Abstract
Background
Acute exacerbation (AE) is the major cause of morbidity and mortality in patients with idiopathic pulmonary fibrosis (IPF). AEs also occur in other forms of fibrosing interstitial lung disease (fILD). The clinical features and prognosis of AE patients with connective tissue diseases (CTDs) associated-ILD has not been fully described.
Methods
We retrospectively reviewed 177 patients with either IPF or a characterized CTD-ILD admitted to Nanjing Drum Tower Hospital with an AE from January 2010 to December 2016.
Results
The study cohort included 107 subjects with AE-IPF and 70 cases with AE-CTD-ILD. Female gender, prior use of corticosteroid and immunosupressants, lower serum albumin, higher D-dimer level, TLC% pred, survival, and treatment with immunosupressants and caspofungin were more common in the CTD-ILD group (all p<0.05). The incidences of AE-CTD-ILD and AE-IPF were similar in our single center (p = 0.526). TLC% pred was the risk factor for AE after ILD diagnosis for 1 year in CTD patients (p = 0.018). Log-rank tests showed patients with CTD-ILD had a significantly lower mortality rate compared with IPF patients after AEs (p = 0.029). No significant difference in survival was noted among CTD subgroups (p = 0.353). The survival was negatively correlated with WBC count, LDH and CT score, (p = 0.006, p = 0.013 and p = 0.035, respectively), and positively correlated with PaO2/FiO2 ratio (p<0.001) in the CTD-ILD group. WBC count and PO2/FiO2 ratio were the independent predictors for survival in AE-CTD-ILD after adjusting for other clinical variates in Cox regression Models (p = 0.038 and p < 0.001, respectively).
Conclusions
The clinical characteristics of patients with AE-CTD-ILD differed from those with AE-IPF, while AE incidences were similar between the two groups. Subjects with AE-CTD-fILD tended to have a better prognosis, and WBC count and PO2/FiO2 ratio were the independent survival predictors for these patients.
Similar content being viewed by others
Background
Idiopathic pulmonary fibrosis (IPF) is a chronic and progressive scarring disease of the lung characterized by worsening dyspnea and lung function over time [1, 2]. Some patients will experience an acute, clinically significant respiratory deterioration characterized by widespread alveolar abnormality [3]. Acute exacerbations (AEs) are a major cause of mortality of patients with IPF. The incidence of AE-IPF ranges from 4 to 20% per year among IPF patients in reported clinical trials [4,5,6,7,8], and up to one-half of IPF patients die from AE [9]. In most cases, in-hospital mortality rate was up to 50% [7]. The etiology of AE-IPF is still unknown. Recent evidence suggests that causes of acute lung injury (ALI) and histopathologic diffuse alveolar damage (DAD) such as infection, aspiration, surgery and air pollution exposure could contribute to the development of AE-IPF [3, 10, 11]. Low forced vital capacity (FVC), diffusing capacity for carbonmonoxide (DLCO) and baseline oxygenation were known risk factors for AE [7, 10, 12,13,14]. Lower baseline FVC and DLCO and more extensive CT abnormalities at the time of AE and worse oxygenation were predictors of mortality [7, 8, 15]. The elevated levels of Kerbs von Lungen-6 and leptin in peripheral blood could be the risk and prognostic factors for AE-IPF [16, 17].
Recently, AE has also been shown to occur in fibrosing interstitial lung diseases (fILD) other than IPF, such as connective tissue disease (CTD) associated ILD, idiopathic nonspecific interstitial lung disease and chronic hypersensitivity pneumonias [18,19,20,21,22]. Park et al. reported AE in CTD-ILD was similar to AE-IPF and most common in older patients with rheumatoid arthritis (RA) [18]. Suda et al. suggested that AE occurred mostly with RA-ILD with a usual interstitial pneumonia (UIP) pattern [19]. Toyoda et al. showed some AE cases in patients with CTD had good response to corticosteroid [23]. To date, there are few reports of AE in CTD-ILD patients. The incidence, clinical characteristics and prognosis of AE patients in CTD-ILD have yet to be fully elucidated.
We conducted a retrospective study of AE in CTD-ILD and IPF in our single center. The aims were to clarify the occurrence, clinical characteristics and prognosis of patients with CTD-ILD who developed AE, and to identify risk and predictive factors in Chinese population.
