Abstract
Background
Dietary nitrate supplementation has been shown to decrease the oxygen cost of exercise and prolong exercise tolerance, as measured by sub-maximal exercise endurance distance and time at 85% V̇O2max, in both elite athletes and normal healthy subjects. Patients with chronic obstructive pulmonary disease (COPD) have reduced quality of life and ability to perform activities of daily living attributable to diminished exercise tolerance, and dietary nitrate may be able to ameliorate this.
Methods
We performed a double-blind, computer-randomized placebo control crossover trial at a tertiary Australian hospital to investigate whether dietary nitrate supplementation as beetroot juice (BR) would augment submaximal exercise endurance in individuals with spirometrically confirmed stable moderate COPD. Volunteers underwent an incremental shuttle walk test to determine V̇O2max followed by a test dose of BR to establish safety in the study population. Participants performed an endurance shuttle walk test (ESWT) at 85% V̇O2max after randomization to either a 3 day wash-in of BR (4.8 mmol twice a day) or placebo (nitrate deplete BR), with a final dose on the morning of testing. They then crossed over after 4 day washout. Repeated measures two sided paired t-tests were employed.
Results
35 participants were recruited with 19 completing the trial. In the initial safety phase, we measured systolic blood pressure over four hours post first dose of BR, and found a mean 10 mmHg decrement maximal at 1 hour. One individual developed symptomatic postural hypotension and was excluded. The primary outcomes of ESWT distance and time to fatigue improved by 11% and 6% respectively; however these differences did not achieve statistical significance (p = 0.494 and 0.693 respectively).
Conclusions
Our study does not support a role for routine dietary nitrate supplementation for enhancement of exercise endurance in COPD.
Trial registration
Australia and New Zealand Clinical Trial Register: ACTRN12611001088932
Similar content being viewed by others
Background
Chronic Obstructive Pulmonary Disease (COPD) is a leading cause of morbidity worldwide, with a substantial and increasing economic and social burden: it is estimated that 64 million people are affected worldwide [1,2]. Exercise intolerance and fatigue are major negative contributors to quality of life in individuals with COPD, for whom the activities of daily living may embody a significant exercise challenge [3].
Pharmacological inorganic nitrate (NO3−) supplementation reduces the O2 cost of and enhances high-intensity exercise tolerance in humans [4]. In a placebo-controlled study, dietary nitrate supplementation with beetroot juice (BR) reduced the O2 cost of submaximal walking and running exercise tolerance in young healthy males [5], and improves submaximal exercise endurance in club level cyclists [6]. A systematic review and meta-analysis of the effect of nitrate supplementation on exercise performance in healthy individuals has demonstrated that there is a significant moderate beneficial effect upon exercise performance as measured by time to exhaustion (effect size = 0.79 (95% CI, 0.23-1.35)). These benefits were more often observed in inactive to recreationally active individuals, and are present in both acute supplementation as well as chronic supplementation at up to at least 15 days [7].
Dietary nitrate supplementation appears to exert its effect via augmentation of the oxygen-independent entero-salivary pathway in which dietary NO3− is reduced to biologically active nitrite (NO2−) and nitrous oxide (NO), resulting in greater NO availability [8]. This pathway is conceptualized as a ‘back-up’ to the oxygen-dependent classical L-arginine NO synthetase system, and is upregulated under acidemic or anaerobic conditions, situations conceivably more commonplace in individuals with COPD [9]. Resultant increased bioavailable NO may lead to enhanced vasodilation and/or O2 distribution within skeletal muscle, possibly by increasing the driving pressure of O2 in the microcirculation [10]. Other proposed mechanisms include increased muscle contractile efficiency and increased mitochondrial efficiency [11].
It has also been established that nitrate supplementation also lowers blood pressure, due to a vasodilatory effect [12,13]. This has safety implications for individuals with COPD who are often older, more frail and osteopenic and in whom a fracture may lead to life threatening complications [14]. The physiology of individuals with COPD greatly differs to healthy individuals, with a chronic inflammatory state resulting in disruption to pulmonary tissues, gas exchange abnormalities, cardiac dysfunction and skeletal muscle deconditioning [15]. Compared to healthy controls, individuals with COPD appear to have preserved baseline levels of nitrate and nitrite, but NO dynamics are altered, with differences in NO synthetase isoforms [16-18].
