Abstract
Background
The prevalence of low-, moderate-, and high-risk obesity has been increasing globally. Our aim was to estimate the societal burden of these three obesity classes in the Austrian population by taking a societal–annual perspective and an individual–life-cycle perspective. Secondly, we sought to identify the respective cost drivers and the effects on life expectancy.
Methods
We used population-weighted survey data on the distribution of body mass index (BMI) and data on relative risks regarding 83 diseases. Using fractional polynomial regressions, we estimated relative risks per BMI unit for about 30 cost-intensive diseases up to BMI values of 50. The approach for the cost analysis was based on the use of population-attributable fractions applied to direct medical and indirect cost data. Macro-disease-specific data regarding cost factors came from cost-of-illness statistics and administrative sources.
Results
About 8.2% of deaths and 4.6% of health expenditure are attributable to obesity in Austria in 2019, causing 0.61% of GDP loss. A third of annual direct and indirect costs came from class 2 and class 3 obesity. From an individual perspective, life-cycle costs of class 2 and class 3 obesity were 1.9 and 3.6 times the costs of class 1 obesity, respectively. At the age of 45, people with high-risk obesity are about to lose approximately 4.9 life years and 9.7 quality-adjusted life years.
Conclusions
We have extended the method of population-attributable fractions, allowing us to estimate differences in the life-cycle direct medical and indirect costs between low-, moderate-, and high-risk obesity. We found that the lifetime costs of obesity are strikingly different between obesity classes, which has been veiled in the societal—annual perspective. Our findings provide a foundation for evaluating public health interventions aimed at combating obesity in order to mitigate the escalating individual and societal burden resulting from obesity prevalence and complications in the future.
Similar content being viewed by others
Explore related subjects
Find the latest articles, discoveries, and news in related topics.Introduction
The complications associated with obesity increase with increasing weight. [1,2,3,4,5,6,7,8] The degree of obesity can be measured by the body mass index (BMI) and categorized into three obesity classes. It has turned out in former cost analyses that class 2 (moderate-risk) and class 3 (high-risk) obesity are the main driving factors of increasing health care expenses [9, 10] and increase missed workdays per year [11]. In Austria, the prevalence of class 2 and 3 obesity – drawn from the medical examination for military service for males at age 18 – has doubled from 2003 to 2018. [12] Against this backdrop, we aim to examine Austria’s societal burden of each obesity class for the year 2019 as well as the cost burden over the life cycle.
Studies from other countries have reported lifetime costs, but they have not differentiated between obesity classes in this regard. [13,14,15] Our analysis distinguishes between obesity classes and estimates direct medical costs and indirect costs (due to missed workdays) over the life cycle, assuming a patient remains with class 1, class 2, or class 3 obesity. We use population-weighted survey data on the distribution of BMI in Austria and data from epidemiological studies on relative risks regarding 83 obesity-related diseases. To capture the costs of high-risk obesity, we apply polynomial regressions estimating relative risks per BMI value (up to a BMI of 50) for 23 cost-intensive obesity-related diseases regarding morbidity risk and for the 11 most relevant obesity-related diseases regarding mortality risk.
Our estimates of the lifetime cost of obesity provide an important basis for evaluating public health interventions, with a focus on different degrees of obesity. Prevention and treatment measures will be needed – from a young age – in order to tackle the progress of obesity and the associated costs over the life cycle.
Methods
Prevalence
We have drawn on data about body weight and height from the Austrian Health Interview Survey (n = 14 606) [16], representing the Austrian adult population. We calculated the BMI for each observation after adjusting self-reported body weight data with correcting factors according to an Austrian validity study on self-reported body weight and height. [17] In the next step, we calculated the prevalence of overweight (BMI 25–29.9), class 1 obesity (BMI 30–34.9), class 2 obesity (BMI 35–39.9), and class 3 obesity (BMI 40+) for 5-year-age-groups of the male and female population, respectively.