Methods
Study subjects
This retrospective cohort study was approved by the Ethics Committee of Nanjing Drum Tower Hospital and conducted in compliance with the Helsinki Declaration (1989) (NO.2016–160-01). We retrospectively reviewed 3280 new patients with ILD admitted to our hospital from January 2010 to December 2016, and 177 consecutive patients with an AE of underlying fILD were enrolled in our study (Fig. 1). The follow-up period was from 6 months to 7 years. The final cohort included 107 cases of AE-IPF and 70 cases of AE-CTD-ILD. All subjects had an underlying UIP or possible UIP pattern on chest high resolution computed tomography (HRCT) as defined by the American Thoracic Society (ATS)/European Respiratory Society (ERS) guidelines [1, 2]. The diagnosis of the underlying CTD and interstitial pneumonia with autoimmune features (IPAF) was according to the established criteria [23,24,25,26,27,28,29,30]. IPAF means patients with ILD that had features of autoimmunity, yet falling short of a characterizable CTD, was included in CTD-ILD. The definition of AE was based on the updated international criteria for AE-IPF [3]. Briefly, AE-IPF and AE-CTD-fILD were defined as an acute, clinically significant respiratory deterioration characterized by evidence of new widespread alveolar abnormality with: ① Previous or concurrent diagnosis of IPF or a characterized CTD-fILD; ② Acute worsening or development of dyspnea typically 1 month duration; ③ CT with new bilateral ground-glass opacity and/or consolidation superimposed on a background pattern consistent with UIP or possible UIP pattern; ④ Deterioration not fully explained by cardiac failure or fluid overload [3]. All baseline and follow-up clinical data and chest imaging findings were obtained from hospital medical records. Vital status was obtained from medical records or telephone interview. Baseline clinical variables were obtained at the time of AE. The pulmonary function tests (PFTs) were conducted up to 1 month prior or after the initial diagnosis as AE-PF.
HRCT scanning
Chest HRCT examination was performed within 24 h of the diagnosis of respiratory failure with 1.0–1.5 mm thick sections with appropriate window settings (window width: 1600, window level: − 600). The findings were blindly reviewed and scored by two senior radiologists without any knowledge of clinical data. The images were assessed for the presence and extent of ground glass opacity, consolidation, traction bronchiectasis, reticulation, honeycombing and emphysema. The overall extent of abnormalities was determined for per lung using a 4-point scale (0 = no involvement, 1 = 1–25% involvement, 2 = 26–50% involvement, 3 = 51–75% involvement, and 4 = 76–100% involvement) according to the published studies [31, 32]. The HRCT of each case was evaluated as UIP pattern and possible UIP (P-UIP) pattern. Those with an inconsistent with UIP pattern were excluded from this study [1, 33].
Statistical analysis
Data are presented as mean ± standard deviation (SD) for continuous variables or percentages for categorical variables. Chi-squared and Fisher’s exact tests were used for categorical data, and the unpaired t-test and Kruskal-Wallis test were used for continuous data. A multivariate Cox regression model was used to identify significant variables capable of predicting AE or acting as prognostic factors. Bivariate correlation analysis was used to identify the relationship between total survival time and clinical variables. The incidence of AE was obtained from the Kaplan-Meier (K-M) survival curve by treating AE as the death variable. Survival was also evaluated using the log rank test. P < 0.05 was considered to represent statistical significance. Statistical analyses were performed by using IBM SPSS version 19 (SPSS, Inc., Chicago IL, USA) and Prism version 5 (GraphPad, SanDiego, CA, USA).
Results
Clinical characteristics
The baseline clinical features of subjects with AE-CTD-fILD (n = 70) and AE-IPF (n = 107) were summarized in Table 1. The average ages were similar. Female gender, prior use of corticosteroid and immunosupressants were more common in the CTD-ILD group (p<0.001, p = 0.011 and p<0.001, respectively). Serum albumin (ALB), D-dimer and TLC% pred also differed between the two groups (p<0.001, p = 0.023 and p = 0.012, respectively). Subjects with AE-CTD-ILD were treated with immunosupressants and caspofungin more frequently than AE-IPF subjects after AEs (p<0.001 and p = 0.009, respectively).
AE occurrence in patients with CTD-ILD
The incidence of AE-IPF in patients with idiopathic interstitial pneumonia (IIP) was 3.97–11.06% (6.67%) and the incidence of AE-CTD-fILD in CTD-ILD cases was 1.92–7.89% (5.99%) per year in our single center from January 2010 to December 2016 (Fig. 2A). The difference of AE occurrence between the two groups was not significant (p = 0.526) (Fig. 2B).