Three recent reports have reported the effects of acute and short term dietary nitrate supplementation on exercise endurance in individuals with COPD, with mixed results [19-21].
We performed a randomized, double blind placebo control crossover trial in order to test the hypothesis that short term dietary nitrate supplementation (3 days of 4.8 mmol twice daily) would exert a beneficial effect on exercise tolerance as measured by submaximal walk test endurance in individuals with moderate severity, stable COPD.
Methods
Institutional ethics review was sought and received from the Southern Health Human Research Ethics Committee (11335A, approval date 24/1/2012). The trial was registered with the Australia and New Zealand Clinical Trial Registry (ACTRN12611001088932, registered 20/10/2011). All participants gave written informed consent prior to participation and refrained from antibacterial products including mouthwash and toothpaste during the study period. Apart from abstaining from beetroot products, no dietary restrictions were imposed. Participants’ medications remained unchanged for the duration of the trial.
BR was chosen as the source of dietary nitrate for this study for its ready commercial availability and use in prior literature. BEET-IT shots (James White Drinks, Ipswich, UK) were used for this study as the 0.3gm (4.8 mmol) dose of NO3− in each 70 mL shot approximates the 0.34-0.38gm (5.5 mmol-6.2 mmol) NO3− dose as described by prior investigators [4,5,22] without the requirement for a 500 mL daily fluid intake. Physically identical nitrate-deplete placebo shots (PL) were obtained from the manufacturer (0.00035-0.0013gm (0.0056-0.020 mmol) NO3− dose) (personal communication, Pinho, A., James White Drinks, Ipswisch, UK). The dose administered was 70 mL (0.3gm NO3−), twice daily.
Participants
Voluntary participants were recruited at a tertiary metropolitan Australian medical center either directly from the outpatient pulmonary function test laboratory or by direct referral from thoracic physicians. Inclusion criteria were Global Initiative for Chronic Obstructive Lung Disease (GOLD) class II stable COPD (‘moderate’ severity: FEV 1/FVC < 0.7, FEV 1 50-79% predicted) [1], age 45–80 and physical ability to perform an endurance shuttle walk test. GOLD II severity was chosen as a group who would be likely to experience exercise limitation but who would still be able to perform exercise testing. Exclusion criteria included a history of acute exacerbation of COPD in the preceding month, oral corticosteroid therapy, long term domiciliary O2 therapy, beta blocker therapy, ischemic heart disease or congestive cardiac failure and musculoskeletal problems limiting exercise.
Baseline measurements
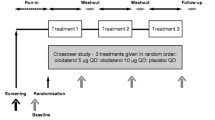
At baseline (visit 1), participants underwent pre- and post-bronchodilator spirometry and measurement of carbon monoxide diffusing capacity as per American Thoracic Society/European Respiratory Society standard guidelines for pulmonary function testing [23] (Figure 1).
Study design. Visits 1 and 2 occurred approximately 1 week apart and collected baseline and safety data on participants. Visits 3 and 4 were exactly 7 days apart per study protocol. Prior to visit 3, participants consumed either beetroot (BR) or placebo (PL) twice a day for three days. They underwent an endurance shuttle walk test during visit 3 and then abstained from BR for 4 days to allow washout. In the crossover phase, participants were given to whichever juice (BR or PL) they had not previously been exposed to. This was taken twice a day for three days prior to visit 4, at which time another endurance shuttle walk test was performed.
During the same visit, participants undertook an incremental shuttle walk test (ISWT) [24], a commonly used field walking test designed to simulate a maximal cardiopulmonary exercise test in individuals with COPD. This involved timed, accelerating shuttle walking around cones 9 m apart, forming a 10 m course. Standardized procedures were adopted as mandated by the ISWT protocol: these included uniform instructions and coaching and a repeat test to account for a learning effect. The best distance was recorded and used to estimate V̇O2max and to calculate the walking speed representing 85% of maximal exercise performance for use in the endurance shuttle walk test (ESWT) [25].
