Abstract
Background
Most patients with heart failure (HF) have multimorbidity which may cause difficulties with self-management. Understanding the resources patients draw upon to effectively manage their health is fundamental to designing new practice models to improve outcomes in HF. We describe the rationale, conceptual framework, and implementation of a multi-center survey of HF patients, characterize differences between responders and non-responders, and summarize patient characteristics and responses to the survey constructs among responders.
Methods
This was a multi-center cross-sectional survey study with linked electronic health record (EHR) data. Our survey was guided by the Chronic Care Model to understand the distribution of patient-centric factors, including health literacy, social support, self-management, and functional and mental status in patients with HF. Most questions were from existing validated questionnaires. The survey was administered to HF patients aged ≥ 30 years from 4 health systems in PCORnet® (the National Patient-Centered Clinical Research Network): Essentia Health, Intermountain Health, Mayo Clinic, and The Ohio State University. Each health system mapped their EHR data to a standardized PCORnet Common Data Model, which was used to extract demographic and clinical data on survey responders and non-responders.
Results
Across the 4 sites, 10,662 patients with HF were invited to participate, and 3330 completed the survey (response rate: 31%). Responders were older (74 vs. 71 years; standardized difference (95% CI): 0.18 (0.13, 0.22)), less racially diverse (3% vs. 12% non-White; standardized difference (95% CI): -0.32 (-0.36, -0.28)), and had higher prevalence of many chronic conditions than non-responders, and thus may not be representative of all HF patients. The internal reliability of the validated questionnaires in our survey was good (range of Cronbach’s alpha: 0.50–0.96). Responders reported their health was generally good or fair, they frequently had cardiovascular comorbidities, > 50% had difficulty climbing stairs, and > 10% reported difficulties with bathing, preparing meals, and using transportation. Nearly 80% of patients had family or friends sit with them during a doctor visit, and 54% managed their health by themselves. Patients reported generally low perceived support for self-management related to exercise and diet.
Conclusions
More than half of patients with HF managed their health by themselves. Increased understanding of self-management resources may guide the development of interventions to improve HF outcomes.
Similar content being viewed by others
Introduction
Heart failure (HF) is a major public health problem, currently affecting more than 6 million Americans and projected to increase to more than 8 million Americans by 2030 [1]. HF is one of the most frequent causes of hospitalizations in the United States [2,3,4], and is associated with significant mortality, morbidity, and healthcare expenditures, particularly among those aged 65 and older [5]. HF patients are often elderly, and most have multimorbidity [6], which may precipitate acute decompensation and increase the risk of non-fatal complications, healthcare utilization, and death [7,8,9,10].
As a chronic disease that coexists with multiple other conditions, HF may cause self-management difficulties in patients who may also have knowledge deficits about HF or a lack of social support to manage their illness [11, 12]. Patients with limited health literacy or social support may have more difficulties accessing healthcare services, interacting with the medical system and communicating with physicians, may less effectively participate in decision-making, and may have more difficulties with self-care including managing medications and adhering to healthy behaviors related to physical activity and diet [11, 13, 14]. Furthermore, self-care behaviors, including managing medications, adherence to a healthy diet and physical activity, and monitoring for changes in symptoms may need to be adapted over time as HF progresses, which may hinder effective self-care [15].
Understanding the social and self-management resources that patients draw upon to effectively manage their health is fundamental to design new practice models to improve outcomes in HF. Patients with HF who have more effective self-care behavior have improved quality of life and lower rates of HF and all-cause hospital readmissions [16,17,18], yet randomized controlled trials targeting dietary and exercise interventions have exhibited no to modest improvements in outcomes in patients with HF [19, 20]. Thus, we implemented a survey guided by the Chronic Care Model [21] to understand the distribution of patient-centric factors, including health literacy, social support, and self-management, as well as self-reported functional and mental status in patients with HF. We selected existing validated questionnaires measuring social support, health literacy, and self-management because these are conceptually essential to successfully manage HF.