The prevalence of obesity among children and adolescents came from three sources: for all girls between the ages of 5 and 19 and boys between 5 and 14, we used measured data surveyed by the Austrian Child Obesity Surveillance Initiative [18]; for boys between 15 and 19, we used measured data from the medical examination for the military service; and, for children under the age of 5 we have drawn on German data [19] because Austrian data was not available for this age group. To estimate costs arising from obesity-associated pregnancy and birth complications, we further applied Austrian data on obesity prevalence among expecting mothers. [20]
In 2019, 14.1% of the Austrian adult population fell into class 1 obesity (BMI 30–34.9), another 4% into class 2 obesity (BMI 35–39.9) and 1.4% into class 3 obesity (BMI ≥ 40). This corresponds to 999,934 adults with class 1 obesity, 284,394 adults with class 2 obesity, and 97,182 adults with class 3 obesity. The prevalence among expecting mothers is 7.6%, 2.7%, and 0.1%, respectively. For children and adolescents between 5 and 19 years, we did not distinguish between obesity classes: 6.7% of girls and 10.1% of boys (10.4% of boys in the age group 15–19) are classified as obese according to WHO child growth standards, which corresponds to 112,971 children and adolescents. [21] The distribution of BMI categories by age group for men and women is shown in the Supplementary Table S1.
Relative risks
We used relative risk data drawn from a systematic literature search regarding relative risks associated with obesity. The search is described in the Supplementary Figure S1. We conducted and validated for methodological quality with the help of a physician specializing in obesity. The resulting selection of 449 meta-studies, provided 95% confidence intervals for relative morbidity and mortality risks for comorbidities in association with obesity in the general population. The selection also included pregnancy and birth complications and health consequences for children but excluded any obesity-related disease severities, complications, or disease recurrences as well as preliminary stages of diseases such as dyslipidemia, hyperuricemia, and prediabetes/insulin resistance.
We extracted data either from those meta-studies, which distinguished their results between obesity classes, or from additional underlying cohort studies. If neither a meta-analysis nor a cohort study revealed the increasing risks above BMI values of 35, we applied equal relative risks to all obesity classes in our study. We used evidence regarding 83 obesity-related diseases summarized in Table 1; for 23 diseases (written in bold letters in the table), we obtained relative risk data per obesity class, which covered the most important cost factors according to former studies [22]. All diseases and the corresponding mean (95% CI) relative risks and study sources are summarized in the Supplementary Materials.
Population-attributable fraction
To estimate the number of sick people and deaths that can be attributed to obesity, we calculated the obesity-attributable proportion of each disease based on prevalence data for obesity and relative risk data for each obesity-related disease. Most epidemiological studies did not report relative risks by age, but some studies differentiated between sexes; BMI data, in contrast, was available by age and sex. Thus, the respective attributable fractions (AF) of a certain age (a) group (of one sex) resulted from the corresponding sex-age-specific prevalence (p) for certain BMI values (i.e. the superscript values in the formula), and the (sex-age)-BMI-specific relative risk (RR) for disease (d) using the following formula. [23]
We employed polynomial regression results on relative risks per BMI value for 32 diseases with respect to morbidity and 12 diseases with respect to mortality; the corresponding regression results reported in the Supplementary Material. For the remaining diseases we did not differentiate relative risks between single BMI values or obesity classes but assumed constant risks for any BMI values above 30. In our life-cycle model, we only estimated the costs per obesity class (instead of single BMI values). Therefore, the formula contained data with respect to one obesity class in the counter.
Furthermore, the uncertainty of relative risks with respect to comorbidities was accounted for by randomly drawing relative risk values 100 times from an assumed lognormal distribution with a 0.2 standard deviation. The analysis was done with STATA 16.0.
The life-cycle model
The idea of the life-cycle model is to sum up obesity-attributable costs from an individual’s age in the base year until his or her expected end of life. In practice, we calculated the costs of a hypothetical population without obesity, considering survival probabilities without excess body weight and the consequential non-obesity-associated health expenditure in the case of survival. 2019 serves as the base year. The difference in the observed costs of 2019 and the hypothetical costs yielded the costs of obesity. Thus, the life-cycle costs of obesity depend on the probability of entering the next age group (in 5-year intervals) and on the discount rate applied to future costs.