The ESWT [26] is a constant paced standardized test of endurance capacity developed for use in patients with COPD. It has face and construct validity as the exercise testing regime suitable validated for use in patients with COPD as a measure reflective of the workload undertaken by an individual with COPD in the course of their daily activities. Participants performed the walk under controlled conditions by walking at a constant predetermined rate around cones 9 m apart (10 m shuttle walk) to a constant, timed audio recording until exhaustion. The ESWT permits measurement of walking time and distance at the speed that represents their estimated 85% V̇O2max; hence it is a measure of the individual’s sub-maximal exercise endurance capacity.
Previous studies have demonstrated that it is not necessary to perform repeat determination of either the ISWT or the ESWT in this patient group [27].
Initial ESWT and safety phase
During visit 2, which occurred approximately a week after visit 1, an initial ESWT was undertaken. Immediately after the ESWT, a Modified Borg Rating Scale for Perceived Dyspnea was recorded [28]. Following this, a safety phase was performed during which participants consumed, un-blinded, active BR and underwent measurement of sitting and standing blood pressure, heart rate and pulse oximetry saturations at 0, 0.5, 1 and 4 hours post dose. The rationale for this was that BR has been previously demonstrated to decrease resting systolic blood pressure [5]. As pre-specified, if participants became symptomatic or had a >20 mmHg systolic blood pressure fall in response to the test dose, they were excluded from the remainder of the study protocol.
Double blinded randomized placebo controlled phase
Participants were randomized to receive either (active) BR or PL first in a double blind fashion by computerized allocation performed by an independent clinical trials pharmacist.
For three days prior to visit 3, participants consumed their allocated dose of juice (70 mL) twice per day. They consumed a final dose of juice on the morning of visit 3, approximately an hour prior to the performance of the ESWT. Per protocol, visits 3 and 4 occurred strictly 7 days apart.
Following visit 3, participants abstained from juice supplementation for four days, allowing a ‘wash-out’ period between dosing regimes. They then consumed whichever of BR or PL they had not previously been exposed to. This was again taken twice a day for three days prior to visit 4, with a final supplement on the morning of visit 4. Visit 4 also consisted of an ESWT.
The timing of supplementation was based on another randomized double-blind placebo-controlled crossover study [29], however washout was shortened from 10 to 7 days for logistical reasons.
Trial supplement adherence was checked by self-report and bottle count.
Sample size estimation
A recent meta-analysis of the effect of nitrate supplementation on exercise performance in healthy individuals has demonstrated that there is a significant moderate beneficial effect upon exercise performance as measured by time to exhaustion with an effect size of 0.79 (95% CI, 0.23-1.35) [7]. Eaton et al. (2006) reported that the baseline performance by moderate severity COPD patients on the ESWT was 313 meters, with a standard deviation of 193 meters, and the reported improvement in performance in response to exercise rehabilitation of 92% of baseline (302 meters). However, using the reported 92% improvement in ESWT performance in response to pulmonary rehabilitation as the minimum clinically important difference (MCID) results in an effect size of 1.6, leading to a sample size requirement of only 6–7 patients in a cross-over study in order for the study to have an 80% power at the 5% significance level. A more conservative estimate of the MCID was used instead. We chose 55% improvement on the baseline performance in the ESWT as the MCID resulting in a more conservative estimate of the required sample size: 20 patients [30].
Outcomes and statistical analysis
The pre-specified primary endpoints were distance walked (meters) and time to fatigue (minutes) on the ESWT on BR versus that on PL, with comparison by two-sided paired repeated sample t-tests. Secondary endpoints included safety phase data, Borg dyspnea scores and blood pressure. Analysis was performed using SPSS Statistics for Windows, Version 20.0 (Armonk, NY, IBM Corp, 2011). Results are presented as mean ± standard deviation, and statistical significance accepted if alpha <0.05.
Results
Recruitment
Between March 2012 and October 2013, 131 eligible participants were identified, 35 of whom agreed to participate in the study, 23 of whom completed the safety phase, and 19 went on to complete all four visits (Table 1 and Figure 1). One patient was excluded at enrolment as he was detected to have asymptomatic sick sinus syndrome. The most common reason for attrition during the study period was intercurrent illness, predominantly acute exacerbations of COPD.