The current study was designed to comprehensively assess barriers to successful self-management in a real-world population of patients with HF from multiple health care systems with linkage to data from the electronic health record to inform the design of future interventions for improvement of patient outcomes. The survey was administered to HF patients from 4 health care systems in the United States participating in a Patient-Centered Outcomes Research Institute (PCORI) clinical data research network. The purpose of this article is to describe the rationale and conceptual framework of the survey, the constructs included in the survey, the implementation of the survey at the 4 participating sites, and the linkage of clinical data to survey responses. Herein we describe the response rate for our survey and reasons for non-response, characterize differences between survey responders and non-responders, and summarize patient characteristics and responses to the survey constructs among responders.
Methods
Patient-Eentered Eetwork of Eearning Eealth Eystems
The Patient-Centered Network of Learning Health Systems (LHSNet) was a clinical data research network of 9 participating organizations, including 6 health systems, encompassing data on nearly 10 million patients [22]. LHSNet was funded by PCORI from 2015 to 2019. More details on the LHSNet clinical data research network partners and organization structure has been previously published [22]. Briefly, each of the participating sites mapped their data to a standardized National Patient-Centered Clinical Research Network (PCORnet) Common Data Model, allowing efficient execution of data queries across LHSNet and PCORnet [23,24,25]. The LHSNet developed computable phenotypes for a common disease (HF) [26] and a rare disease (osteogenesis imperfecta), and designed surveys for these 2 patient cohorts, along with a weight cohort (overweight and obesity) [27] based on the PCORnet obesity algorithm. Four health systems from LHSNet – Essentia Health, Intermountain Health, Mayo Clinic, and The Ohio State University – participated in the HF survey.
Identification of patients with heart failure
A series of computable phenotypes for HF were developed utilizing data elements available in the PCORnet Common Data Model [26]. The computable phenotypes varied in complexity, ranging from a single diagnosis code to inclusion of diagnosis code(s) plus prescription medications and NT-proBNP. For this study, we chose a computable phenotype that had higher positive predictive value to reduce false positive diagnoses. HF was identified in patients aged 30 years or older using the algorithm that required 2 or more HF diagnostic codes (International Classification of Diseases, Ninth Revision (ICD-9) 428.xx and ICD-10 I50.xx) and 1 or more HF-related prescription drugs (aldosterone antagonists; HF specific beta blockers including bisoprolol, carvedilol, and metoprolol succinate; loop diuretics; digoxin; angiotensin converting enzyme inhibitors; angiotensin receptor blockers; sucabatril/valsartan; ivabradine; and hydralazine/isosorbide dinitrate in Black patients). We required the 2 or more HF diagnostic codes to occur more than 30 days apart and within a 2 year period, and the HF-related prescription to occur within the same 2 year period. The performance of this algorithm was tested against a Framingham criteria-validated cohort of patients with HF in Olmsted County, MN and found to have a sensitivity of 56.1%, specificity of 99.6%, positive predictive value of 80.7%, and negative predictive value of 98.7% [26]. We restricted our sampling frame for the HF survey to patients with recent diagnoses of HF (first ever diagnosis of HF on or after 1/1/2013 at 1 of the participating sites and on or after 1/1/2015 for the other 3 participating sites).
Selection of survey questions
We used the Chronic Care Model as a conceptual model to guide the selection of questions for our survey. The Chronic Care Model is one approach to improving chronic illness care and proposes to change reactive acute-oriented care to care that is proactive and patient-centered. The Chronic Care Model elucidates the requisite elements for improving chronic illness care, including health systems and community requirements, specifically highlighting the importance of self-management support [21, 28, 29]. Both health literacy and social support affect how patients interact with the medical system, participate in decision-making, and practice self-care behaviors [11, 13, 14], and are thus instrumental in improving self-management. Effective self-management has been associated with improved health outcomes in chronic diseases, such as asthma, hypertension, and diabetes [30, 31]. These key patient-centric factors, social support, health literacy, and self-management are conceptually essential to successfully manage HF. A prior meta-analysis reported reduced combined endpoint of HF-related hospitalization or all-cause death, reduced HF-related hospitalization alone, and modestly improved HF-related quality of life for HF patients receiving self-management interventions [18].