We implemented discount rates of 3% and 0%. The former reflects the time preference of a society, incorporating the fact that costs in real terms change when GDP per capita rises, [24, 25] and is recommended for Austria. [26] However, we also estimated lifetime costs without discounting to better highlight the long-term harm and related costs of obesity. [27] In the latter case, future costs were only weighted by the probability of survival.
The probability of survival was calculated as the opposite of the probability of mortality, given by the mortality rate (ratio of the number of dead to the average number of surviving within a 5-year age cohort) in relation to the starting population of 100,000. We estimated the survival probabilities for a hypothetical population in which obesity does not exist (i.e., obesity-attributable fractions are subtracted from the observed deaths); finally, we applied the survival probabilities derived for individual age groups to future cost factors. The model, thereby, reflects additional medical costs and productivity gains in this hypothetical population in which obesity-associated deaths were avoided.
Finally, the results of the life-cycle model express costs per head at constant price levels in 2019. Because the Austrian prevalence of obesity is projected to vary over time and will also depend on future (access to) treatment options, [28] we preferred to estimate the life-cycle costs per untreated person (who we defined to stay in his or her obesity class over life) instead of the entire population of the year 2019.
Cost factors
Our cost analysis included direct medical and indirect costs. Our medical cost data covered public and private expenses for diagnosis, medical treatment, prevention, rehabilitation, care as well as any related resource consumption (e.g., administrative costs). In our model, indirect costs arose from absenteeism due to sick leave, entry into the disability pension, or death during working age. We did not estimate productivity losses due to discrimination in the labor market, lower skill acquisition, or lower health-related in-job productivity [29, 30] because quantitative evidence is limited in these regards.
Data on cost factors were macro-data aggregated for 5-year age groups by sex (Supplementary Tables S2 – S7). For the calculation of medical costs, disease-specific cost account data was not available for Austria. Therefore, we have drawn on disease-specific cost shares (defined by ICD-10 codes) from the German cost-of-illness database [31] and applied them to Austrian data on health expenditure by age and sex [32]. Since the Austrian data did not include preventive care, governance, and health system and financing administration we applied a proportional adjustment factor over all age groups so that the sum over all age groups equaled Austria’s total current expenditure on health care of 2019. To evaluate the indirect costs associated with obesity, we used Austrian gross income data [33] (including employer contribution to social insurance), full- and part-time employment rate data [34, 35], and disease-specific data on entries into the disability pension scheme [36], disease-specific data on deaths [35], and disease-specific data on sick leave (provided by the Austrian Health Insurance Fund and the Social Insurance Institution for Self-Employed Persons).
Additionally, we estimated the obesity-associated bed occupancy in hospitals. The data on in-patient hospital stays (i.e., patients) and hospital days were provided by the Federal Ministry of Social Affairs, Health, Care and Consumer Protection for the year 2019. Firstly, we applied the estimated age- and sex-related population-attributable fractions to in-patient hospital stays of each obesity-associated disease and, secondly, multiplied the obesity-attributable hospital stays with the Austrian average in-patient hospital days per corresponding disease.
Results
Deaths
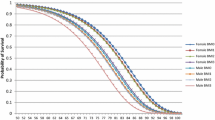
The health consequences of obesity become more apparent with age. Figure 1 illustrates the distribution of deaths over age groups per 1000 people with different obesity classes. For example, in the age group 45–49, about one death occurred among 1000 people with obesity, regardless of their degree of obesity. In the older age group 65–69, we estimated that there were about 3 deaths per 1000 people with class 1 obesity, compared to 6 deaths with class 2 obesity and 9 deaths with class 3 obesity.
Obesity caused approximately 4000 deaths in the Austrian population under the age of 85 in 2019, which was about 8.2% of the total deaths of the respective population. Class 1 obesity – 72% of the adult population with obesity – caused 57% of all obesity-associated deaths and class 2 and 3 obesity – 28% of the adult population with obesity – caused together 43% of all obesity –associated deaths.
Costs
We estimated annual obesity-attributable costs of €2410 million, which were about 0.61% of Austria’s GDP in 2019. Relative to the population with obesity of different degrees, costs related to class 2 and class 3 obesity, which were about 38% of total obesity-associated costs, were disproportionately higher than the costs related to class 1 obesity.
