmHg and absence of clinical evidence of left atrial hypertension or bilateral infiltrates on chest radiography and oxygenation index (PaO2/FiO2 ≤ 200) [27]. There were no limitations on dose or duration of ulinastatin. The intervention comparisons were made between ulinastatin plus conventional treatment and conventional treatment. The primary efficacy outcomes were mortality, rate of ventilator associated pneumonia (VAP), duration of mechanical ventilation, length of intensive care unit (ICU) and hospital stay. Secondary efficacy outcomes were PaO2/FiO2, respiratory rate, serum inflammatory factors (TNF-a, IL-1β, IL-6, IL-8).
Data extraction and quality assessment
Both the study selection (XYZ and WJ) and data extraction processes (XYZ and WL) were performed by two authors independently. Disagreements were resolved through consensus or arbitration by a third author (XZ or FL). Data for basic characteristics of included trials extracted from full-text articles included the following terms: first author, year of publication, mean age or range, the number of patients, intervention information, Jadad score and duration of intervention. We obtained mean ± standard deviation values for continuous variables in the original manuscripts for the meta-analysis. Trials in which specific endpoints were not reported were excluded from the pooled analyses of the specific endpoints that were reported. Study quality was assessed by the Jadad scale, which assesses adequacy of randomization, blinding and attrition. The Jadad scale ranges from 0 to 5 points, with a low-quality study receiving a score of 2 or less and a high-quality study having a score of at least 3 [28].
Statistical analysis
Data analyses were performed in Rev. Man 5.3 software. The results were expressed as standard mean difference (SMD), weighted mean difference (WMD), relative risk (RR) with 95% confidence interval (CI), I2 value, and Egger test’s P value. I2 value serves as a marker of inter-trial heterogeneity [29]. If I2 < 50%, the fixed-effect model (Mantel-Haenszel) was employed without considering inter-trial heterogeneity. Otherwise, sensitivity analyses were used to identify the sources of inter-trial heterogeneity. In sensitivity analyses, we serially left one study out and analyzed heterogeneity on the basis of masking within the trial in order to judge the stability of effective values. If effective values were stable in sensitivity analyses, the random-effect model (Inverse Variance) was used. If effective values were unstable in sensitivity analyses, we tended to give up performing a meta-analysis and just made a descriptive analysis. Finally, publication bias was formally assessed by using funnel plot and Egger’s regression analysis (with P < 0.05 defined as having publication bias [30]).
Results
Study characteristics
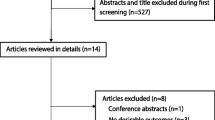
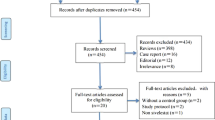
A total of 672 potentially relevant RCTs were identified and screened, using the process shown in Fig. 1. We retrieved 90 RCTs for detailed evaluation, out of which 33 RCTs involving 2344 patients satisfied the selection criteria [31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63]. The included trials were published between 2003 and 2017. The median number of patients was 71 (range 36–160), with three trials [47, 48, 61] having more than 100 patients. Treatment duration ranged from 3 to 30 days. The conventional therapy included mechanical ventilation, anti-infective therapy, nutritional support, treatment of primary diseases, etc. Although all the trials announced the randomization, 7 RCTs adequately described randomization procedures [31, 33, 38, 42, 43, 45, 49, 61], and 1 study [60] explicitly mentioned the method of allocation concealment using opaque envelope. Table 2 displays the quality and characteristics of these studies.