Four participants were assessed to be too fit for the trial following the initial ISWT. On the basis of completing the ISWT, these excluded participants’ predicted 85% V̇O2max exceeded the measurement ceiling of the ESWT (approximately 15 ml/min/kg). Any potential increase in walk distance therefore could not be measured with the ESWT. Therefore these four subjects were excluded. In the design phase, it was not foreseen that individuals with moderate COPD would be able to complete the ISWT.
Baseline data
Of the 19 participants who completed the trial, the mean age was 67 and 14 were female. Their mean FEV1 was 62.0 ± 6.9% predicted, with TLCO of 53.9 ± 13.9% predicted. The mean estimated V̇O2max was 14.9 ± 3.8 ml/min/kg, with a calculated predicted 85% V̇O2max of 12.6 ± 3.2 ml/min/kg.
Numbers analyzed and outcomes
The safety phase (n = 23) confirmed the known blood pressure effects of BR supplementation. Compared to sitting blood pressure at the start of the safety phase, there was a maximal mean systolic blood pressure decrement of 10 mmHg on standing at 1 hour (7.3%) (p = 0.001), accompanied by significant compensatory increases in heart rate at 0 hours (mean increase 6.2 ± 8.0 beats per minute, p = 0.001) and 0.5 hours (3.8 ± 7.5 beats per minute, p = 0.027) (Tables 2 and 3). One participant developed symptomatic postural hypotension at 4 hours (30 mmHg drop on sitting to standing at 4 hours) and was excluded from the trial. Diastolic blood pressure remained unchanged throughout the observation period (Additional file 1: Table S1).
19 participants completed the crossover paired sample phase of the trial (visits 3 and 4). Adherence to trial supplementation was excellent based on self report and bottle count, with the majority of participants reporting full compliance (n = 18) on both occasions and the remaining subject reporting only one missed dose during the three day wash-in period.
Participants walked a mean distance of 721.6 ± 587.5 m on PL and 800.0 ± 584.3 m on BR (difference: 79 m, 11%) (Table 4). The median for PL was 520 m (interquartile range 360-795 m), and for BR, the median was 560 m (interquartile range 420-925 m) (Figure 2). These changes were not significant (p = 0.494, 95% CI −314.3 to 175.5 m). Similarly, walking time was not changed between PL (9.9 ± 7.5 minutes) and BR (10.5 ± 6.0 minutes) (difference = 0.6 minutes, 6%, p = 0.693). After walking, there was no difference in dyspnea score (Borg scale) or systolic blood pressure between the PL and BR treatment arms. These results did not change after removal of outliers (analysis not shown).
Discussion
The results of this study indicate that dietary nitrate supplementation in individuals with moderate COPD a) does not significantly increase submaximal exercise endurance as measured by ESWT distance, time or dyspnea score and b) decreases resting systolic blood pressure with c) compensatory increases in resting heart rate.
Limitations
Unfortunately given logistical (funding) constraints, it was impractical to measure serum NO3− and NO2− levels: ideally, these would have been measured at baseline and post supplementation, with additional analyses carried out to determine whether improvements in exercise endurance were associated with increased NO3− and/or NO2−, and whether medications or comorbidities interacted with this effect. We observed a decrement in blood pressure in the active arm, suggestive of successful nitrate supplementation, however, quantitative determination of NO3− and/or NO2− may have provided definite confirmation. Confirmation of return of NO3− and NO2− to baseline values after the four day washout period may also have been useful.
Our trial also suffered from a high attrition rate: from 35 subjects, only 19 completed the trial. This is significantly higher than in other trials of dietary nitrate supplementation in individuals with COPD, in which withdrawals were limited to a maximum of 1 per trial [19-21]. The attrition rate in our study is reflective of the burden of illness in individuals with COPD, with multiple drop-outs due to underlying illness or intercurrent illness. 10 of our patients withdrew due to medical illness (predominantly COPD exacerbations), and in the safety phase, one withdrew with symptomatic postural hypotension induced by BR, confirming the reported [5,12] effect of dietary nitrates on blood pressure. These withdrawals may reflect the tertiary nature of our center, with a high proportion of more unwell patients.