The direct medical costs of obesity yielded 4.6% of Austria’s total health expenditure in 2019. €1200 million came from patients with class 1 obesity, €550 million from patients with class 2 obesity, and €180 million from patients with class 3 obesity. The indirect costs of one year per obesity class (not taking into account future production loss from premature death) yielded €290 million, €138 million, and €57 million, respectively (see Table 2).
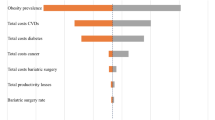
The degree of obesity is mainly related to costs from circulatory system diseases and endocrine, nutritional, and metabolic diseases. Overall, the highest proportions of medical costs are caused by diseases of the circulatory system (35%) and endocrine, nutritional, and metabolic diseases (23%). These diseases are followed by diseases of the musculoskeletal system and connective tissue (11%), diseases of the respiratory system (8%), diseases of the digestive system (7%), mental and behavioral disorders (6%), neoplasms (5%), and diseases of the genitourinary system (3%). However, the rank of disease-related cost drivers differs between cost factors (see Fig. 2). Diseases of the circulatory system were responsible for the most obesity-related deaths (49%). Regarding sick days, the primary cost drivers were diseases of the musculoskeletal system and connective tissue (46%), whereas obesity-related diseases of the nervous system (25%) are an additional reason for eligibility for disability pension.
In 2019, Austria lost approximately 4300 full-time equivalents, of which almost 40% were attributable to moderate-, or high-risk obesity. Two thirds are lost due to sick leave and one third due to disability and death. From an annual perspective, the highest proportion of productivity loss was due to sick days; losses due to disability and deaths, however, are more significant in terms of long-term loss of productivity, which we only considered in the cost analysis of the life-cycle model. From an individual perspective, sick days at the age of 30 were not significantly different between obesity classes but diverged at older ages (see Fig. 3).
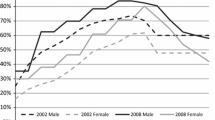
Finally, we examined the share of obesity-associated inpatient hospital days (see Fig. 4). In absolute terms, obesity-associated hospital days increased until the age of 80. Hospitalization tends to increase with age, but the obesity-related share of total inpatient hospital days peaks at 3.5% below the age of 70. From the age of 30, class 2 and class 3 obesity accounted for about 38% of obesity-associated hospital days.
The life-cycle model
Figure 5 shows the multiples of costs of class 2 and class 3 obesity relative to class 1 obesity from the societal annual perspective and an individual life-cycle perspective. From a societal perspective, annual cost of class 2 and class 3 obesity are 0.5 and 0.2 of the costs of class 1 obesity, respectively. In contrast, from an individual perspective life-cycle costs of class 2 and class 3 obesity are 1.9 and 3.6 times the costs of class 1 obesity, respectively. The figure, therefore, emphasizes that the rise in the degree of obesity matters, although it is veiled in the societal perspective given the higher prevalence of class 1 obesity in the population.
It has turned out that direct medical costs over the life cycle were positive for all age groups and both sexes, which means that an earlier onset of morbidity and mortality does not offset higher medical expenses in old age for the population without obesity (Supplementary Tables S9 and S10 show the increasing life-cycle costs per person at different ages). Applying a discount rate of 3% has resulted in increasing lifetime costs with age because the complications, i.e., costs, occur sooner and gain more weight. For instance, at the age of 45, direct and indirect life-cycle costs of a man rise from approximately €45,000 to €88,000 to €165,000 with each obesity class; the values are lower at the age of 30, but about the same at the age of 60. In contrast, life-cycle costs without discounting future cost (but considering the probability of survival) generally decrease with age. For instance, at the age of 45, direct and indirect life-cycle costs of a man without discounting rise from approximately €90,000 to €180,000 to €330,000 with each obesity class, the values are lower at the age of 60, but about the same at the age of 30.
Furthermore, we estimated the remaining life years of people with different risks due to their body mass index by assuming that these people did not change between normal weight, overweight, and obesity classes over their life cycles (see Fig. 6).