Primary efficacy outcomes
The primary efficacy outcomes on which we focused were directly associated with clinical benefit, including mortality and VAP rate, duration of mechanical ventilation, and length of stay (ICU stay, hospital stay). A total of 24 RCTs [31, 33, 34, 36, 38,39,40,41,42,43,44,45, 48, 49, 52,53,54,55,56, 59,60,61,62,63] (1686 patients) reporting patients’ mortality, the results of meta-analysis confirmed that ulinastatin significantly decreased mortality (RR = 0.51, 95% CI: 0.43~0.61, P < 0.0001, I2 = 0%, Pegger < 0.001, Fig. 2a). Similarly, in a total of 7 RCTs [31, 43, 54, 60,61,62,63] (487 patients) reporting patients’ VAP rate, the results of meta-analysis found that ulinastatin significantly decreased patients’ VAP rate (RR = 0.50, 95% CI: 0.36~0.69, P < 0.0001, I2 = 0%, Pegger = 0.873, Fig. 2b). Moreover, ulinastatin also significantly shortened duration of mechanical ventilation (SMD = -1.29, 95% CI: -1.76~-0.83, P < 0.0001, I2 = 87%, Pegger = 0.221, 9 RCTs including 714 patients [33, 37,38,39, 45, 46, 48, 51, 59] Fig. 3a), ICU stay (SMD = -1.38, 95% CI: -1.95~-0.80, P < 0.0001, I2 = 89%, Pegger = 0.339, 7 RCTs including 594 patients [35, 38, 42, 43, 46, 50, 53], Fig. 3b), and hospital stay (SMD = -1.70, 95% CI: -2.63~-0.77, P < 0.0001, I2 = 92%, Pegger = 0.029, 5 RCTs including 302 patients [37, 39, 46, 51, 62], Fig. 3c). The overall results were similar after sequentially excluding each individual study. The test for heterogeneity was not significant for mortality and VAP rate and fixed-effects model was used. However, there was significant inter-trial heterogeneity in duration of mechanical ventilation, ICU stay and hospital stay and random effect model was used. Egger’s regression analysis found publication bias existed in mortality and hospital stay.
Secondary efficacy outcomes
Secondary efficacy outcomes on which we focused were indirectly associated with clinical benefit, including PaO2/FiO2, respiratory rate, and serum inflammatory factors (TNF-a, IL-1β, IL-6, IL-8). A total of 26 RCTs [31, 32, 34,35,36,37,38, 40,41,42,43,44,45,46, 48, 50, 52, 53, 55,56,57, 59,60,61,62,63] including 1824 patients reported PaO2/FiO2. Compared with conventional therapy, ulinastatin significantly increased patients’ PaO2/FiO2 (SMD = 2.04, 95% CI: 1.62~2.46, P < 0.00001, I2 = 93%, Pegger < 0.001), which was confirmed by the results of meta-analysis (Table 3). Moreover, the findings of meta-analysis on patients’ secondary efficacy outcomes after ulinastatin treatment (Table 3) suggested that ulinastatin significantly decreased patients’ respiratory rate (SMD = -1.08, 95% CI: -1.29~-0.88, P < 0.0001, I2 = 60%, Pegger = 0.001, 15 RCTs including 1117 patients [31, 34,35,36, 43, 47,48,49,50, 52,53,54, 60, 61, 63]) and serum inflammatory factors (TNF-α: SMD = -3.06, 95% CI: -4.34~-1.78, P < 0.0001, I2 = 97%, Pegger < 0.001, 8 RCTs including 600 patients [33, 43,44,45,46, 56,57,58]; IL-1β: SMD = -3.49, 95% CI: -4.64~-2.34, P < 0.0001, I2 = 78%, 2 RCTs including 137 patients [46, 58]; IL-6: SMD = -2.39, 95% CI: -3.34~-1.45, P < 0.0001, I2 = 94%, Pegger = 0.002, 7 RCTs including 523 patients [33, 39, 45, 46, 56,57,58]; IL-8: SMD = -2.43, 95% CI: -3.86~-1.00, P < 0.0001, I2 = 95%, Pegger = 0.015, 4 RCTs including 286 patients [43, 44, 57, 58]). Though significant heterogeneity existed, the overall results of all the outcomes were similar after sequentially excluding each individual study. Egger’s regression analysis found publication bias existed in these outcomes.