In univariate Cox analysis, prior corticosteroids use was the independent risk factor for AE among in subjects with CTDs after ILD diagnosis for 1 year (HR: 0.415, 95% CI: 0.215–802, p = 0.009), and pulmonary arterial hypertension (PAH) was the independent risk factor for AE occurrence in IPF patients (HR: 0.959, 95% CI: 0.921–1.000, p = 0.048) (Table 2). After considering the clinical significance and adjusting other clinical variables, prior corticosteroids and immunosuppressant use, FVC, TLC% pred, PAH and body mass index (BMI) were included in the multivariate Cox analysis. The findings showed only TLC% pred was associated with the occurrences of AE among CTD-ILD patients (HR: 0.668, 95% CI: 0.505–0.938; p:0.018). However, none of above clinical variables could predict the occurrence of AE in AE-IPF group (Table 2).
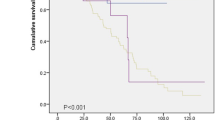
Survival
The median survival time of AE-CTD-ILD patients was 35.0 ± 4.2 days. Kaplan-Merier method analysis revealed that AE-CTD-ILD subjects had a significantly better overall survival than AE-IPF patients (log-rank test, p = 0.029) (Fig. 3A). The differences of cumulative proportion survival (CPS) less than 180 days after AE were not significant between the two groups (Additional file 1: Table S1). Among 70 cases of AE-CTD-ILD, 25 (35.7%) were primary Sjogren syndrome (PSS), 16 (22.9%) were RA, 6 (8.6%) were mixed connective tissue disease (MCTD), 5 (7.1%) were antineutrophil cytoplasmic antibodyassociated vasculitis (AAV), 8 (11.4%) were PM/DM and 10 (14.3%) were IPAF (Fig. 3B). There was no difference in survival among different CTDs subgroups (log-rank, p = 0.353) (Fig. 3C).
Prognostic factors
Bivariate correlation analysis demonstrated that overall survival time was negatively correlated with WBC counts, LDH, and CT score (r = − 0.323, p = 0.006; r = − 0.296, p = 0.013 and r = − 0.252, p = 0.035), respectively, and positively correlated to PaO2/FiO2 (r = 0.407, < 0.001) in AE-CTD-ILD group. In AE-IPF group, the findings were similar to CTD-ILD patients except for a negative correlation with the maximal dosage of corticosteroid and no correlation with PaO2/FiO2 (Table 3).
The univariate analysis showed that WBC count, CRP, LDH, ALB, D-dimmer, PaO2/FiO2, CT score, treatment with MV, maximal dosage of methylprednisolone, immunosuppressant, immunoglobulin, co-trimoxazole and caspofungin for AE were associated with survival in all 177 cases (all p<0.05, respectively), while only WBC count, PaO2/FiO2 and CT score were the independent risk factors for survival in these patients (HR 1.051, 95%CI 1.013–1.090, p = 0.001; HR 0.991, 95%CI 0.987–0.994, p<0.001 and HR 1.337, 95%CI 1.063–1.682, p<0.013, respectively) (Table 4). As shown in Table 5, similar to all 177 patients, WBC count, PaO2/FiO2 ratio and CT score in AE-IPF group were also the independent risk factors for survival by a multivariate Cox regression analysis (HR 1.067, 95%CI 1.028–1.108, p = 0.001; HR 0.993, 95%CI 0.989–0.998, p = 0.002 and HR 1.531, 95%CI 1.170–2.002, p = 0.002, respectively).
In AE-CTD-ILD group, WBC counts, LDH, PaO2/FiO2 ratio, treatment with MV, immunoglobulin, and caspofungin for AE were associated with the survival by univariate Cox model (all p<0.05, respectively). After considering the clinical significance and adjusting other clinical variables, WBC count, PaO2/FiO2, CT score and maximal dosage of methylprednisolone were included in multivariate Cox model. The findings showed that WBC count and PaO2/FiO2 ratio were the independent prognostic factors for survival by a multivariate Cox regression analysis (HR 1.074, 95%CI 1.004–1.150, p = 0.038 and HR 0.989, 95%CI 0.984–0.994, p<0.001, respectively) (Table 5).
Discussion
The present study retrospectively compared the clinical, chest imaging and follow-up data between 70 subjects with AE-CTD-ILD and 107 subjects with AE-IPF. The clinical features of patients with AE-CTD-ILD differed from those of AE-IPF. Subjects with AE-CTD-ILD had a better survival than AE-IPF patients, while the survival of AE cases in the CTD subgroups didn’t show any difference. WBC count and PO2/FiO2 ratio could predict the survival of AE-CTD-ILD patients independently.