For instance, at the age of 45, a man and woman with normal weight are expected to live another 36.7 and 40.4 years, respectively. Class 1 obesity reduces life expectancy by 1.7 years for men and 1.3 years for women; class 2 obesity reduces life expectancy by 2.7 and 2.1 years; and class 3 obesity by 4.9 and 3.7 years for men and women, respectively. Applying EQ-5D weights in relation to the BMI, which came from a sample of UK residents at the age of 45 or older [37], gave us the approximated remaining quality-adjusted life years in relation to BMI status. A man and woman with normal weight at the age of 45 are expected to live another 29.5 and 33.5 quality-adjusted years, respectively. About 5 quality-adjusted life years are expected to be lost for men as well as women at the age of 45 with class 1 obesity, 6.3 years with class 2 obesity, and 9.7 years with class 3 obesity.
Discussion
The prevalence of obesity, mainly, moderate-, and high-risk obesity, has been increasing in Austria. In 2019, 1.49 million Austrians (16.8% of the total population) were classified with obesity. About 8.2% of deaths and 4.6% of health expenditure were attributable to obesity, causing 0.61% of GDP loss. From an individual perspective, life-cycle costs of class 2 and class 3 obesity were 1.9 and 3.6 times the costs of class 1 obesity, respectively. At the age of 45, people with lifetime high-risk obesity were about to lose approximately 4.9 life years and 9.7 quality-adjusted life years.
Compared to studies from other countries, we have received similar but higher obesity-associated shares of health expenditure and deaths and lower obesity-associated production losses. [22, 38,39,40] The reason for the former is probably the inclusion of more diseases in our analysis. In our analysis, though, we have not considered the lifetime production loss in the annual perspective, which explains the relatively low share of indirect costs from total costs in our study as well as the relatively low share of total obesity-associated costs as a percentage of total GDP. [41] Instead, we have considered the lifetime consequences in the individual perspective.
Accordingly, one advantage of our study is the extension of the method of population-attributable fractions to assess the expenses among individuals over the life-cycle and differentiate between low-, moderate-, and high-risk obesity. We were able to quantify the long-term harm and costs of high-risk obesity by quantifying the rising relative risks up to BMI values of 50. As we aimed to include a substantial number of morbidity and mortality risks associated with obesity, our findings are particularly extensive in revealing disparities in cost drivers pertaining to medical expenses, deaths, and sick days, as well as disparities in (quality-adjusted) life expectancy associated with low-, moderate-, and high-risk obesity.
Furthermore, we aimed to advance the attributable-fraction-approach and to implement probabilistic relative risk values. Because the attributable fraction approach is not a stochastic approach, variances in the results are usually addressed only in the sensitivity analyses. One way of dealing with this is to implement the lower and upper bounds of the confidence intervals (of all diseases) in a sensitivity analysis. In this study, we went in a different direction. We drew values around the reported mean relative risk values for the cost analysis, assuming a log-normal distribution, which is reasonable given the functional form of the underlying estimations. We also assumened an absolute standard deviation value of 0.2 (with a lower bound of the resulting relative risk value of 1), given that we did not know the standard deviations from the studies presented in the supplementary relative risk table. Consequently, the uncertainty intervals of our cost results only refer to the uncertainty in relative risk values, but not, for instance, to the uncertainty in the prevalence. Because single costs factors are relatively low in numbers (e.g., deaths), our approach of modelling uncertainty intervals, moreover, only make sense regarding aggregated costs. To address these limitations, the implementation of further uncertainties could possibly be considered in future studies.
Further limitations of our study include the fact that we did not include all cost factors to estimate indirect costs. We did not include productivity losses due to discrimination in the labor market, lower skill acquisition, or lower health-related in-job productivity, [29, 30] but quantitative evidence is limited in these regards. Furthermore, we did not include the higher risk of mortality and severity of COVID-19, and we excluded complications or recurrences after surgeries, or the severity of, for instance, cancer. The medical cost data, furthermore, does not include expenses on transportation to health care providers and on informal care. Hence, our results rather depict an underestimate of the actual economic costs caused by obesity.