Discussion
ARDS is a syndrome with acute lung and systemic inflammation, which are because of activation and accumulation of neutrophils and cytokines [7, 64]. ARDS remains a major public health problem that incurs high health care costs and causes major mortality in ICU despite some improvements in conventional therapeutic approach and managements in the past decades, including mechanical ventilation, systemic steroid, and nitric oxide [65]. Ulinastatin, known as a protease inhibitor, is found in the urine, plasma and all organs [66] and has been used to treat acute pancreatitis [67], acute circulatory failure [68], and severe sepsis [69, 70]. However, it remains uncertain whether ulinastatin can be recommended as a standard medication for and ARDS. In animal models, ulinastatin attenuates the pathophysiological process of acute lung injury induced by lipopolysaccharide, scald injury, phosgene and seawater, among other injuries [23, 24, 71,72,73]. These benefits are mainly associated with inhibiting the activation of neutrophils, blocking nuclear factor-κB pathway, which plays a critical role in the regulation of pro-inflammatory (e.g. TNF-α, IL-1β, IL-6), down-regulate chemokines (e.g. IL-8, macrophage inflammatory protein-2), and increases neutrophils apoptosis [5, 23, 24, 67, 68, 71,72,73]. In theory, ulinastatin could be a new option in ARDS treatment. Several clinical trials and systematic reviews [11, 12] have also confirmed the lung protection of ulinastatin. We performed this meta-analysis to present a comprehensive evaluation to date of ulinastatin in patients with ARDS.
The primary efficacy outcomes were directly associated with clinical benefit, including mortality and VAP rate, duration of mechanical ventilation, and length of stay (ICU stay, hospital stay). Over the past decades, a significant decline has been found in mortality of ARDS, but still as high as 45% [4]. The majority of deaths are attributable to sepsis or multiple organ dysfunction rather than primary respiratory causes, but in some cases death is directly related to lung injury [64]. Ulinastatin has an exact lung protection pharmacological mechanism. In our study, compared to conventional therapy, the results clearly showed that ulinastatin could reduce mortality by 49%, which provided convincing evidence that the pharmacological effect of ulinastatin could be translated into a clinical benefit. Additionally, the results provided more evidence to prompt ulinastatin to become a new hope for ARDS treatment. Ulinastatin significantly reduced patients’ VAP rate by 50% and shorten duration of mechanical ventilation (WMD = -4.60 days, 95% CI: -6.83 ~-2.37), which contributed to a reduced risk of death from another perspective. Ulinastatin also shorten more patients’ hospital stay (WMD = -10.09 days, 95% CI: -17.24 ~-2.94) than ICU stay (WMD = -4.18 days, 95% CI: -5.98 ~-2.38), so it might be able to drastically reduce the cost of medical treatment for patients and government.
Secondary efficacy outcomes were indirectly associated with clinical benefit, including PaO2/FiO2, respiratory rate, and serum inflammatory factors (TNF-α, IL-1β, IL-6, IL-8). Our meta-analysis result found ulinastatin could increase patients’ PaO2/FiO2, and the improvement of PaO2/FiO2 has been suggested to be positively related to mortality [65], which was consistent with our findings. Moreover, our study showed ulinastatin could decrease respiratory rate and serum inflammatory factors (TNF-α, IL-1β, IL-6, IL-8), which was consistent with animal models’ results [24, 73, 74]. Although, our study supported ulinastatin to be an effective treatment for ARDS, we found an older study [75] indicates intra-alveolar ulinastatin cannot inhibit polymorphonuclear elastase activity in the lung in postsurgical patients with ARDS. The statistical significance of this conclusion is questionable as the sample size is too small (only 8 patients). Compared to this study, our study increased the sample size to include 2344 patients and took into account multiple clinically relevant outcomes, so our findings were robust and more reliable.