Most questions in the survey were from existing validated questionnaires. Social support was measured by the Patient-Reported Outcomes Measurement Information System (PROMIS) surveys measuring components of instrumental support, informational support, and social isolation [32]. In addition, 2 questions from the National Health and Aging Trends Study were included, which assessed whether a patient has someone accompany them to doctor visits and helps with health care activities [33, 34]. Health literacy was assessed using the Health Literacy Screener [35,36,37]. Patient self-management was assessed by the Chronic Illness Resources Survey [38, 39], which includes 7 subscales assessing support from proximal (family and friends) to distal (neighborhood or community) factors. To ensure we obtained measures of functional and mental status at the time of the survey, we also included a question related to general health [40], a question asking participants to self-report cardiovascular related diseases, a question about activities of daily living (ADLs) and mobility activities, and the PROMIS Health Profile [41, 42], which includes 29 questions related to various aspects of mental and physical health. In total, the survey included 85 questions, which are summarized in Table 1.
Survey implementation
The Mayo Clinic Survey Research Center designed and printed the surveys for all participating sites. The survey was printed in English. A consecutive sample of the first 1000 eligible patients were selected from Intermountain Health, whereas all eligible patients were invited for participation at the other 3 sites. Intermountain Health was responsible for printing and assembling the rest of the survey packet and mailing of the surveys for their patients. For Essentia Health and The Ohio State University, patients who met the eligibility criteria for participation were first sent an introductory letter from the site principal investigator. This introductory letter served to inform the patient of the study, the nature of the collaboration between their site and Mayo Clinic, and allowed the patient the opportunity to opt out of the study (via telephone call to the site principal investigator or by checking a box on the introductory letter and returning it via mail) before being mailed a survey. The patients were given 3 weeks after the introductory letter mailing to opt out; for those who did not opt out, the names and addresses of patients were forwarded to the Mayo Clinic Survey Research Center using a secure file transfer protocol. The Mayo Clinic Survey Research Center assembled the survey packets and mailed the surveys for Mayo Clinic, Essentia Health, and The Ohio State University.
The survey packet included a cover letter, Health Insurance Portability and Accountability Act of 1996 (HIPAA) authorization form, survey, and postage paid and addressed return envelope. In accordance with ethical guidelines, the cover letter included written statements that patient participation in the study will not impact their health care at the participating site and that they could refuse study participation without any negative consequences. In addition, if the patient did not wish to participate, they were allowed the opportunity to check a box on the cover letter indicating ‘I am not willing to participate in this research study’ and return the letter in the postage paid envelope. For all sites, a second mailing of the survey packet was sent to non-responders approximately 4 weeks after the initial mailing. For Mayo Clinic only, telephone contact was attempted approximately 4 weeks after the second mailing; patients were given the opportunity to complete the survey over the phone or were mailed another survey if requested. When completed surveys were returned without an accompanying signed HIPAA form, an additional mailing of the HIPAA form was attempted. If a signed HIPAA form was not obtained, the survey was not used. The surveys were completed between 10/7/2014 and 11/14/2018. The completed surveys for all 4 sites were sent for scanning at the Mayo Clinic Survey Research Center, who returned a survey dataset to the site principal investigator. A SAS query was written and distributed to all sites for scoring of the survey responses.
Clinical data collection
The majority of the remaining data elements, including demographic information and comorbidities, were available from the PCORnet Common Data Model. A SAS query was written and distributed to all sites for collection of demographics and comorbidities present at the survey date (cross-sectional study) to ensure a standardized approach was used at all sites for defining these variables. All height and weight values within the 5 years prior to survey date were obtained and averaged. The most recent values of height and weight were used to calculate body mass index (BMI) as weight (in kg) divided by height (in meters) squared, as long as the values were not ≥ 20% lower or higher than the mean values. If a height and/or weight were ≥ 20% lower or higher than the mean, the value was excluded and the next proximal value that was not ≥ 20% different from the mean was selected. Current, former, and never smoking status were obtained at the date of the survey. Comorbidities were ascertained using the United States Department of Health and Human Services list of 20 chronic conditions for studying multimorbidity [43, 44]. We excluded autism and human immunodeficiency virus due to low prevalence. In addition, we added anxiety to the list of chronic conditions because it is common in patients with HF. For each chronic condition, we required 2 occurrences of a code (either the same diagnostic code or 2 different diagnostic codes within the same code set) separated by more than 30 days and occurring within the 5 years prior to the survey date to rule out false positives due to suspect diagnoses. The ICD-9 and ICD-10 codes used to define the conditions are provided in Additional File 1. Left ventricular ejection fraction was not available in the Common Data Model and was pulled from local electronic medical records. The closest ejection fraction within the year prior to the survey date was obtained. Finally, residence was defined based on the zip code using the primary Rural-Urban Commuting Area (RUCA) classification as metropolitan/urban, micropolitan/large rural, small town/small rural, and rural/isolated rural [45].