Recently, the definition and diagnostic criteria of AE-IPF have been updated. IPF patients manifesting an acute, clinically significant respiratory deterioration characterized by evidence of new widespread alveolar abnormality on chest imaging or histopathology should be considered as having an AE [3]. Data showed that a potential infectious aetiology is up to one-third of patients with ILD and acute respiratory decline [22]. The updated criteria of AE-IPF do not exclude respiratory infection [3]. The causes of acute lung injure (ALI) including infection, aspiration, drugs and surgery may lead to events that are indistinguishable from idiopathic AE of IPF [3]. These events are further classified into “triggered” (ie, postprocedure, drug toxicity, infection, aspiration) or “idiopathic” (ie, no inciting event identified) AEs in the updated guideline [3].
The published reports about AE in patients with CTD-ILD have been relatively small series and show inconsistent results. The incidence of AE in RA-UIP was about 11.1–20% [18, 19]. The mild elevation of mean PAH was very common in CTD-ILD patients [34]. Suda et al. suggested that the clinical characteristics of AE in CTD-ILD were similar to those of IPF with high mortality [19]. Park and Suda reported that AE occurred mostly with RA-UIP with poor outcome [18, 19]. Song and Huie suggested that CTD-UIP group was younger, included more women and nonsmokers, and showed better survival than the IPF-UIP group [7, 22]. The current study was based on the updated criteria of Collard about AE-IPF [3] and comprised a 177 cases of AE patients with IPF and CTD-ILD. The findings showed that the clinical features, treatment and prognosis of patients with AE-CTD-ILD differed from those with AE-IPF and published reports [7, 8, 18, 35].
The incidence of AE in IPF patients has been reported in a number of studies. Due to different definition and diagnostic criteria of AE, AE incidence in IPF varied greatly, estimated to be in the range of 4–20% per year [4,5,6,7,8]. In patients with CTD-ILD, Suda et al. revealed an overall AE incidence of 7.2% and a 1-year incidence of 1.25% [19]. Compared with AE-IPF, the incidence of AE in CTD-ILD seemed to be lower [19]. In our single center, AE incidence was about 5% in all new ILD patients about 6% per year in those with CTD-ILD. AE incidence of fILD patients in our study is similar to the published reports of IPF population in other countries [4,5,6,7,8]. IPF patients with physiologically and functionally advanced disease are thought to be at higher risk of AE [3]. However, little is known about the causative factors of AE in patients with CTD-ILD. In CTD-ILD patients, age and SS overlapped with PM/DM were the independent risk factors for AE [19, 36]. In our study, AE occurrence in CTD-ILD patients within 1 year after ILD diagnosis was weakly associated with TLC% pred, likely due to the limited sample size. A larger sample prospective study of AE-CTD-ILD is therefore needed to clarify this complication.
The poor survival of patients with AE-IPF is a great challenge. The MST of these patients was about 3–4 months [6, 7]. Nearly half of patients with AE-IPF died in the hospital, and patients with both definite AE-IPF and suspected AE-IPF had a similar clinical outcome [6, 7, 22]. The published reports on AE-CTD-ILD differ from AE-IPF and include a relatively small number of cases. Tachikawa et al. concluded that the survival of AE-CTD-ILD was better than that of advanced IPF (90-day mortality at 33% vs. 69%) [37]. Huie et al. suggested that IPF patients had a lower rate of survival as compared with patients with non-IPF fibrotic disease after acute respiratory decline [22]. Suda et al. suggested that AE incidence in CTD-ILD was similar to that of IPF with poor prognosis [19]. In our cohort study, the overall survival was significant better in subjects with AE-CTD-ILD than AE-IPF cases, despite their similar short-term survival. Usui Y.et al. reported that systemic inflammatory response (SIRS) was the most significant predictor for in-hospital mortality in patients with AE of chronic fILD including IPF and non-IPF [38]. Infection at AE was thought to determine the short-term survival in these patients. In current study, the inflammatory indicators, WBC counts, CRP, LDH and CT scores didn’t show any difference between the two groups, implying that infection was similar between the two groups. So, the similar short-term survival may be associated with the similar infection in the two groups [38]. The better overall survival in CTD-ILD patients may be associated with the favorable response to corticosteroids treatment [23]. Yoshimura et al. also suggested that the diagnosis of IPF might predict a favorable prognosis in patients with chronic fILD [39], thereby a better survival in AE-CTD-fILD. The lack of difference in overall survival among different CTD subgroups may be due to the small sample size in our study.