Comparisons
The dose and timing of nitrate used in our study differed to that used by other investigators who have examined its effect on exercise endurance in COPD. Kerley et al. and Berry et al., both of whom found positive effects, used acute doses of 12.9 mmol and 7.58 mmol respectively [19,20]. However, Shepherd et al. used 6.77 mmol twice a day for 2.5 days and found no difference [21]. We used 4.8 mmol twice a day for three days with a further dose on the morning of walk test and found no significant difference. Although a trial in healthy cyclists used a wash-in period and demonstrated benefit (three days 0.1 mmol nitrate/kg/day) [29], it is possible that physiological alterations in aging, or in COPD render wash-in periods less useful, or alternatively, that the dose we used was too low.
The effect of increased adiposity has been proposed as a modifying factor in the response to dietary nitrate, by means that are unknown [21]. The BMI (kg/m2) of our study population (29.1 ± 6.5) was similar to that in Berry et al. (29.2 ± 5.5) who found similar an increase in exercise performance. Kerley et al. had a slightly lighter population and found an increase in exercise endurance with a BMI of 27.3 ± 6.4, whereas Shepherd et al., whose patients were heavier than ours, and found no difference with a BMI of 30.8 ± 3.2. The influence of adiposity on nitrate supplementation’s effect on exercise supplementation remains yet to be defined.
In healthy older individuals, short term dietary nitrate supplementation at 9.6 mmol/day reduced resting blood pressure but there was no effect on walk time, an effect broadly similar to our findings [31]. Interestingly, although decreases in the oxygen cost of submaximal exercise have been relatively consistently found in younger individuals [7], the oxygen cost of submaximal exercise was not decreased in those healthy older individuals, for reasons that are unclear. Additionally, decreases in the oxygen cost of exercise were not found in the studies of nitrate supplementation of individuals with COPD, suggesting that if exercise endurance is increased in COPD, the mechanisms may be different to that in younger individuals [20,21].
We found a heterogeneous response to dietary nitrate in our study population, consistent with Berry et al. who, despite an overall positive effect, found that two of their 11 participants had a decrease in exercise time. This variation in response was also noted by Kerley et al., and its mechanism remains unclear. It may therefore be that individuals with COPD differ in their response to dietary nitrate and that our study population may have been comprised of a greater proportion of non-responders.
Skeletal muscle in individuals with COPD may affected by myriad factors, which include deconditioning, hypoxia, hypercapnia, systemic inflammation, malnutrition, and drug therapy. Multiple abnormalities result, including redox imbalance, autophagy induction, mitochondrial dysfunction, a protein catabolic state with reduced anabolism and structural abnormalities [32]. Intriguingly, it also appears that epigenetic modification may play a role in muscle phenotype and performance in COPD [33]. Additionally, exercise limitation may result from not only skeletal muscle factors, but also lung and haemodynamic factors [34]. These complex, interwoven factors and their varying extents in any given individual with COPD may make prediction of response to dietary nitrate supplementation difficult, and furthermore may render it challenging to precisely identify a mechanism for exercise enhancement, if it exists.
Study design
An important question arising from this study is whether the observed difference in endurance distance (11%) and time to fatigue (6%) is clinically important. A change in distance of 60-115 m has been reported as a minimally clinically important difference in the ESWT in the context of a pharmacological trial [35]. In our study, the observed incremental improvement in ESWT was only 79 m whilst the standard deviation of the observed values for in the baseline ESWT was 583 m. These values represent a much reduced effect size of approximately 14% compared with our initial estimate of 55%. Were the sample size larger and the standard deviation reduced, the change in distance may have been clinically significant. An important question is therefore whether the study was under-powered.
Study sample size estimates prior to this study were based on estimates of effect sizes derived from the published evidence. A meta-analysis of the effect of nitrate supplementation on exercise performance in healthy individuals has demonstrated that there is a significant moderate beneficial effect upon exercise performance as measured by time to exhaustion (effect size = 0.79 (95% CI, 0.23-1.35) [7]). Estimates of the effect size derived from studies of the incremental effect of pulmonary rehabilitation program upon exercise distance and time in the endurance shuttle walk test ESWT yielded an effect size of 1.6 [36]. This was deemed to be a clinically important incremental improvement, and was therefore used as a guide for the choice of effect size for the power calculation for this study. We used the published ESWT data to inform us of the anticipated incremental improvement and the standard deviation for baseline performance.