Statistical analysis
Analyses were performed using SAS statistical software, version 9.4 (SAS Institute Inc., Cary, NC). The response rate was calculated using the American Association for Public Opinion Research (AAPOR) formula 2, as the number of complete and partial surveys divided by the total number of surveys including complete and partial surveys, refusals, non-contacts, others, and cases of unknown eligibility [47]. Internal reliability of each of the validated questionnaires included in our survey was assessed with Cronbach’s alpha using the combined data from all 4 sites. Characteristics of survey responders vs. non-responders were compared using standardized differences, calculated as the difference in means or proportions divided by the standard error; confidence intervals were calculated using methodology by Hedges and Olkin [46]. For the 2 sites utilizing the introductory letter allowing patients to opt out of the study, data were not included for patients who opted out. In addition, data were not available in the PCORnet Common Data Model for some nonresponders at 2 sites (81 at one site and 48 at another site) so their data could not be included in the comparison of responders and non-responders. Among survey responders, summaries of the survey constructs were provided using percentages for categorical variables, and median (25th, 75th percentile) and range for continuous variables. Patients who returned partially completed surveys were retained for analysis; however, missing data was not imputed. For the patients with partial surveys, data was only included for the subscales where all questions were answered and a score could be estimated.
Results
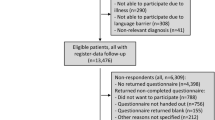
Across the 4 participating sites, 10,662 patients with HF were identified and invited to participate in the survey (Fig. 1). The response rate varied across sites (17.3-44.9%), with an overall response rate of 31.2% (3330 returned surveys). 60% of respondents (1992) returned complete surveys, whereas the remaining returned partial surveys with at least one missing question. Among the 7332 non-responders, the most common reasons for nonparticipants included: no response (3810; 52.0%), refusal (2460; 33.6%), and mental, physical, language, or hearing barrier (464; 6.3%). In addition, 89 patients returned the survey without a signed HIPAA authorization form, and thus the survey could not be used. Responders were older (73.5 vs. 71.2 years), less racially diverse (3.2% vs. 11.5% non-White), less likely to be a current smoker (8.8% vs. 17.5%), less likely to have reduced ejection fraction (EF; 21.7% vs. 27.3% with EF < 40%), and had higher prevalence of many chronic conditions than non-responders (Table 2).
The internal reliability of the validated questionnaires included in our survey was generally good (range of Cronbach’s alpha: 0.50–0.96; Table 1). The majority of responders filled out the survey themselves without assistance (87.6%; Table 3). More than half (58.2%) were married and 22.9% were widowed. Most patients described their health as generally good (42.9%) or fair (33.8%; Table 4). Responders frequently had cardiovascular comorbidities, and more than half had difficulty climbing stairs. In addition, more than 10% of responders reported difficulties with bathing, preparing meals, and using transportation, and 29% of responders reported difficulty with at least 1 activity of daily living (out of a list of 8 activities). Although nearly 80% of patients had family or friends sit with them during a doctor visit, more than half (53.7%) responded that they manage their health mostly by themselves. Patients generally had high health literacy and high levels of both instrumental support (perceived availability of information or advice) and informational support (perceived availability of support for cognitive, material, or task performance; Table 5). In addition, the greatest perceived support/resources for self-management was related to health care (shared decision making, active listening, and efforts to ensure understanding). Participants reported lower perceived support for self-management from community organizations, exercise, diet, and family/friends (which includes questions related to exercising and sharing healthy recipes with friends or family, or having family/friends prepare healthy food for you).
Discussion
We implemented a large, survey of patients with HF seen at 4 health care systems participating in PCORnet® and linked the survey responses with data from the electronic health record. The survey was informed by the Chronic Care Model and included standardized questionnaires measuring constructs related to functional status, mental health, health literacy, social support, and self-management resources. The response rate varied across sites, but was overall 31%. Responders tended to be older and have more chronic conditions than non-responders. In addition, it is noteworthy that the survey respondents were primarily of White race (97%) and had generally high health literacy; thus, our findings should be interpreted in this context. Nevertheless, inclusion of patients with HF seen at 4 health systems may have improved the generalizability of our results over a single center survey.