A number of factors including lower baseline FVC and DLCO, extensive CT abnormalities and CT pattern, worse oxygenation, higher bronchoalveolar lavage neutrophil and lymphocyte percentages at the time of AE were reported to be associated with the survival of patients with AE-IPF [7, 8, 15, 32, 40, 41]. Literature also indicated that described age, TLC, honeycombing score and higher initial MPAP were the independent prognostic factors in AE-CTD-ILD [34, 35]. In current study, WBC count and PO2/FiO2 ratio could predict the survival of patients with AE-CTD-ILD after adjusting for other clinical variables. Now, infection has been regarded as one of the causes for AE in IPF patients [3]. WBC is a non-specific inflammation marker of infection. Infection with elevated WBC counts was correlated with the poor clinical outcomes in AE-CTD-fILD patients in our study. Low PO2/FiO2 ratio could account for the severity of hypoxia and clinical condition at AE, which indicated patients with more severe condition when AE occurred would have worse prognosis. The different risk factors of prognosis in the two groups may be due to the underlying diseases and the conditions at AE occurrence.
Although there is no effective therapy for AE-IPF, supportive care including supplemental oxygen and palliation of symptoms are regarded as important treatment modality. The use of MV is controversial [1, 3]. Corticosteroids are recommended but with somewhat low-quality evidence [1, 3]. There is no consensus on the dosage and course of therapy. A slight survival benefit was reported for the treatment of AE-IPF combining cyclosporine with systemic glucocorticoids as compared to that without cyclosporine in a small retrospective study [41]. The therapy of steroids combined with cyclophosphosphamide for patients with AE-CTD-ILD appeared better than those without any immunosuppressant [7, 42]. However, there is no evidence supporting the benefit of a specific immunosuppressant. Addition of intravenous thrombomodulin and polymyxin B immobilized fiber column perfusion to conventional treatment was shown to improve the survival in patients with AE-IPF [39, 41]. Rituximab combined with plasma exchange and intravenous immunoglobulin also showed certain benefits [42]. All patients in our study used corticosteroids after AEs, but the maximal dosage was different for each case according to the clinical severity and the extent of damage on chest CT. The overall survival time was not correlated with the maximal dosage of methylprednisolone both in CTD-ILD and IPF patients when AE occurred. So, we speculated that the higher dosage of corticosteroids would not be beneficial for AE patients, and the clinical outcomes of AE patients with pulmonary fibrosis mainly depend on the underlying clinical conditions and the extent of lung injury at AE.
The current study has some limitations. It was designed retrospectively and the stable cases of pulmonary fibrosis were not available as controls. Histological evidence was not available for the diagnosis of pulmonary fibrosis. It was unable to get the exact number of cases with IPF patients. A definite cause of death was not available for each case, although most patients died of AE. A prospective, multicenter, multinational study of a larger cases cohort would be helpful to provide enough data for the epidemiology in AE-CTD-ILD.
Conclusions
In summary, the current study demonstrated that the clinical characteristics of patients with AE-CTD-ILD differed from those of AE-IPF. The incidence of AE-CTD-ILD was similar to that of AE-IPF. Subjects with AE-CTD-fILD had a better prognosis than those with AE-IPF. However, the survival of AE patients in each group was identical. WBC count and PO2/FiO2 were the independent predictors for the survival in patients with CTD-fILD. A higher dosage of corticosteroids would not be beneficial for the survival of fILD patien.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- AE:
-
Acute exacerbation
- ALB:
-
Albumin
- BNP:
-
B-type natriuretic peptide
- CRP:
-
C reactive protein
- CTDs:
-
Connective tissue diseases
- DLCO:
-
Diffusing capacity of the lung for CO2
- ESR:
-
Erythrocyte sedimentation rate
- fILD:
-
Fibrosing interstitial lung disease
- FVC:
-
Forced vital capacity
- HRCT:
-
High resolution computed tomography
- IPF:
-
Idiopathic pulmonary fibrosis
- LDH:
-
Lactate dehydrogenase
- MV:
-
Mechanical ventilation
- NAC:
-
N-acetylcysteine
- PAH:
-
Pulmonary arterial hypertension; BMI: body mass index
- PaO2/FiO2 :
-
Oxygenation index
- P-UIP:
-
Possible UIP
- TLC:
-
Total lung capacity
- UIP:
-
Usual interstitial pneumonia
- WBC:
-
White blood cell
References
Raghu G, Collard HR, Egan JJ, Martinez FJ, Behr J, Brown KK, Colby TV, Cordier JF, Flaherty KR, Lasky JA. An official ATS/ERS/JRS/ALAT statement: idiopathic pulmonary fibrosis: evidence-based guidelines for diagnosis and management. American Journal of Respiratory & Critical Care Medicine. 2011;183(6):788–824.