In addition, for our estimate we had to rely on prevalence and cost assumptions due to data restrictions. For instance, we had to use different data sources to approximate obesity prevalence of children and adolescents and relied on shares of the German cost-of-disease-statistics. Our model and findings would be improved if we had harmonized data and cost-of-disease-statistics for Austria available. Overall, we provided novel insights into lifetime costs, the cost drivers of low-, moderate-, and high-risk obesity, and the reduction of life years. Thus, our findings raise awareness about the importance of furthering public health prevention (e.g., campaigns and programs for a healthy lifestyle) and treatment programs (e.g., access to medication and behavior therapy). In this regard, our findings may serve as a foundation for assessing public health interventions.
Data availability
The datasets used and analysed during the current study are not publicly available, but can be provided by the corresponding author on reasonable request.
Abbreviations
- AF:
-
Attributable fraction
- BMI:
-
Body mass index
- CI:
-
Confidence interval
- EQ-5D:
-
European Quality of Life 5 Dimensions
- GDP:
-
Gross domestic product
- QALY:
-
Quality-adjusted life years
- ICD-10:
-
International Statistical Classification of Diseases and Related Health Problems
- LY:
-
Life years
- RR:
-
Relative risk
- SD:
-
Standard deviation
References
Aune, Aune N, Aune V. Body mass index, abdominal fatness and the risk of gallbladder disease. Eur J Epidemiol. 2015;30(9):1009–19.
Aune D, Saugstad OD, Henriksen T, Tonstad S. Maternal body mass index and the risk of fetal death, stillbirth, and infant death: a systematic review and meta-analysis. Obstet Gynecol Surv. 2014;69(8):449–50. https://doi.org/10.1097/01.ogx.0000453816.54481.36.
Aune D, Sen A, Norat T, et al. Body mass index, abdominal fatness, and heart failure incidence and mortality: a systematic review and dose-response meta-analysis of prospective studies. Circulation. 2016;133(7):639–49.
Aune D, Sen A, Schlesinger S, et al. Body mass index, abdominal fatness, fat mass and the risk of atrial fibrillation: a systematic review and dose–response meta-analysis of prospective studies. Eur J Epidemiol. 2017;32(3):181–92.
Aune D, Mahamat-Saleh Y, Norat T, Riboli E. High body mass index and central adiposity is associated with increased risk of acute pancreatitis: a Meta-analysis. Dig Dis Sci. 2021;66(4):1249–67.
Herrington WG, Smith M, Bankhead C et al. G Remuzzi ed. 2017 Body-mass index and risk of advanced chronic kidney disease: prospective analyses from a primary care cohort of 1.4 million adults in England. PLoS ONE 12 3 e0173515 https://doi.org/10.1371/journal.pone.0173515.
Järvholm B, Lewold S, Malchau H, Vingård E. Age, bodyweight, smoking habits and the risk of severe osteoarthritis in the hip and knee in men. Eur J Epidemiol. 2005;20(6):537–42. https://doi.org/10.1007/s10654-005-4263-x.
Prospective Studies Collaboration. Body-mass index and cause-specific mortality in 900 000 adults: collaborative analyses of 57 prospective studies. Lancet. 2009;373(9669):1083–96. https://doi.org/10.1016/S0140-6736(09)60318-4.
Andreyeva T, Sturm R, Ringel JS. Moderate and severe obesity have large differences in health care costs. Obes Res. 2004;12(12):1936–43. https://doi.org/10.1038/oby.2004.243.
Cawley J, Meyerhoefer C, Biener A, Hammer M, Wintfeld N. Savings in medical expenditures associated with reductions in body mass index among US adults with obesity, by Diabetes Status. PharmacoEconomics. 2015;33(7):707–22. https://doi.org/10.1007/s40273-014-0230-2.
Andreyeva T, Luedicke J, Wang YC. State-level estimates of obesity-attributable costs of Absenteeism. J Occup Environ Med. 2014;56(11):1120–7. https://doi.org/10.1097/JOM.0000000000000298.
Gensthaler L, Felsenreich DM, Jedamzik J, et al. Trends of overweight and obesity in male adolescents: prevalence, socioeconomic status, and impact on cardiovascular risk in a central European country. Obes Surg. 2022;32(4):1024–33. https://doi.org/10.1007/s11695-021-05867-z.