Patients with HF have many comorbidities which often complicate their management, and the complexity of treatment plans for these patients may encumber self-management. Self-management encompasses three sets of tasks, including medical management of the condition; maintaining, changing, and creating new behaviors; and coping with emotions commonly experienced by having a chronic condition [48]. In HF, self-management behaviors include activities such as smoking cessation, adhering to a healthy diet, exercising, and taking medication (self-care maintenance); daily weighing, monitoring blood pressure, and observing changes in fatigue, shortness of breath, and activity level (self-care monitoring); and adjusting medications such as diuretics, adapting activity level and diet, and consulting a health care professional in response to changes in symptoms when they occur (self-care management) [15].
In the current study, most patients with HF had high health literacy, as well as informational and instrumental support. However, patients reported generally low perceived support for self-management related to self-care maintenance practices of exercise and diet, important modifiable risk factors in the long-term management of HF. Recent HF guidelines from the American Heart Association/American College of Cardiology/Heart Failure Society of America with class I level of evidence say we should provide multidisciplinary education and support to promote self-care in patients with HF and to reduce potential medical and social barriers to self-care [49]. Recommendations from the Heart Failure Association of the European Society of Cardiology outlines lifestyle and behavior recommendations, which include diet and physical activity, to promote self-care maintenance and management in patients with HF [15]. Evidence from systematic reviews and meta-analyses shows that patients with HF who have more effective self-care behavior have lower rates of HF and all-cause hospital readmissions and modestly improved quality of life [16,17,18]. Furthermore, large multi-center randomized controlled trials showed modest effects of exercise training on the composite endpoints of all-cause mortality or hospitalization and cardiovascular mortality or HF hospitalization [20], but no effect of a dietary intervention to reduce sodium intake on outcomes in patients with HF [19]. Thus, increased understanding of self-management resources for lifestyle behaviors may be needed to design interventions to improve outcomes in patients with HF.
Future directions
Although the data in the current study are primarily cross-sectional, due to the availability of patient identifiers at each of the sites, it would be possible to pull in additional data from the electronic health record to obtain follow-up information and relevant patient outcomes for future studies. Furthermore, linkage to other data sources could bring in additional data that may not be available in the medical records, such as publicly available data on neighborhood characteristics from the Census or American Community Survey, measures of neighborhood socioeconomic status, or information on causes of death from the National Death Index. In addition, the available data on survey non-responders would allow further research to understand participation bias and to inform future design and implementation of surveys to improve the generalizability of survey findings. Finally, a unique aspect to our survey implementation was the utilization of a single survey research center for survey administration at 3 of the 4 sites. Our process can serve as a guide for future multi-center surveys, but additional research may be warranted to identify methods to improve inclusion of sites who may lack resources to facilitate survey administration and to streamline processes for multi-center surveys utilizing a single survey research center.
Limitations and strengths
Some limitations deserve mention. First, we relied on a computable phenotype to identify patients with HF using electronic health record data, so some misclassification in the diagnosis of HF may have occurred. Second, our survey was long, including 85 questions, which may have affected our response rate (31%) and may have contributed to return of partial surveys with at least one missing question (40% of respondents). Third, we observed some notable differences in characteristics of responders vs. non-responders. Fourth, the vast majority of respondents were non-Hispanic Whites. Taken collectively, these may have affected the external validity of our results. Thus, the results of our study should be interpreted in this context because our findings may not be generalizable to all patients with HF. Fifth, we did not include questions in the survey related to medical management of HF or treatment burden in general, which may influence self-management. Nevertheless, we leveraged the PCORnet infrastructure to efficiently identify a cohort of HF patients with similar inclusion criteria across 4 health systems, and implemented a large survey to gather critical information not included in electronic medical records, and linked our survey data with data from the electronic health record.
Conclusions
We implemented a survey guided by the Chronic Care Model to understand the distribution of patient-centric factors, including health literacy, social support, self-management, and functional and mental status in patients with HF. Most patients with HF in this study described their health as generally good or fair, had high health literacy and social support, and most frequently endorsed health care as the greatest perceived support/resource for self-management. However, patients reported generally low perceived support for self-management related to exercise and diet, indicating that knowledge and social support may not be sufficient for effective self-management. Furthermore, more than half of patients with HF manage their health by themselves. Increased understanding of self-management resources for lifestyle behaviors such as diet and exercise, in particular, may guide the development of interventions to improve HF outcomes.
