Abstract
Background
Any immunological mechanisms induced by influenza virus could cause severe secondary bacterial superinfection such as those by Streptococcus pyogenes [group A streptococcus (GAS)], Streptococcus pneumoniae or Staphylococcus aureus. Over recent years, the frequency of pleural empyema has increased in children with influenza infection.
We present a severe case of acute empyema caused by S.pyogenes after influenza A infection.
Case presentation
A previously healthy 39-year old woman was diagnosed as influenza A and received oral Oseltamivir 75 mg twice daily for 5 days. She had no vaccination of influenza A. Although her influenza A infection improved, she complained of fever and cough to our institute. Chest radiography showed encapsulated pleural effusion of the left lung and pleural effusion which was consistent with acute empyema. Then, she was diagnosed as having acute empyema and was admitted to our institute. Streptococcus pyogenes was identified by pleural fluid culture on day 4. thus, MNZ was changed to clindamycin (CLDM) 600 mg three times a day. While thoracic drainage with intrapleural urokinase and combination antibiotic therapy of ceftriaxone and CLDM were performed, her general condition and chest radiographic findings were not improved. She received video-assisted thoracic debridement on day 10. After the operation, the antibiotic therapy was changed to ABPC 6 g daily iv. Due to good clinical course, the antibiotic therapy was switched to oral amoxicillin 500 mg three times daily on day 28. Then, she was discharged.
Conclusion
Influenza A virus infection could lead to severe GAS infection, while the latter can occur in otherwise healthy individual as well. Physician must consider the possibility of severe GAS infection after influenza A infection.
Similar content being viewed by others
Background
Streptococcus pyogenes is a common cause of pharyngitis, deep neck abscesses, and skin and soft tissue infections [1]. It can occasionally cause toxic shock syndrome, severe pneumonia or meningitis, resulting in a high mortality of up to 20%. Also, it has been reported that S.pyogenes is a common bacterial pathogen of viral co-infection with varicella [2], Epstein-Barr virus [3], and influenza A virus (IAV) [4].
We present a severe case of acute empyema caused by S.pyogenes after influenza A infection.
Case presentation
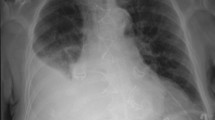
A previously healthy 39-year old woman was diagnosed as having influenza A virus infection by rapid influenza diagnostic test (RIDT) in a clinic, and received oral Oseltamivir 75 mg twice daily for 5 days. The clinical course is shown in Fig. 1. While influenza like illness was improved once, fever and cough recurred on day 7 after the onset of flu. At this time, RIDT was performed, showing that the result was negative at the clinic. She complained of fever, cough and the left chest pain and presented to our institute on day 14 after the onset of the flu. RIDT was performed and the result was again negative. The data representing the inflammatory reactions were elevated (Table 1) and the chest radiography showed encapsulated pleural effusion of the left lung (Figs 2 and 3). Pleural fluid from the initial thoracentesis was pus, and showed an increase in cell counts with neutrophil predominance. Thus, she was diagnosed as having acute empyema. Thoracic drainage with intrapleural urokinase and antibiotic therapy of ceftriaxone (CTRX) 2 g and metronidazole (MNZ) were started. Pleural fluid cultures from the initial thoracentesis grew Streptococcus pyogenes on day 4. Thus, MNZ was changed to clindamycin (CLDM) 600 mg three times a day. On day 10 after the antibiotic therapy with thoracic drainage was started, she received video-assisted thoracic debridement due to worsening of the patients’ general condition and infiltrations by chest radiography. After the operation, the patient’s condition improved and antibiotic de-escalation was performed to ampicillin 6 g daily iv. Due to patient’ good condition, antibiotic therapy was switched to oral amoxicillin 500 mg three times daily on day 28. Then, she was discharged. During this six months, recurrence of the infection was not observed.
Discussion
The group A streptococcus (GAS) can colonize humans asymptomatically or often causes infections of the pharynx and the skin. It could cause invasive infections including bacteremia, pneumonia, and toxic shock syndrome (TSS), resulting in the high mortality of up to 20% [1].
Over recent years, the frequency of pleural empyema has increased in children with influenza A infection [5,6,7]. This tendency could be explained by influenza virus induced-immunological mechanism. Barthelemy documented that influenza A virus-induced release of interleukin (IL)-10 inhibited the antimicrobial activities of invariant natural killer T cells during invasive pneumococcal superinfection [8]. Robinson reported that influenza A virus inhibits bacterial-induced IL-1β production and impairs host defense against bacterial infection [9]. These cytokines by influenza virus and regulatory T cell could play an important role in the immunological mechanisms underlying influenza virus-GAS superinfection. Some reported that secondary bacterial superinfection could occur in one week after influenza infection [10, 11]. In our case, respiratory symptoms surfaced in 7 days after influenza A infection as previous reports. It is consistent with clinical course of secondary bacterial superinfection after influenza infection.