Although, our study suggested that ulinastatin was relatively effective for the treatment of ARDS and provided a justification for large, well-designed, RCTs to examine the effects of ulinastatin in ARDS, by limiting the study population to patients with ARDS, and increasing the sample size, and expanding the research outcomes, but there were still several limitations. First, publication bias existed in mortality and secondary efficacy outcomes, which probably stemmed from small-study effects [76], all of the trials published in Chinese and the exclusion of trials published as abstracts and conference articles. Second, significant heterogeneity was shown for all the continuous outcomes (ie, duration of mechanical ventilation, ICU stay, hospital stay). Mostly, potential sources of heterogeneity have been identified by sensitivity analyses and random effect model, but some residual heterogeneity still existed in this meta-analysis, which might have originated from the included studies’ low quality (most studies’ Jadad score of only 2), small sample size (range 36–160), or variations in conventional interventions. Therefore, more large-scale multicenter, well designed, RCTs are needed to verify ulinastatin’s efficacy. Lastly, no information on safety was provided by the trials included in our meta-analysis, which is especially needed for future studies.
Conclusions
The findings of this meta-analysis seemly support ulinastatin to be an effective treatment for ARDS. This drug might reduce mortality, ventilator associated pneumonia rate, shortening duration of mechanical ventilation, length of ICU stay and hospital stay in ARDS patients, which needs large, well-designed, RCTs to confirm.
Availability of data and materials
The datasets analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- ARDS:
-
Acute respiratory distress syndrome
- CI:
-
Confidence interval
- ICU:
-
Intensive care unit
- IL-6:
-
Interleukin-6
- IL-8:
-
Interleukin-8
- PaO2/FiO2 :
-
Ratio of arterial oxygen partial pressure to fractional inspired oxygen
- RCTs:
-
Randomized controlled trials
- RR:
-
Relative risk
- SMD:
-
Standard mean difference
- TNF-α:
-
Tumor necrosis factor-α
- VAP:
-
Ventilator associated pneumonia
- WMD:
-
Weighted mean difference
References
Henderson WR, Chen L, Amato MBP, Brochard LJ. Fifty years of research in ARDS. Respiratory mechanics in acute respiratory distress syndrome. Am J Respir Crit Care Med. 2017;196(7):822–33.
Li G, Malinchoc M, Cartin-Ceba R, Venkata CV, Kor DJ, Peters SG, Hubmayr RD, Gajic O. Eight-year trend of acute respiratory distress syndrome: a population-based study in Olmsted County, Minnesota. Am J Respir Crit Care Med. 2011;183(1):59–66.
Beitler JR, Schoenfeld DA, Thompson BT. Preventing ARDS: progress, promise, and pitfalls. Chest. 2014;146(4):1102–13.
Force ADT, Ranieri VM, Rubenfeld GD, Thompson BT, Ferguson ND, Caldwell E, Fan E, Camporota L, Slutsky AS. Acute respiratory distress syndrome: the Berlin definition. JAMA. 2012;307(23):2526–33.
Abraham E. Neutrophils and acute lung injury. Crit Care Med. 2003;31(4 Suppl):S195–9.
Kawabata K, Hagio T, Matsuoka S. The role of neutrophil elastase in acute lung injury. Eur J Pharmacol. 2002;451(1):1–10.
Wang T, Zhu Z, Liu Z, Yi L, Yang Z, Bian W, Chen W, Wang S, Li G, Li A, et al. Plasma neutrophil Elastase and Elafin as prognostic biomarker for acute respiratory distress syndrome: a multicenter survival and longitudinal prospective observation study. Shock. 2017;48(2):168–74.
Fitch PM, Roghanian A, Howie SE, Sallenave JM. Human neutrophil elastase inhibitors in innate and adaptive immunity. Biochem Soc Trans. 2006;34(Pt 2):279–82.
Fujishima S, Morisaki H, Ishizaka A, Kotake Y, Miyaki M, Yoh K, Sekine K, Sasaki J, Tasaka S, Hasegawa N, et al. Neutrophil elastase and systemic inflammatory response syndrome in the initiation and development of acute lung injury among critically ill patients. Biomed Pharmacother. 2008;62(5):333–8.