Data availability
The participants of this study did not provide written informed consent for storage of their data on an individual level in repositories or journals. However, requests to access the data set from qualified researchers trained in human subject confidentiality protocols will be considered. Access to the data will require approval of the proposed use of the data and establishment of data use agreements with each of the participating health systems. Researchers who wish to access the data set should send a request to the corresponding author.
Abbreviations
- AAPOR:
-
American Association for Public Opinion Research
- ADLs:
-
activities of daily living
- BMI:
-
body mass index
- HIPAA:
-
Health Insurance Portability and Accountability Act of 1996
- HF:
-
heart failure
- LHSNet:
-
Patient-Centered Network of Learning Health Systems
- PCORI:
-
Patient-Centered Outcomes Research Institute
- PCORnet:
-
The National Patient-Centered Clinical Research Network
- PROMIS:
-
Patient-Reported Outcomes Measurement Information System
- RUCA:
-
Rural-Urban Commuting Area
References
Benjamin EJ, Muntner P, Alonso A, et al. Heart disease and stroke statistics-2019 update: a report from the American Heart Association. Circulation. 2019;139(10):e56–66.
Jackson SL, Tong X, King RJ, Loustalot F, Hong Y, Ritchey MD. National burden of heart failure events in the United States, 2006 to 2014. Circ Heart Fail. 2018;11(12):e004873.
Jencks SF, Williams MV, Coleman EA. Rehospitalizations among patients in the Medicare fee-for-service program. N Engl J Med. 2009;360(14):1418–28.
McDermott KW, Elixhauser A, Sun R. Trends in hospital inpatient stays in the United States, 2005–2014. HCUP Statistical Brief #225. June 2017. Agency for Healthcare Research and Quality, Rockville, MD; Available from: http://www.hcup-us.ahrq.gov/reports/statbriefs/sb225-Inpatient-US-Stays-Trends.pdf
Yancy CW, Jessup M, Bozkurt B, et al. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice guidelines. J Am Coll Cardiol. 2013;62(16):e147–239.
Chamberlain AM, St Sauver JL, Gerber Y, et al. Multimorbidity in heart failure: a community perspective. Am J Med. 2015;128(1):38–45.
Ahluwalia SC, Gross CP, Chaudhry SI, et al. Impact of comorbidity on mortality among older persons with advanced heart failure. J Gen Intern Med. 2012;27(5):513–9.
Braunstein JB, Anderson GF, Gerstenblith G, et al. Noncardiac comorbidity increases preventable hospitalizations and mortality among Medicare beneficiaries with chronic heart failure. J Am Coll Cardiol. 2003;42(7):1226–33.
Metra M, Zaca V, Parati G, et al. Cardiovascular and noncardiovascular comorbidities in patients with chronic heart failure. J Cardiovasc Med. 2011;12(2):76–84.
Page RL 2nd, Lindenfeld J. The comorbidity conundrum: a focus on the role of noncardiovascular chronic conditions in the heart failure patient. Curr Cardiol Rep. 2012;14(3):276–84.
Magnani JW, Mujahid MS, Aronow HD, et al. Health literacy and cardiovascular disease: fundamental relevance to primary and secondary prevention: a scientific statement from the American Heart Association. Circulation. 2018;138(2):e48–74.
Toukhsati SR, Driscoll A, Hare DL. Patient self-management in chronic heart failure - establishing concordance between guidelines and practice. Card Fail Rev. 2015;1(2):128–31.
Graven LJ, Grant JS. Social support and self-care behaviors in individuals with heart failure: an integrative review. Int J Nurs Stud. 2014;51(2):320–33.
Kitko L, McIlvennan CK, Bidwell JT, et al. Family caregiving for individuals with heart failure: a scientific statement from the American Heart Association. Circulation. 2020;141(22):e864–78.
Jaarsma T, Hill L, Bayes-Genis A, et al. Self-care of heart failure patients: practical management recommendations from the Heart Failure Association of the European Society of Cardiology. Eur J Heart Fail. 2021;23(1):157–74.