The sample size we chose reflected favorably with the sample sizes chosen for recently reported studies of dietary nitrate supplementation in COPD, in which sample size ranged from 11–15 [19-21]. Our trial, with a final sample size of 19, did not demonstrate an effect of dietary nitrate supplementation in COPD, concordant with the results of Shepard et al. (n = 13) [21]. This contrasts with the results of Berry et al. and Kerley et al., who found a positive effect on exercise performance for dietary nitrate supplementation in COPD, and whose sample sizes were 15 and 11 respectively [20,31].
Conclusion
Our results do not support a role for the routine use of acute dietary nitrate supplementation in individuals with GOLD stage II COPD for the purposes of physical activity limitation. Whilst this trial, to our knowledge, is the largest trial of nitrate supplementation in individuals with COPD, other investigators have reported positive effects on exercise endurance for dietary nitrate supplementation in COPD. A consistent finding is decreased blood pressure, and with the development of symptomatic postural hypotension seen in one individual in our study, the role and risk-benefit ratio of acute dietary nitrate supplementation remains undefined. Overall, it is difficult draw a firm conclusion as to whether acute dietary supplementation for exercise endurance in COPD is effective given study heterogeneity (nitrate dose and form, study setting, exercise methodology).
Future directions
Given the discordance between this study and other recently published literature, further studies should recruit larger patient cohorts, in order to minimize the effect of type II error and to examine an interaction between nitrate, medications and comorbidities. The identification of a dose–response relationship for both exercise endurance and hypotension would be important if an effect for the former were to be confirmed and nitrate were to be extended to routine clinical use. Further pharmacodynamics studies could also establish whether tolerance develops. An important step would be to identify whether there are distinct phenotypes or subpopulations of COPD that can be classified into nitrate responders or non-responders, and in whom hypotension may be an issue.
An important study to perform in the future may be to examine for a potential interaction in effects on submaximal exercise endurance between a pulmonary rehabilitation program and dietary nitrate supplementation. Such a study will likely need to be a multicenter randomized double blind placebo controlled study enrolling large numbers of subjects to be recruited given the likely drop out rate may approach 50%. The results of this study may help inform sample size estimates for such a study.
Abbreviations
- BR:
-
Beetroot juice
- COPD:
-
Chronic obstructive pulmonary disease
- ESWT:
-
Endurance shuttle walk test
- FEV1:
-
Forced expiratory volume in 1 second
- FVC:
-
Forced vital capacity
- ISWT:
-
Incremental shuttle walk test
- NO:
-
Nitrous oxide
- NO2−:
-
Nitrite
- NO3−:
-
Nitrate
- PL:
-
Placebo
- TLCO:
-
Transfer factor for carbon monoxide
- V̇O2max:
-
Maximum oxygen uptake
References
Global Initiative for Chronic Obstructive Lung Disease (GOLD). Global Strategy for the Diagnosis, Management and Prevention of COPD. 2014.
World Health Organization. The Global Burden of Disease: 2004 Update. Geneva, Switzerland: World Health Organization; 2008.
Breslin E, van der Schans C, Breukink S, Meek P, Mercer K, Volz W, et al. Perception of fatigue and quality of life in patients with COPD. Chest. 1998;114:958–64.
Bailey SJ, Winyard P, Vanhatalo A, Blackwell JR, DiMenna FJ, Wilkerson DP, et al. Dietary nitrate supplementation reduces the O2 cost of low-intensity exercise and enhances tolerance to high-intensity exercise in humans. J Appl Physiol. 2009;107:1144–55.
Lansley KE, Winyard PG, Fulford J, Vanhatalo A, Bailey SJ, Blackwell JR, et al. Dietary nitrate supplementation reduces the O2 cost of walking and running: a placebo-controlled study. J Appl Physiol. 2011;110:591–600.