Pierrakos C, Karanikolas M, Scolletta S, Karamouzos V, Velissaris D. Acute respiratory distress syndrome: pathophysiology and therapeutic options. J Clin Med Res. 2012;4(1):7–16.
Leng YX, Yang SG, Song YH, Zhu X, Yao GQ. Ulinastatin for acute lung injury and acute respiratory distress syndrome: a systematic review and meta-analysis. World J Crit Care Med. 2014;3(1):34–41.
Li S, Fnag M, Wang Y, Yu T, Li W, Yang W. Effects of ulinastatin in patients with acute respiratory distress syndrome: a meta-analysis. Chin J Clin (Electronic Edition). 2016;15(10):2319–24.
Tsujino T, Komatsu Y, Isayama H, Hirano K, Sasahira N, Yamamoto N, Toda N, Ito Y, Nakai Y, Tada M, et al. Ulinastatin for pancreatitis after endoscopic retrograde cholangiopancreatography: a randomized, controlled trial. Clin Gastroenterol Hepatol. 2005;3(4):376–83.
Atal SS, Atal S. Ulinastatin - a newer potential therapeutic option for multiple organ dysfunction syndrome. J Basic Clin Physiol Pharmacol. 2016;27(2):91–9.
Linder A, Russell JA. An exciting candidate therapy for sepsis: ulinastatin, a urinary protease inhibitor. Intensive Care Med. 2014;40(8):1164–7.
Wang T, Liu Z, Wang Z, Duan M, Li G, Wang S, Li W, Zhu Z, Wei Y, Christiani DC, et al. Thrombocytopenia is associated with acute respiratory distress syndrome mortality: an international study. PLoS One. 2014;9(4):e94124.
Inoue K, Takano H, Yanagisawa R, Sakurai M, Shimada A, Yoshino S, Sato H, Yoshikawa T. Protective role of urinary trypsin inhibitor in acute lung injury induced by lipopolysaccharide. Exp Biol Med (Maywood). 2005;230(4):281–7.
Inoue K, Takano H, Shimada A, Yanagisawa R, Sakurai M, Yoshino S, Sato H, Yoshikawa T. Urinary trypsin inhibitor protects against systemic inflammation induced by lipopolysaccharide. Mol Pharmacol. 2005;67(3):673–80.
Xu CE, Zou CW, Zhang MY, Guo L. Effects of high-dose ulinastatin on inflammatory response and pulmonary function in patients with type-a aortic dissection after cardiopulmonary bypass under deep hypothermic circulatory arrest. J Cardiothorac Vasc Anesth. 2013;27(3):479–84.
Gao DN, Zhang Y. Research progress of neutrophil elastase in the mechanism of acute lung injury. Chin Crit Care Med. 2006;18(8):510–2.
Ma PP, Zhu D, Liu BZ, Zhong L, Zhu XY, Wang H, Zhang X, Gao YM. Hu XX: [neutrophil elastase inhibitor on proliferation and apoptosis of U937 cells]. Zhonghua Xue Ye Xue Za Zhi. 2013;34(6):507–11.
Yuan L, Zhu X. The role of neutrophil elastase and its inhibitors in acute respiratory distress syndrome: an update. Zhonghua Wei Zhong Bing Ji Jiu Yi Xue. 2014;26(5):364–8.
Tao Z, Hu FQ, Li CF, Zhang T, Cao BZ, Cui LQ. Effect of ulinastatin, a human urinary protease inhibitor, on heatstroke-induced apoptosis and inflammatory responses in rats. Exp Ther Med. 2017;13(1):335–41.
Luo Y, Che W, Zhao M. Ulinastatin post-treatment attenuates lipopolysaccharide-induced acute lung injury in rats and human alveolar epithelial cells. Int J Mol Med. 2017;39(2):297–306.
Sun R, Li Y, Chen W, Zhang F, Li T. Total ginsenosides synergize with ulinastatin against septic acute lung injury and acute respiratory distress syndrome. Int J Clin Exp Pathol. 2015;8(6):7385–90.