Travis WD, Costabel U, Hansell DM, King TE Jr, Lynch DA, Nicholson AG, Ryerson CJ, Ryu JH, Selman M, Wells AU, et al. An official American Thoracic Society/European Respiratory Society statement: update of the international multidisciplinary classification of the idiopathic interstitial pneumonias. Am J Respir Crit Care Med. 2013;188(6):733–48.
Collard HR, Ryerson CJ, Corte TJ, Jenkins G, Kondoh Y, Lederer DJ, Lee JS, Maher TM, Wells AU, Antoniou KM. Acute Exacerbation of Idiopathic Pulmonary Fibrosis. An International Working Group Report. American Journal of Respiratory & Critical Care Medicine. 2016;194(3).
Ryerson CJ, Cottin V, Brown KK, Collard HR. Acute exacerbation of idiopathic pulmonary fibrosis: shifting the paradigm. Eur Respir J. 2015;46(2):512–20.
Atkins CP, Loke YK, Wilson AM. S16 outcomes in idiopathic pulmonary fibrosis: a meta-analysis from placebo controlled trials. Respir Med. 2014;108(2):376–87.
Collard HR, Yow E, Richeldi L, Anstrom KJ, Glazer C: Suspected acute exacerbation of idiopathic pulmonary fibrosis as an outcome measure in clinical trials. Respiratory Research,14,1(2013-07-13) 2013, 14(1):1–7.
Song JW, Hong SB, Lim CM, Koh Y, Kim DS. Acute exacerbation of idiopathic pulmonary fibrosis: incidence, risk factors and outcome. Eur Respir J. 2011;37(2):356.
Simon-Blancal V, Freynet O, Nunes H, Bouvry D, Naggara N, Brillet PY, Denis D, Cohen Y, Vincent F, Valeyre D, et al. Acute exacerbation of idiopathic pulmonary fibrosis: outcome and prognostic factors. Respiration; international review of thoracic diseases. 2012;83(1):28–35.
Natsuizaka M, Chiba H, Kuronuma K, Otsuka M, Kudo K, Mori M, Bando M, Sugiyama Y, Takahashi H. Epidemiologic survey of Japanese patients with idiopathic pulmonary fibrosis and investigation of ethnic differences. Am J Respir Crit Care Med. 2014;190(7):773–9.
Collard HR, Moore BB, Flaherty KR, Brown KK, Kaner RJ, King TE Jr, Lasky JA, Loyd JE, Noth I, Olman MA, et al. Acute exacerbations of idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2007;176(7):636–43.
Johannson KA, Vittinghoff E, Lee K, Balmes JR, Ji W, Kaplan GG, Kim DS, Collard HR. Acute exacerbation of idiopathic pulmonary fibrosis associated with air pollution exposure. Eur Respir J. 2014;43(4):1124–31.
Mura M, Porretta MA, Bargagli E, Sergiacomi G, Zompatori M, Sverzellati N, Taglieri A, Mezzasalma F, Rottoli P, Saltini C, et al. Predicting survival in newly diagnosed idiopathic pulmonary fibrosis: a 3-year prospective study. Eur Respir J. 2012;40(1):101–9.
Kondoh Y, Taniguchi H, Ebina M, Azuma A, Ogura T, Taguchi Y, Suga M, Takahashi H, Nakata K, Sugiyama Y, et al. Risk factors for acute exacerbation of idiopathic pulmonary fibrosis--extended analysis of pirfenidone trial in Japan. Respir Investig. 2015;53(6):271–8.
Reichmann WM, Yu YF, Macaulay D, Wu EQ, Nathan SD. Change in forced vital capacity and associated subsequent outcomes in patients with newly diagnosed idiopathic pulmonary fibrosis. Bmc Pulmonary Medicine. 2015;15(1):167.
Kim DS, Park JH, Park BK, Lee JS, Nicholson AG, Colby T. Acute exacerbation of idiopathic pulmonary fibrosis: frequency and clinical features. Eur Respir J. 2006;27(1):143–50.
Ohshimo S, Ishikawa N, Horimasu Y, Hattori N, Hirohashi N, Tanigawa K, Kohno N, Bonella F, Guzman J, Costabel U. Baseline KL-6 predicts increased risk for acute exacerbation of idiopathic pulmonary fibrosis. Respir Med. 2014;108(7):1031–9.
Cao M, Swigris JJ, Xin W, Min C, Qiu Y, Mei H, Xiao Y, Cai H. Plasma Leptin is elevated in acute exacerbation of idiopathic pulmonary fibrosis. Mediat Inflamm. 2016;2016(7):1–7.