Hamilton D, Dee A, Perry IJ. The lifetime costs of overweight and obesity in childhood and adolescence: a systematic review: lifetime costs of childhood obesity. Obes Rev. 2018;19(4):452–63. https://doi.org/10.1111/obr.12649.
Sonntag D, Ali S, Lehnert T, Konnopka A, Riedel-Heller S, König HH. Estimating the lifetime cost of childhood obesity in Germany: results of a Markov Model: lifetime cost of childhood obesity. Pediatr Obes. 2015;10(6):416–22. https://doi.org/10.1111/ijpo.278.
Sonntag D, Jarczok MN, Ali S, DC-Obesity. A New Model for estimating Differential Lifetime costs of overweight and obesity by socioeconomic status: DC-Obesity: a new obesity model. Obesity. 2017;25(9):1603–9. https://doi.org/10.1002/oby.21913.
ATHIS, Gesundheitsbefragung, Published. online 2019. https://www.sozialministerium.at/Themen/Gesundheit/Gesundheitssystem/Gesundheitsberichte/%C3%96sterreichische-Gesundheitsbefragung-2014-(ATHIS).html
Großschädl F, Haditsch B, Stronegger WJ. Validity of self-reported weight and height in Austrian adults: sociodemographic determinants and consequences for the classification of BMI categories. Public Health Nutr. 2012;15(1):20–7. https://doi.org/10.1017/S1368980011001911.
Weghuber D, Maruszczak K. Childhood obesity surveillance initiative (COSI). Bundesminist Für Soz Gesundh Pflege Konsum. Published online 2021. Accessed July 6, 2022. https://www.sozialministerium.at/Themen/Gesundheit/Kinder--und-Jugendgesundheit/COSI.html
Schienkiewitz A, Demerow S, Schaffrath Rosario A. Prävalenz von Untergewicht, Übergewicht und Adipositas bei Kindern und Jugendlichen in Deutschland – Einordnung der Ergebnisse aus KiGGS Welle 2 nach internationalen Referenzsystemen. Robert Koch-Inst. Published online 2018. https://doi.org/10.17886/RKI-GBE-2018-080
Schildberger B, Hofer K, Harrasser A, Leitner H. Der Einfluss Von mütterlicher Adipositas auf ausgewählte geburtshilfliche parameter. Z Für Geburtshilfe Neonatol. 2021;225(03):267–74. https://doi.org/10.1055/a-1327-4619.
WHO. Child growth standars. Published 2023. Accessed July 5. 2023. https://www.who.int/tools/child-growth-standards
Andersson E, Eliasson B, Steen Carlsson K. Current and future costs of obesity in Sweden. Health Policy. 2022;126(6):558–64. https://doi.org/10.1016/j.healthpol.2022.03.010.
Wegner C, Gutsch A, Hessel F, Wasem J. Rauchen-attributable Produktivitätsausfallkosten in Deutschland - Eine Partielle Krankheitskostenstudie Unter Zugrundelegung Der Humankapitalmethode. Gesundheitswesen. 2004;66(07):423–32. https://doi.org/10.1055/s-2004-813326.
Severens JL, Milne RJ. Discounting Health outcomes in economic evaluation: the ongoing debate. Value Health. 2004;7(4):397–401. https://doi.org/10.1111/j.1524-4733.2004.74002.x.
Haacker M, Hallett TB, Atun R. On discount rates for economic evaluations in global health. Health Policy Plan Published Online Oct. 2019;18:czz127. https://doi.org/10.1093/heapol/czz127.
European Commission. Commission Implementing Regulation (EU) 2015/207 of 20 January 2015. 2018. Accessed March 15, 2023. https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=celex%3A32015R0207
Polasky S, Dampha NK. Discounting and Global Environmental Change. Annu Rev Environ Resour. 2021;46(1):691–717. https://doi.org/10.1146/annurev-environ-020420-042100.
Janssen F, Bardoutsos A, Vidra N. Obesity prevalence in the long-term future in 18 European countries and in the USA. Obes Facts. 2020;13(5):514–27. https://doi.org/10.1159/000511023.