Qiu Y, Lin J, Yang Y, Zhou J, Gong LN, Qin Z, Du L. Lack of efficacy of Ulinastatin therapy during cardiopulmonary bypass surgery. Chin Med J. 2015;128(23):3138–42.
Bernard GR, Artigas A, Brigham KL, Carlet J, Falke K, Hudson L, Lamy M, Legall JR, Morris A, Spragg R. The American-European consensus conference on ARDS. Definitions, mechanisms, relevant outcomes, and clinical trial coordination. Am J Respir Crit Care Med. 1994;149(3 Pt 1):818–24.
Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJ, Gavaghan DJ, McQuay HJ. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials. 1996;17(1):1–12.
Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–60.
Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629–34.
Zeng BI, Peng WH, Wang RJ, Zhang WJ, Xu LF. Clinical Effect Observation of Mechanical Ventilation Combined with Ulinastatin in Treatment of Acute Respiratory Distress Syndrome. Prog Mod Biomed. 2014;2(14):286–288+329.
Tian ZT, Li H, Zhang SL, Li WW. Effects of ulinastatin on oxygenation index, serum hs-CRP level and extravascular lung water index in ARDS patients. Shandong Med. 2014;6(54):65–6.
Cao YY, Li YF, Li GC. Effect of ulinastatin on oxygenation index and inflammatory factors in ARDS patients. China Med Eng. 2014;12(22):139–40.
Zhang CG, Jiang X, Liu SJ. Effects of ulinastatin on oxygenation index and mortality in ARDS patients. Hainan Med J. 2011;16(22):8–10.
Zhang YL, Pan LW, Zhuang R, Lin MX, Ying BY, Ruan H. Changes of plasma matrix metalloproteinase-2 and C-reactive protein in patients with acute respiratory distress syndrome after trauma and the effect of ulinastatin on them. Zhejiang J Trauma Surg. 2009;1(14):6–8.
Zhou MH, Ren GL, Jiao FF. Study on clinical eficacy of ulinastatin in patients with acute respiratory distress syndrome. J Binzhou Med Coll. 2011;2(34):122–4.
Wang ZH. Effect of ulinastatin on arterial blood gas and lactate clearance in patients with acute respiratory distress syndrome. Chin J Clin (Electronic Edition). 2017;2(45):58–60.
Ji M, Chen T, Wang B, Chen M, Ding Q, Chen L, Fang Y, Yu X, Chen Y, Wang X, et al. Effects of ulinastatin combined with mechanical ventilation on oxygen metabolism, inflammation and stress response and antioxidant capacity of ARDS. Exp Ther Med. 2018;15(6):4665–70.
Zhang CQ, Wang YY, Gao ZL. Control clinical study of the effect of ulinastatin on prognosis of acute respiratory distress syndrome patient. Chin J Clin Pract Med. 2010;3(4):18–20.
Huang ZX, Tao H, Xu W, Xu XK, Jin Z, Zhang J, Wei JD, He C, Li WF, Lin ZF. The impact of ulinastatin injection in patients plasma levels of CRP, PCT and lactate in ARDS patients with severe sepsis. J Pract Med. 2015;10(31):1692–4.
Liu JX, Z.H. X, Huang YJ, Li JY, Wang RH, Jia XJ. Clinical study of ulinastatin for acute respiratory distress syndrome. J Guangxi Univ Tradit Chin Med. 2012;4(15):21–3.
Miu SX, Geng YQ, Song YK. Clinical efficacy of ulinastatin combined with lung recruitment in the treatment of acute respiratory distress syndrome. Chin J High Med Educ. 2016;4:132–5.
Yan ZH, Zhao BQ, Cao SW. Effect of ulinastatin combined with mechanical ventilation on lung protection in patients with acute respiratory distress syndrome. J Clin Med Pract. 2015;17(19):112–3.
Ding HH, Liu JD, Peng WP, Lin PH. Clinical efficacy of ulinastatin combined with mechanical ventilation in patients with acute respiratory distress syndrome. Guide China Med. 2014;30(12):42–3.