Park IN, Dong SK, Shim TS, Lim CM, Sang DL, Koh Y, Kim WS, Kim WD, Jang SJ, Colby TV. Acute exacerbation of interstitial pneumonia other than idiopathic pulmonary fibrosis. Chest. 2007;132(1):214–20.
Suda T, Kaida Y, Nakamura Y, Enomoto N, Fujisawa T, Imokawa S, Hashizume H, Naito T, Dai H, Takehara Y. Acute exacerbation of interstitial pneumonia associated with collagen vascular diseases. Respir Med. 2009;103(6):846–53.
Miyazaki Y, Tateishi T, Akashi T, Ohtani Y, Inase N, Yoshizawa Y. Clinical predictors and histologic appearance of acute exacerbations in chronic hypersensitivity pneumonitis. Chest. 2008;134(6):1265.
Olson AL, Huie TJ, Groshong SD, Cosgrove GP, Janssen WJ, Schwarz MI, Brown KK, Frankel SK. Acute exacerbations of fibrotic hypersensitivity pneumonitis: a case series. Chest. 2008;134(4):844–50.
Huie TJ, Olson AL, Cosgrove GP, Janssen WJ, Lara AR, Lynch DA, Groshong SD, Moss M, Schwarz MI, Brown KK. A detailed evaluation of acute respiratory decline in patients with fibrotic lung disease: Aetiology and outcomes. Respirology. 2010;15(6):909–17.
Toyoda Y, Hanibuchi M, Kishi J, Kawano H, Morizumi S, Sato S, Kondo M, Takikura T, Tezuka T, Goto H. Clinical features and outcome of acute exacerbation of interstitial pneumonia associated with connective tissue disease. Journal of Medical Investigation. 2016;63(3–4):294.
Arnett FC, Edworthy SM, Bloch DA, DJ MS, Fries JF, Cooper NS, Healey LA, Kaplan SR, Liang MH, Luthra HS. The American rheumatism association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988;31(3):315–24.
Aletaha D, Neogi T, Silman AJ, Funovits J, Felson DT, Bingham CO 3rd, Birnbaum NS, Burmester GR, Bykerk VP, Cohen MD, et al. Rheumatoid arthritis classification criteria: an American College of Rheumatology/European league against rheumatism collaborative initiative. Arthritis Rheum. 2010;62(9):2569–81.
Bohan A, Peter JB. Polymyositis and dermatomyositis (first of two parts). N Engl J Med. 1975;292(7):344–7.
Tan EM, Cohen AS, Fries JF, Masi AT, McShane DJ, Rothfield NF, Schaller JG, Talal N, Winchester RJ. The 1982 revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1982;25(11):1271–7.
Hochberg MC. Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1997;40(9):1725.
Fujibayashi T, Sugai S, Miyasaka N, Hayashi Y, Tsubota K. Revised Japanese criteria for Sjogren's syndrome (1999): availability and validity. Mod Rheumatol. 2004;14(6):425–34.
Fischer A, Antoniou KM, Brown KK, Cadranel J, Corte TJ, du Bois RM, Lee JS, Leslie KO, Lynch DA, Matteson EL, et al. An official European Respiratory Society/American Thoracic Society research statement: interstitial pneumonia with autoimmune features. Eur Respir J. 2015;46(4):976–87.
Lynch DA, Godwin JD, Safrin S, Starko KM, Hormel P, Brown KK, Raghu G, King TE Jr, Bradford WZ, Schwartz DA, et al. High-resolution computed tomography in idiopathic pulmonary fibrosis: diagnosis and prognosis. Am J Respir Crit Care Med. 2005;172(4):488–93.
Akira M, Kozuka T, Yamamoto S, Sakatani M. Computed tomography findings in acute exacerbation of idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2008;178(4):372–8.
Raghu G, Remy-Jardin M, Myers JL, Richeldi L, Ryerson CJ, Lederer DJ, Behr J, Cottin V, Danoff SK, Morell F, Flaherty KR, Wells A, Martinez FJ, Azuma A, Bice TJ, Bouros D, Brown KK, Collard HR, Duggal A, Galvin L, Inoue Y, Jenkins RG, Johkoh T, Kazerooni EA, Kitaichi M, Knight SL, Mansour G, Nicholson AG, Pipavath SNJ, Buendía-Roldán I, Selman M, Travis WD, Walsh S, Wilson KC; American Thoracic Society, European Respiratory Society, Japanese Respiratory Society, and Latin American Thoracic Society. Diagnosis of Idiopathic Pulmonary Fibrosis. An Official ATS/ERS/JRS/ALAT Clinical Practice Guideline. Am J Respir Crit Care Med. 2018;198(5):e44–68.