McAlister FA, Stewart S, Ferrua S, McMurray JJ. Multidisciplinary strategies for the management of heart failure patients at high risk for admission: a systematic review of randomized trials. J Am Coll Cardiol. 2004;44(4):810–9.
Jovicic A, Holroyd-Leduc JM, Straus SE. Effects of self-management intervention on health outcomes of patients with heart failure: a systematic review of randomized controlled trials. BMC Cardiovasc Disord. 2006;6:43.
Jonkman NH, Westland H, Groenwold RH, et al. Do self-management interventions work in patients with heart failure? An individual patient data meta-analysis. Circulation. 2016;133(12):1189–98.
Ezekowitz JA, Colin-Ramirez E, Ross H, et al. Reduction of dietary sodium to less than 100 mmol in heart failure (SODIUM-HF): an international, open-label, randomised, controlled trial. Lancet. 2022;399(10333):1391–400.
O’Connor CM, Whellan DJ, Lee KL, et al. Efficacy and safety of exercise training in patients with chronic heart failure: HF-ACTION randomized controlled trial. JAMA. 2009;301(14):1439–50.
Coleman K, Austin BT, Brach C, Wagner EH. Evidence on the Chronic Care Model in the new millennium. Health Aff. 2009;28(1):75–85.
Finney Rutten LJ, Alexander A, Embi PJ, et al. Patient-Centered Network of Learning Health Systems: developing a resource for clinical translational research. J Clin Transl Sci. 2017;1(1):40–4.
PCORnet Common Data Model. (CDM) https://pcornet.org/pcornet-common-data-model/.
Fleurence RL, Curtis LH, Califf RM, Platt R, Selby JV, Brown JS. Launching PCORnet, a national patient-centered clinical research network. J Am Med Inf Assoc. 2014;21(4):578–82.
Qualls LG, Phillips TA, Hammill BG, et al. Evaluating foundational data quality in the National Patient-Centered Clinical Research Network (PCORnet(R)). EGEMS (Wash DC). 2018;6(1):3.
Tison GH, Chamberlain AM, Pletcher MJ, et al. Identifying heart failure using EMR-based algorithms. Int J Med Inf. 2018;120:1–7.
Croghan IT, Phelan SM, Bradley DP, et al. Needs assessment for weight management: the Learning Health System Network experience. Mayo Clin Proc Innov Qual Outcomes. 2018;2(4):324–35.
Barr VJ, Robinson S, Marin-Link B, et al. The expanded Chronic Care Model: an integration of concepts and strategies from population health promotion and the Chronic Care Model. Hosp Q. 2003;7(1):73–82.
Wagner EH. Chronic disease management: what will it take to improve care for chronic illness? Eff Clin Pract. 1998;1(1):2–4.
Lorig KR, Ritter P, Stewart AL, et al. Chronic disease self-management program: 2-year health status and health care utilization outcomes. Med Care. 2001;39(11):1217–23.
Damush TM, Jackson GL, Powers BJ, et al. Implementing evidence-based patient self-management programs in the Veterans Health Administration: perspectives on delivery system design considerations. J Gen Intern Med. 2010;25(Suppl 1):68–71.
Hahn EA, DeWalt DA, Bode RK, et al. New English and Spanish social health measures will facilitate evaluating health determinants. Health Psychol. 2014;33(5):490–9.
Freedman VA, Kasper JD. Cohort Profile: the National Health and Aging trends Study (NHATS). Int J Epidemiol. 2019;48(4):1044–g5.
National Health and Aging Trends Study (NHATS.) https://www.nhats.org/researcher/nhats.
Chew LD, Bradley KA, Boyko EJ. Brief questions to identify patients with inadequate health literacy. Fam Med. 2004;36(8):588–94.
Chew LD, Griffin JM, Partin MR, et al. Validation of screening questions for limited health literacy in a large VA outpatient population. J Gen Intern Med. 2008;23(5):561–6.
Wallston KA, Cawthon C, McNaughton CD, Rothman RL, Osborn CY, Kripalani S. Psychometric properties of the brief health literacy screen in clinical practice. J Gen Intern Med. 2014;29(1):119–26.