Wei M, Zhong Q, Zhen HN, Hu HQ, Wan SB. Clinical efficacy of ulinastatin combined with continuous blood purification in 48 patients with acute respiratory distress syndrome. Herald Med. 2015;7(34):910–3.
Huang QS, Zhang LX, Li Y. Clinical trial of ulinastatin combined with mechanical ventilation in patients with acute respiratory distress syndrome. Chin J Clin Pharmacol. 2016;14(32):1268–71.
He B. Clinical efficacy of ulinastatin in the treatment of acute respiratory distress syndrome. Med Front. 2012;28:11–2.
Gu JP, Yu J, Wang JS, Yang L, Liu L, Wang ZY. Clinical study of ulinastatin for acute respiratory distress syndrome. Prog Mod Biomed. 2012;14(12):2695–7.
Mo ZM. Clinical efficacy of ulinastatin in the treatment of acute respiratory distress syndrome. China Foreign Med Treat. 2016;21(35):155–7.
Xue HX, Yang AN, Zhao YD. Clinical efficacy of ulinastatin in the treatment of acute respiratory distress syndrome. Shandong Med. 2008;46(48):75.
Huang HT. Clinical efficacy of ulinastatin in the treatment of acute respiratory distress syndrome. Med Aesthetics Cosmetology. 2014;10(23):270.
Tan C, Huang W, Chang Z. Clinical study of ulinastatin for acute respiratory distress syndrome. Guide China Med. 2010;34(8):129–30.
Ou SQ, Ma Y, Wen YM, Tao Y. Clinical study of ulinastatin for acute respiratory distress syndrome. Chongqing Med. 2008;12(37):1336–7.
Wu YQ, Zhao JC, Yang K, Hu XY. Effect of noninvasive ventilator combined with ulinastatin on immunity, liver and kidney function in patients with acute respiratory distress syndrome. Hebei Med J. 2016;9(38):1327–9.
He C, Huang ZX, Tao H, Xu W, Xu XK, Jin M, Wei JD, Zhang J, Li WF, Lin ZF. Changes of extra-vascular lung water and effects of ulinastatin in serious septic patients with acute respiratory distress syndrome. Chin J Respir Crit Care Med. 2015;3(14):291–4.
Ye YY, Zhang W, Huang HX, Jia LP, Wang CF. Changes of extra-vascular lung water in severe sepsis with acute respiratory distress syndrome and the effect of ulinastatin. J Clin Pulmon Med. 2016;12(21):2264–7.
Ye QD, Huang Q, Li XM. Clinical efficacy of ulinastatin in the treatment of acute respiratory distress syndrome. Guide China Med. 2012;18(10):87–8.
Zhu GY, Xie J, Li T, Jiang ZM, Qiu J, Wang YP. Effects of ulinastatin on TNF-α, IL-1β, IL-6 and IL-8 in ARDS patients. Shandong Med. 2003;25(43):27.
Hu MH, Xu XJ, Jin D, Ji CL, Chen YB, Zhang G. Effect of ulinastrtin on pulmonary endothelial permeability in patients with acute respiratory distress syndrome. Clin Educ Gen Pract. 2009;3(7):229–31 234.
Duan PL. Application of ventilator combined with ulinastatin in acute respiratory distress syndrome. Med Recapitulate. 2015;4(20):740–2.
Liu YX, Teng XH, Song L. Application of ventilator combined with ulinastatin in acute respiratory distress syndrome. Med Recapitulate. 2015;18(21):3424–6.
Lin B. Clinical effect of ulinastatin on treatment of acute respiratory distress syndrome. Clin J Chin Med. 2015;6(7):35–7.
Hu Y. Clinical diagnosis and treatment experience of 30 patients with severe acute respiratory distress syndrome. Mod Diagn Treat. 2014;17(25):4005–6.
Ware LB, Matthay MA. The acute respiratory distress syndrome. N Engl J Med. 2000;342(18):1334–49.