Lundborg P, Nystedt P, Rooth DO. Body size, skills, and income: evidence from 150,000 teenage siblings. Demography. 2014;51(5):1573–96. https://doi.org/10.1007/s13524-014-0325-6.
García Villar J, Quintana-Domeque C. Income and body mass index in Europe. Econ Hum Biol. 2009;7(1):73–83. https://doi.org/10.1016/j.ehb.2009.01.006.
DESTATIS. Cost of illness. Accessed May 25. 2022. https://www.destatis.de/EN/Themes/Society-Environment/Health/Cost-Illness/_node.html (2015), Accessed 29 August 2022.
Statistik Austria. Personal health expenditure by age and gender in Austria. 2019. Published 2023. Accessed October 24, 2023. https://www.statistik.at/en/statistics/population-and-society/health/health-care-and-expenditure/health-expenditure
Statistik, Austria. Lohnsteuerstatistik 2019.; 2020.
AMS, Arbeitsmarktlage. 2019. Published online 2019. Accessed April 14, 2023. https://www.ams.at/arbeitsmarktdaten-und-medien/arbeitsmarkt-daten-und-arbeitsmarkt-forschung/berichte-und-auswertungen#berichte
Statistik Austria. STATcube – Statistische Datenbank. Published 2021. Accessed August 17. 2022. http://www.statistik.at/web_de/services/statcube/index.html (2019), Accessed 15 August 2022.
Dachverband. Jahresbericht der österreichischen Sozialversicherung 2019. Published online 2020.
Sach TH, Barton GR, Doherty M, Muir KR, Jenkinson C, Avery AJ. The relationship between body mass index and health-related quality of life: comparing the EQ-5D, EuroQol VAS and SF-6D. Int J Obes. 2007;31(1):189–96. https://doi.org/10.1038/sj.ijo.0803365.
Laxy M, Stark R, Peters A, Hauner H, Holle R, Teuner C. The non-linear relationship between BMI and health care costs and the resulting cost fraction attributable to obesity. Int J Environ Res Public Health. 2017;14(9):984. https://doi.org/10.3390/ijerph14090984.
Konnopka A, Bödemann M, König HH. Health burden and costs of obesity and overweight in Germany. Eur J Health Econ. 2011;12(4):345–52. https://doi.org/10.1007/s10198-010-0242-6.
Lette M, Bemelmans WJE, Breda J, Slobbe LCJ, Dias J, Boshuizen HC. Health care costs attributable to overweight calculated in a standardized way for three European countries. Eur J Health Econ. 2016;17(1):61–9. https://doi.org/10.1007/s10198-014-0655-8.
Okunogbe A, Nugent R, Spencer G, Powis J, Ralston J, Wilding J. Economic impacts of overweight and obesity: current and future estimates for 161 countries. BMJ Glob Health. 2022;7(9):e009773. https://doi.org/10.1136/bmjgh-2022-009773.
Acknowledgements
We thank our colleague Miriam Reiss who contributed to writing the funding proposal. We thank Miriam Reiss and Johanna Brix who supported us in validating the literature search on relative risks. We thank Berthold Reichardt (Austrian Health Insurance Fund), Werner Bohuslav (Federal Ministry of Social Affairs, Health, Care and Consumer Protection), Beate Mayer (Austrian Accident Insurance Fund), Lukas Eichinger (Social Insurance Institution for Self-Employed Persons), and Barbara Leitner (Statistics Austria) who provided data from administrative sources.
Funding
Funding for this study was provided by the Anniversary Fund of the Oesterreichische Nationalbank, the central bank of the Republic of Austria, grant number 18468.
Author information
Authors and Affiliations
Contributions
TC conceived the idea for the study and acquired funding. TC and SR designed the study, SR acquired and prepared the data, did the analyses, and prepared the initial draft of the manuscript. TC supervised the work and contributed to the interpretation of the data and to the revision of the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it.The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder.To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Reitzinger, S., Czypionka, T. Low-, moderate-, and high-risk obesity in association with cost drivers, costs over the lifecycle, and life expectancy. BMC Public Health 24, 2069 (2024). https://doi.org/10.1186/s12889-024-19574-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12889-024-19574-8