Glasgow RE, Toobert DJ, Barrera M Jr., Strycker LA. The Chronic Illness Resources Survey: cross-validation and sensitivity to intervention. Health Educ Res. 2005;20(4):402–9.
Glasgow RE, Strycker LA, Toobert DJ, Eakin E. A social-ecologic approach to assessing support for disease self-management: the Chronic Illness Resources Survey. J Behav Med. 2000;23(6):559–83.
Hays RD, Bjorner JB, Revicki DA, Spritzer KL, Cella D. Development of physical and mental health summary scores from the patient-reported outcomes measurement information system (PROMIS) global items. Qual Life Res. 2009;18(7):873–80.
Cella D, Choi SW, Condon DM, et al. PROMIS((R)) Adult Health Profiles: efficient short-form measures of seven health domains. Value Health. 2019;22(5):537–44.
Hays RD, Spritzer KL, Schalet BD, Cella D. PROMIS((R))-29 v2.0 profile physical and mental health summary scores. Qual Life Res. 2018;27(7):1885–91.
Goodman RA, Posner SF, Huang ES, Parekh AK, Koh HK. Defining and measuring chronic conditions: imperatives for research, policy, program, and practice. Prev Chronic Dis. 2013;10:E66.
US Department of Health and Human Services. Multiple chronic conditions - a strategic framework: optimum health and quality of life for individuals with multiple chronic conditions. 2010. Washington, DC. Accessed at: https://www.hhs.gov/sites/default/files/ash/initiatives/mcc/mcc_framework.pdf
Rural-urban commuting area codes: United States Department of Agriculture. Economic Research Service; https://www.ers.usda.gov/data-products/rural-urban-commuting-area-codes.aspx.
Hedges LV, Olkin I. Statistical methods for Meta-Analysis. San Diego, CA: Academic; 1985.
The American Association for Public Opinion Research. Standard Definitions: Final Dispositions of Case Codes and Outcome Rates for Surveys. 9th edition: AAPOR. 2016. https://www.aapor.org/AAPOR_Main/media/publications/Standard-Definitions20169theditionfinal.pdf
Lorig KR, Holman H. Self-management education: history, definition, outcomes, and mechanisms. Ann Behav Med. 2003;26(1):1–7.
Heidenreich PA, Bozkurt B, Aguilar D, et al. 2022 AHA/ACC/HFSA guideline for the management of heart failure: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice guidelines. Circulation. 2022;145(18):e895–1032.
Acknowledgements
We thank Jill M. Killian and Susan A. Weston for their assistance with data analysis, and Deborah S. Strain for her assistance in preparation and formatting of the manuscript.
Funding
This work was supported by grants from the National Heart, Lung, and Blood Institute (R01 HL120859) and the Patient Centered Outcomes Research Institute - PCORI (CDRN-1501-26638). The content is solely the responsibility of the authors; the funding sources played no role in the design, conduct, or reporting of this study.
Author information
Authors and Affiliations
Contributions
AMC, EMH, IVH, BDH, CPB, KDR, SMM, and VLR made substantial contributions to the conception and design of the work.AMC EMH, IVH, BDH performed the data acquisition and analysis, and AMC, EMH, IVH, BDH, CPB, KDR, SMM, VLR interpreted the data.All authors drafted the work or substantively revised it.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study was approved by the Mayo Clinic Institutional Review Board (17-008773), which served as the IRB of record for the other 3 relying institutions participating in the LHSNet HF survey study. For this minimal risk study, the Mayo Clinic Institutional Review Board granted a waiver of written informed consent. All elements of informed consent were presented to participants in the cover letter that accompanied the survey packet in the mail. By completing and returning the survey, the Mayo Clinic Institutional Review Board considered this implied consent. The signed HIPAA form allowed linkage of the survey data to electronic health record data for patients who completed the survey. If a survey was returned without a signed HIPAA form, the survey was not used. All methods were carried out in accordance with relevant guidelines and regulations, and the Declaration of Helsinki.
Competing interests
The authors declare no competing interests.
Consent for publication
Not applicable.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Chamberlain, A.M., Hade, E.M., Haller, I.V. et al. A large, multi-center survey assessing health, social support, literacy, and self-management resources in patients with heart failure. BMC Public Health 24, 1141 (2024). https://doi.org/10.1186/s12889-024-18533-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12889-024-18533-7