Cho YJ, Moon JY, Shin ES, Kim JH, Jung H, Park SY, Kim HC, Sim YS, Rhee CK, Lim J, et al. Clinical practice guideline of acute respiratory distress syndrome. Tuberc Respir Dis (Seoul). 2016;79(4):214–33.
Pugia MJ, Lott JA. Pathophysiology and diagnostic value of urinary trypsin inhibitors. Clin Chem Lab Med. 2005;43(1):1–16.
Wang LZ, Luo MY, Zhang JS, Ge FG, Chen JL, Zheng CQ. Effect of ulinastatin on serum inflammatory factors in Asian patients with acute pancreatitis before and after treatment: a meta-analysis. Int J Clin Pharmacol Ther. 2016;54(11):890–8.
Song J, Park J, Kim JY, Kim JD, Kang WS, Muhammad HB, Kwon MY, Kim SH, Yoon TG, Kim TY, et al. Effect of ulinastatin on perioperative organ function and systemic inflammatory reaction during cardiac surgery: a randomized double-blinded study. Korean J Anesthesiol. 2013;64(4):334–40.
Feng Z, Shi Q, Fan Y, Wang Q, Yin W. Ulinastatin and/or thymosin alpha1 for severe sepsis: a systematic review and meta-analysis. J Trauma Acute Care Surg. 2016;80(2):335–40.
Karnad DR, Bhadade R, Verma PK, Moulick ND, Daga MK, Chafekar ND, Iyer S. Intravenous administration of ulinastatin (human urinary trypsin inhibitor) in severe sepsis: a multicenter randomized controlled study. Intensive Care Med. 2014;40(6):830–8.
Shen J, Gan Z, Zhao J, Zhang L, Xu G. Ulinastatin reduces pathogenesis of phosgene-induced acute lung injury in rats. Toxicol Ind Health. 2014;30(9):785–93.
Gao C, Liu Y, Ma L, Wang S. Protective effects of ulinastatin on pulmonary damage in rats following scald injury. Burns. 2012;38(7):1027–34.
Rui M, Duan YY, Zhang XH, Wang HL, Wang DP. Urinary trypsin inhibitor attenuates seawater-induced acute lung injury by influencing the activities of nuclear factor-kB and its related inflammatory mediators. Respiration. 2012;83(4):335–43.
Inoue K, Takano H, Yanagisawa R, Yoshikawa T. Protective effects of urinary trypsin inhibitor on systemic inflammatory response induced by lipopolysaccharide. J Clin Biochem Nutr. 2008;43(3):139–42.
Nakane M, Iwama HJST. Intra-alveolar urinary trypsin inhibitor cannot inhibit polymorphonuclear elastase activity in the lung in postsurgical patients with acute respiratory distress syndrome. Surg Today. 1999;29(10):1030–3.
Harbord RM, Egger M, Sterne JA. A modified test for small-study effects in meta-analyses of controlled trials with binary endpoints. Stat Med. 2006;25(20):3443–57.
Acknowledgements
Not applicable.
Funding
This work was supported by the National Science and Technology Major Project (2018ZX10101004), National Natural Science Foundation of China (No. 81372043), the Beijing Natural Science Foundation (No. 7162199) and TECHPOOL Research Fund (No. 01201113). The funders have no role in the design of the study and collection, analysis, and interpretation of data and in writing the manuscript.
Author information
Authors and Affiliations
Contributions
XZ and FL designed the study. XYZ, WJ, WL and ZZ collected data and performed data analysis. XYZ and ZZ drafted the manuscript. XZ, FL and ZZ revised the manuscript. All authors reviewed and edited the final paper. XZ and XYZ had full access to all of the data in the study. All authors have read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Zhang, X., Zhu, Z., Jiao, W. et al. Ulinastatin treatment for acute respiratory distress syndrome in China: a meta-analysis of randomized controlled trials. BMC Pulm Med 19, 196 (2019). https://doi.org/10.1186/s12890-019-0968-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12890-019-0968-6