Previous report documented that vaccination of influenza A virus could prevent secondary GAS infection. In one study, mice were vaccinated against influenza virus and subsequently infected with an H1N1 virus 2 days prior to infection with GAS [12, 13]. The mortality of mice vaccinated and unvaccinated with IAV were 15 and 78%, respectively. Similarly, during the observation period of 1993–2001, the incidence of S.pyogenes-varicella coinfections reduced from 27% of pre-varicella vaccination era to only 2% after the 1995 when the varicella vaccine became available [14].
These results suggest that vaccination of IAV could prevent secondary GAS infection. Our patient had not received IAV vaccination, and thus, severe GAS infection could occur after influenza A infection. Vaccination of IAV is important not only for preventing influenza infection, but also for secondary infections after flu such as pneumococcal, or GAS infection.
As for treatments, the patient empirically received combination therapy of CTRX and MNZ. S. pyogenes has the cell wall M-protein which inhibits complement activation and decreases phagocytosis. Furthermore, S.pyogenes produce exotoxins that are capable of direct T-cell activation without antigen processing by antigen-processing cells, resulting in cytokine storm. Clindamycin (CLDM) would be effective to deactivate M-protein and these exotoxins, resulting in a favorable outcome [15, 16]. Some documented that efficacy and rationale for use of intravenous immunoglobulin therapy (IVIG) in streptococcal TSS [17, 18]. It has been reported that several mechanisms were thought to be opsonization of GAS for phagocytic killing, neutralization of streptococcal toxins, inhibition of T cell proliferation, and inhibition of inflammatory cytokines such as TNF-alpha and IL-6 [19, 20]. While its efficacy has been reported, IVIG is not standardized evidence-based in the treatment for invasive GAS infections.
In this case, combination therapy with CLDM as initial treatment or IVIG should have been considered for prevention of exacerbation of patient’s condition.
In conclusion, influenza A virus infection could lead to severe GAS infection, while the latter can occur in otherwise healthy individual as well. Physician must consider the possibility of severe GAS infection after influenza A infection. Also, vaccination of IAV could prevent secondary severe GAS infection.
Abbreviations
- CLDM:
-
Clindamycin
- CTRX:
-
Ceftriaxone
- GAS:
-
Group A streptococcus
- IAV:
-
Influenza A virus
- IL:
-
Interleukin
- IVIG:
-
Intravenous immunoglobulin therapy
- MNZ:
-
Metronidazole
- RIDT:
-
Rapid influenza diagnostic test
- TSS:
-
Toxic shock syndrome
References
Carapetis JR, Steer AC, Mulholland EK, Weber M. The global burden of group a streptoccal diseases. Lanset Infect Dis. 2005;5:685–94.
Aebi C, Ahmed A, Ramilo O. Bacterial complications of primary varicella in children. ClinInfectDis. 1996;23:698–705.
Rush MC, Simon MW. Occurrence of Epstein-Barr virus illness in children diagnosed with group a streptococcal pharyngitis. Clin Pediatr. 2003;42:417–20.
Jean C, Louie JK, Glaser CA, Harriman K, Hacker JK, Aranki F, et al. Invasive group a streptococcal infection concurrent with 2009 H1N1 influenza. Clin Infec Dis. 2010;50:e59–62.
Ampofo K, Herbener A, Blaschke AJ, Heyrend C, Poritz M, Korgenski K, et al. Association of 2009 pandemic influenza a (H1N1) infection and increased hospitalization with parapneumonic empyema in children in Utah. Pediatr Infect Dis J. 2010;29:905–9.
Krenke K, Urbankowska E, Urbankowski T, Lange J, Kulus M. Clinical characteristics of 323 children with parapneumonic pleural effusion and pleural empyema due to community acquired pneumonia. J Infect Chemother. 2016;22:292–7.
Deceuninck G, Quach C, Panagopoulos M, Thibeault R, Côté-Boileau T, Tapiéro B, et al. Pediatric pleural empyema in the province of Quebec: analysis of a 10-fold increase between 1990 and 2007. J Pediatric Infect Dis Soc. 2014;3:119–26.