Lansley KE, Winyard PG, Bailey SJ, Vanhatalo A, Wilkerson DP, Blackwell JR, et al. Acute dietary nitrate supplementation improves cycling time trial performance. Med Sci Sports Exerc. 2011;43:1125–31.
Hoon MW, Johnson NA, Chapman PG, Burke LM. The effect of nitrate supplementation on exercise performance in healthy individuals: a systematic review and meta-analysis. Int J Sport Nutr Exerc Metab. 2013;23:522–32.
Lundberg JO, Carlström M, Larsen FJ, Weitzberg E. Roles of dietary inorganic nitrate in cardiovascular health and disease. Cardiovasc Res. 2011;89:525–32.
Gilchrist M, Winyard PG, Benjamin N. Dietary nitrate – Good or bad? Nitric Oxide. 2010;22:104–9 [Inorganic Nitrate and Nitrite: Physiology, Pathophysiology and Therapeutics].
Ferreira LF, Behnke BJ. A toast to health and performance! Beetroot juice lowers blood pressure and the O2 cost of exercise. J Appl Physiol. 2011;110:585–6.
Zafeiridis A. The effects of dietary nitrate (beetroot juice) supplementation on exercise performance: A review. Am J Sports Sci. 2014;2:97–110.
Kapil V, Milsom AB, Okorie M, Maleki-Toyserkani S, Akram F, Rehman F, et al. Inorganic nitrate supplementation lowers blood pressure in humans: role for nitrite-derived NO. Hypertension. 2010;56:274–81.
Cosby K, Partovi KS, Crawford JH, Patel RP, Reiter CD, Martyr S, et al. Nitrite reduction to nitric oxide by deoxyhemoglobin vasodilates the human circulation. Nat Med. 2003;9:1498–505.
Agustí AGN, Noguera A, Sauleda J, Sala E, Pons J, Busquets X. Systemic effects of chronic obstructive pulmonary disease. Eur Respir J. 2003;21:347–60.
Corsonello A, Antonelli Incalzi R, Pistelli R, Pedone C, Bustacchini S, Lattanzio F. Comorbidities of chronic obstructive pulmonary disease. Curr Opin Pulm Med. 2011;17 Suppl 1:S21–8.
Cella G, Saetta M, Baraldo S, Turato G, Papi A, Casoni G, et al. Endothelial Cell Activity in Chronic Obstructive Pulmonary Disease Without Severe Pulmonary Hypertension. Clin Appl Thromb. 2005;11:435–40.
Nadeem A, Raj HG, Chhabra SK. Increased Oxidative Stress and Altered Levels of Antioxidants in Chronic Obstructive Pulmonary Disease. Inflammation. 2005;29:23–32.
Malerba M, Radaeli A, Olivini A, Damiani G, Ragnoli B, Montuschi P, Ricciardolo FLM: Exhaled nitric oxide as a biomarker in COPD and related comorbidities. BioMed Res Int 2014. doi:10.1155/2014/271918
Kerley CP, Cahill K, Bolger K, McGowan A, Burke C, Faul J, et al. Dietary nitrate supplementation in COPD: An acute, double-blind, randomized, placebo-controlled, crossover trial. Nitric Oxide. 2015;44:105–11.
Berry MJ, Justus NW, Hauser JI, Case AH, Helms CC, Basu S, Rogers Z, Lewis MT, Miller GD: Dietary nitrate supplementation improves exercise performance and decreases blood pressure in COPD patients. Nitric Oxide. 2014. doi:10.1016/j.niox.2014.10.007
Shepherd AI, Wilkerson DP, Dobson L, Kelly J, Winyard PG, Jones AM, Benjamin N, Shore AC, Gilchrist M: The effect of dietary nitrate supplementation on the oxygen cost of cycling, walking performance and resting blood pressure in individuals with chronic obstructive pulmonary disease: A double blind placebo controlled, randomised control trial. Nitric Oxide. 2015. doi:10.1016/j.niox.2015.01.002.
Bailey SJ, Fulford J, Vanhatalo A, Winyard PG, Blackwell JR, DiMenna FJ, et al. Dietary nitrate supplementation enhances muscle contractile efficiency during knee-extensor exercise in humans. J Appl Physiol. 2010;109:135–48.