Takahashi K, Taniguchi H, Ando M, Sakamoto K, Kondoh Y, Watanabe N, Kimura T, Kataoka K, Suzuki A, Ito S, et al. Mean pulmonary arterial pressure as a prognostic indicator in connective tissue disease associated with interstitial lung disease: a retrospective cohort study. BMC Pulm Med. 2016;16(1):55.
Jin WS, Do KH, Kim MY, Jang SJ, Colby TV, Dong SK. Pathologic and radiologic differences between idiopathic and collagen vascular disease-related usual interstitial pneumonia. Chest. 2009;136(1):23–30.
Tomiyama F, Watanabe R, Ishii T, Kamogawa Y, Fujita Y, Shirota Y, Sugimura K, Fujii H, Harigae H. High prevalence of acute exacerbation of interstitial lung disease in Japanese patients with systemic sclerosis. Tohoku J Exp Med. 2016;239(4):297.
Tachikawa R, Tomii K, Ueda H, Nagata K, Nanjo S, Sakurai A, Otsuka K, Kaji R, Hayashi M, Katakami N, et al. Clinical features and outcome of acute exacerbation of interstitial pneumonia: collagen vascular diseases-related versus idiopathic. Respiration; international review of thoracic diseases. 2012;83(1):20–7.
Usui Y, Kaga A, Sakai F, Shiono A, Komiyama K, Hagiwara K, Kanazawa M. A cohort study of mortality predictors in patients with acute exacerbation of chronic fibrosing interstitial pneumonia. BMJ Open. 2013:3(7).
Abe S, Azuma A, Mukae H, Ogura T, Taniguchi H, Bando M, Sugiyama Y. Polymyxin B-immobilized fiber column (PMX) treatment for idiopathic pulmonary fibrosis with acute exacerbation: a multicenter retrospective analysis. Intern Med. 2012;51(12):1487–91.
Sakamoto S, Homma S, Miyamoto A, Kurosaki A, Fujii T, Yoshimura K. Cyclosporin a in the treatment of acute exacerbation of idiopathic pulmonary fibrosis. Intern Med. 2010;49(2):109–15.
Kataoka K, Taniguchi H, Kondoh Y, Nishiyama O, Kimura T, Matsuda T, Yokoyama T, Sakamoto K, Ando M. Recombinant human Thrombomodulin in acute exacerbation of idiopathic pulmonary fibrosis. Chest. 2015;148(2):436–43.
Donahoe M, Valentine VG, Chien N, Gibson KF, Raval JS, Saul M, Xue J, Zhang Y, Duncan SR. Autoantibody-targeted treatments for acute exacerbations of idiopathic pulmonary fibrosis. PLoS One. 2015;10(6):e0127771.
Acknowledgements
We would like to thank the subjects and their families who participated in this study and all people who participated in the clinical management. We also would like to thank Prof. Kevin K, Brown (Department of medicine, National Jewish Health, USA) and Prof. Yin Chen (Department of Pharmacology, University of Arizona, Tucson, USA) for reviewing and editing this manuscript. The authors were fully responsible for all content and editorial decisions, were involved at all stages of development and provided their approval on the final version.
Funding
This study was partially supported by the National Natural Science Foundation of China (Grant 81200049 and Grant 81670059 to Dr. Cao) and the Nanjing Medical Science and Technique Development Foundation (Grant QRX17005 to Dr. Cao) in the design of the study and collection, analysis, and interpretation of data and in writing the manuscript.
Author information
Authors and Affiliations
Contributions
MC. conceived and designed the study, had full access to all of the data, and took responsibility for the integrity of the data and the accuracy of the data analysis. MC and JS analyzed and contributed to the statistic of the data. MC, JS, XQ, Dandan Wang, YX, and HC contributed to the collection clinical data. Dongmei Wang and YW contributed to evaluating the chest HRCT; MC prepared and reviewed the manuscript. All other authors revised the manuscript and approved the final draft.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study was approved by the Ethics Committee of Nanjing Drum Tower Hospital (NO.2016–160-01) in compliance with the Helsinki Declaration along with established written informed consent. This research was carried out in accordance with the approved guidelines. Written informed consent was obtained from all participants before inclusion.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Additional file 1: Table S1.
Comparison of the CPS at different time after AE.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Cao, M., Sheng, J., Qiu, X. et al. Acute exacerbations of fibrosing interstitial lung disease associated with connective tissue diseases: a population-based study. BMC Pulm Med 19, 215 (2019). https://doi.org/10.1186/s12890-019-0960-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12890-019-0960-1