Barthelemy A, Ivanov S, Fontaine J, Soulard D, Bouabe H, Paget C, et al. Influenza a virus-induced release of interleukin-10 inhibits the anti-microbial activities of invariant natural killer T cells during invasive pneumococcal superinfection. Mucosal Immunol. 2017;10:460–9.
Robinson KM, Choi SM, McHugh KJ, Mandalapu S, Enelow RI, Kolls JK, et al. Influenza a exacerbates Staphylococcus aureus pneumonia by attenuating IL-1β production in mice. J Immunol. 2013;191:5153–9.
Ochi F, Tauchi H, Jogamoto T, Miura H, Moritani T, Nagai K, et al. Sepsis and pleural empyema caused by streptococcus pyogenes after influenza a virus infection. Case reports in pediatrics. 2018;2018:4509847. https://doi.org/10.1155/2018/4509847 eCollection 2018.
Robinson KM, Kolls JK, Alcorn JF. The immunology of influenza virus-associated bacterial pneumonia. Curr Opin Immunol. 2015;34:59–67.
Okamoto S, Kawabata S, Fujitaka H, Uehira T, Okuno Y, Hamada S. Vaccination with formalin-inactivated influenza vaccine protects mice against lethal influenza Streptococcus pyogenes superinfection. Vaccine. 2004;22:2887–93.
Chaussee MS, Sandbulte HR, Schuneman MJ, Depaula FP, Addengast LA, Schlenker EH, et al. (2011). In activated and live, attenuated influenza vaccines protect mice against influenza: Streptococcus pyogenes super- infections. Vaccine 2011;29:3773-3781.
Patel RA, Binns HJ, Shulman ST. Reduction in pediatric hospitalizations for varicella-related invasive group a streptococcal infections in the varicella vaccine era. J Pediatr. 2004;144:68–74.
Carapetis JR, Jacoby P, Carville K, Ang SJ, Curtis N, Andrews R. Effectiveness of clindamycin and intravenous immunoglobulin, and risk of disease in contacts, in invasive group a streptococcal infections. CID. 2014;59:358.
Stevens DL, Gibbons AE, Bergstrom R, Winn V. The eagle effect revisited: efficacy of clindamycin, erythromycin, and penicillin in the treatment of streptococcal myositis. J Infect Dis. 1988;158:23.
Darenberg J, Ihendyane N, Sjölin J, Aufwerber E, Haidl S, Follin P, et al. Intravenous immunoglobulin G therapy in streptococcal toxic shock syndrome: a European randomized, double-blind, placebo-controlled trial. Clin Infect Dis. 2003;37:333–40.
Linnér A, Darenberg J, Sjölin J, Henriques-Normark B, Norrby-Teglund A. Clinical efficacy of polyspecific intravenous immunoglobulin therapy in patients with streptococcal toxic shock syndrome: a comparative observational study. Clin Infect Dis. 2014;59:851–7.
Kaul R, McGeer A, Norrby-Teglund A, Kotb M, Schwartz B, O'Rourke K, et al. Intravenous immunoglobulin therapy for streptococcal toxic shock syndrome--a comparative observational study The Canadian Streptococcal Study Group. Clin Infect Dis. 1999;28:800.
Norrby-Teglund A, Kaul R, Low DE, McGeer A, Newton DW, Andersson J, et al. Plasma from patients with severe invasive group a streptococcal infections treated with normal polyspecific IgG inhibits streptococcal superantigen-induced T cell proliferation and cytokine production. J Immunol. 1996;156:3057–64.
Acknowledgments
We are grateful for the diligent and thorough critical reading of our manuscript by Dr. Yoshihiro Ohkuni, Chief Physician, Taiyo and Mr. John Wocher, Executive Vice President and Director, International Affairs/International Patient Services, Kameda Medical Center (Japan).
Funding
None declared.
Availability of data and materials
All the data supporting our findings is contained within the manuscript.
Author information
Authors and Affiliations
Contributions
NA, HW, YK, YY, HM carried out the clinical follow up. NA draft the manuscript. NA, DS and HS performed microbial testing and NA, HW, YK, YY, HM performed laboratory analysis. HK, AS and MH supervised the antibiotic and antiviral therapy. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Written informed consent was obtained from the patient for publication of this report.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Asai, N., Suematsu, H., Sakanashi, D. et al. A severe case of Streptococcal pyogenes empyema following influenza A infection. BMC Pulm Med 19, 25 (2019). https://doi.org/10.1186/s12890-019-0787-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12890-019-0787-9