Miller MR. Standardisation of spirometry. Eur Respir J. 2005;26:319–38.
Dyer CAE, Singh SJ, Stockley RA, Sinclair AJ, Hill SL. The incremental shuttle walking test in elderly people with chronic airflow limitation. Thorax. 2002;57:34–8.
Singh SJ, Morgan MD, Hardman AE, Rowe C, Bardsley PA. Comparison of oxygen uptake during a conventional treadmill test and the shuttle walking test in chronic airflow limitation. Eur Respir J. 1994;7:2016–20.
Revill S, Morgan M, Singh S, Williams J, Hardman A. The endurance shuttle walk: a new field test for the assessment of endurance capacity in chronic obstructive pulmonary disease. Thorax. 1999;54:213–22.
McKeough ZJ, Leung RWM, Alison JA. Shuttle walk tests as outcome measures: Are two incremental shuttle walk tests and two endurance shuttle walk tests necessary? Am J Phys Med Rehabil Assoc Acad Physiatr. 2011;90:35–9.
Burdon JG, Juniper EF, Killian KJ, Hargreave FE, Campbell EJ. The perception of breathlessness in asthma. Am Rev Respir Dis. 1982;126:825–8.
Larsen FJ, Weitzberg E, Lundberg JO, Ekblom B. Effects of dietary nitrate on oxygen cost during exercise. Acta Physiol. 2007;191:59–66.
Bland M. An Introduction to Medical Statistics. 3rd ed. Oxford: Oxford University Press; 2000 [Oxford Medical Publications].
Kelly J, Fulford J, Vanhatalo A, Blackwell JR, French O, Bailey SJ, et al. Effects of short-term dietary nitrate supplementation on blood pressure, O2 uptake kinetics, and muscle and cognitive function in older adults. Am J Physiol-Regul Integr Comp Physiol. 2013;304:R73–83.
Barreiro E, Sieck G. Muscle dysfunction in COPD. J Appl Physiol. 2013;114:1220–1.
Barreiro E, Sznajder JI. Epigenetic regulation of muscle phenotype and adaptation: a potential role in COPD muscle dysfunction. J Appl Physiol. 2013;114:1263–72.
Vogiatzis I, Zakynthinos S: Factors Limiting Exercise Tolerance in Chronic Lung Diseases. In Comprehensive Physiology. John Wiley & Sons, Inc.; 2012
Pepin V, Laviolette L, Brouillard C, Sewell L, Singh SJ, Revill SM, et al. Significance of changes in endurance shuttle walking performance. Thorax. 2010;66:thx.2010.146159.
Eaton T, Young P, Nicol K, Kolbe J. The endurance shuttle walking test: a responsive measure in pulmonary rehabilitation for COPD patients. Chron Respir Dis. 2006;3:3–9.
Acknowledgements
The authors would like to acknowledge Helen Kopp, Clinical Trials Pharmacist, for managing the randomization and pharmaceutical support. Lawrence Mallinson, Amanda Park and Armanda Pinho at James White Drinks (Suffolk, UK) assisted in supply of nitrate-deplete placebo beetroot juice. Adrian O'Connor at Trialia Foods (Noble Park, Melbourne) Australia, supplied the active beetroot juice gratis. The trial was funded from by the Research Fund, Department of General Medicine, Monash Medical Centre.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests, financial or non-financial.
Author contributions
DC, PB, PF and AC conceived the study. PL, JB, SB and TY recruited subjects, performed all tests and collected primary data. PL and DC performed statistical analysis. PL and DC drafted the initial manuscript. All authors read and approved the final manuscript.
Additional file
Additional file 1: Table S1.
Safety phase data for diastolic blood pressure (DBP) on standing (mmHg) (n=23 unless otherwise stated).
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Leong, P., Basham, J.E., Yong, T. et al. A double blind randomized placebo control crossover trial on the effect of dietary nitrate supplementation on exercise tolerance in stable moderate chronic obstructive pulmonary disease. BMC Pulm Med 15, 52 (2015). https://doi.org/10.1186/s12890-015-0057-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12890-015-0057-4