Abstract
Background
The relationship between integrated lifestyles, mental status and their impact on overall well-being has attracted considerable attention. This study aimed to evaluate the association between lifestyle factors, depression and diabetic retinopathy (DR) in adults aged 18–64 years.
Methods
A cohort of 3482 participants diagnosed with diabetes was drawn from the National Health and Nutrition Examination Survey (NHANES) spanning the years 1999–2018. DR was defined based on self-reported diabetic retinopathy diagnoses by professional physicians, relying on Diabetes Interview Questionnaires. Subgroup analysis was employed to assess lifestyle and psychological factors between participants with DR and those without, both overall and stratified by diabetic duration. Continuous variables were analyzed using the student’s t test, while weighted Rao-Scott χ2 test were employed for categorical variables to compare characteristics among the groups.
Results
Of the 3482 participants, 767 were diagnosed with diabetic retinopathy, yielding a weighted DR prevalence of 20.8%. Patients with DR exhibited a higher prevalence of heavy drinking, depression, sleep deprivation, and insufficient physical activity compared to those without DR. Furthermore, multivariable logistic regression analysis revealed that sleeping less than 5 h (OR = 3.18, 95%CI: 2.04–4.95, p < 0.001) and depression (OR = 1.35, 95%CI:1.06–1.64, p = 0.025) were associated with a higher risk of DR, while moderate drinking (OR = 0.49, 95%CI: 0.32–0.75, p = 0.001) and greater physical activity (OR = 0.64, 95%CI: 0.35–0.92, p = 0.044) were identified as protective factors.
Conclusions
Adults aged 18–64 years with DR exhibited a higher prevalence of lifestyle-related risk factors and poorer mental health. These findings underscore the need for concerted efforts to promote healthy lifestyles and positive emotional well-being in this population.
Similar content being viewed by others
Background
Diabetes has rapidly become one of the most prevalent diseases globally. While diabetes prevention is crucial, mitigating diabetic vascular complications that contribute significantly to considerable morbidity and mortality is an even more pressing concern [1]. Diabetic retinopathy (DR), a major microvascular complication, has now become a leading cause of blindness among working-age adults [2]. According to The Vision Loss Expert Group, DR ranks sixth among the most prevalent preventable causes of vision impairment, potentially impacting as many as 160.5 million people worldwide [3]. Consequently, the prevention and timely treatment of DR carry immense clinical significance.
Numerous publications have established the importance of not only controlling traditional metabolic factors such as hyperglycemia, hypertension and hyperlipemia, but also maintaining a healthy lifestyle as a crucial strategy for preventing DR [4,5,6,7]. Despite significant advances in the development of medications targeting traditional metabolic factors effectively preventing the occurrence of diabetic complications, the promotion lifestyle risk management remains an area where progress is needed, particularly among adults aged 18–64 years [8]. The prevalence of diabetes in this age group is anticipated to increase by 18% over the next decade, reaching 417 million by 2030 [9]. In addition to the substantial medical costs associated with diabetes, it may also result in work disability [10], with indirect economic losses potentially surpassing the expenses incurred for treatment [11]. Therefore, assessing diabetes-related lifestyle factors among adults aged 18–64 years is crucial for preventing DR. However, to our knowledge, research in this field is still lacking.
This study aims to assess the differences in lifestyle-related and mental factors among adults aged 16–64 years diagnosed with diabetes, both with and without DR, and to explore their association with the development of DR.
Methods
Data resource and study population
National Health and Nutrition Examination Survey (NHANES), an ongoing, biennial cross-sectional survey, utilized a complex multistage probability sampling design to investigate the nutritional and health status of the civilian, non-institutionalized U.S. population [12, 13]. Data collected involved physical examinations, laboratory tests, and questionnaires. Informed written consent was obtained from all participants, and the study received ethical approval from the National Center for Health Statistics. The research adhered to the Tenets of the Declaration of Helsinki.
Our study encompassed 3482 participants aged 18–64 years from NHANES 1999–2018, as illustrated in Fig. 1. All participants were non-pregnant and had received a diagnosis of diabetes from a physician or other health professional at 18 years of age or older (excluding diagnosis made solely during pregnancy)
A comprehensive flow chart of the study design
A total of 101,316 participants were enrolled in NHANES 1999–2018. 56163 participants who wasn’t working-age adults were excluded. 41650 participants without self-reported diabetes were excluded. After excluding 21 pregnant participants, 3482 participants were included.
Definitions of diabetes, diabetic retinopathy and lifestyle-related factors
Diabetes in this study was defined based on self-reported diagnoses confirmed by professional physicians, rather than relying on glycosylated hemoglobin A1c (HbA1c) and self-reported results. This approach was selected due to the significant impact of diabetic duration as a risk factor for DR, with newly-diagnosed diabetes not adequately reflecting its substantial influence. Diabetic retinopathy was identified through self-reported diagnosed retinopathy, as confirmed by a dichotomous item as presented consistent with previous literature [14,15,16,17]. In the Diabetes Interview Questionnaires, participants diagnosed with diabetes were asked “Has a doctor ever told you/SP that diabetes has affected your/his/her eyes or that you/s/he had retinopathy (ret-in-op-ath-ee)?” Affirmative responses were considered indicative of self-reported DR. The duration of diagnosed diabetes was calculated as the age at examination minus the age at diagnosis, then subcategorized into three groups: 0–10 years, 11–19 years and ≥ 20 years.
Lifestyle-related variables in NHANES encompassed smoking status, drinking status, physical activity, healthy eating index (HEI), sleeping duration, and self-reported sleeping disorder. Smoking status (never smoker, former smoker, current smoker) was determined by using Smoking-Cigarette Use questionnaires, with never smoking defined as lifetime consumption of fewer than 100 cigarettes. Drinking status (no drinking, mild to moderate drinking, heavy drinking) was based on self-reported drinking frequency and quantity, following officially provided units (1 drink contains 14 g of ethanol) [18]. Mild to moderate drinking was defined as < 1 drink/day for women and < 2 drinks/day for men, while heavy drinking was ≥ 1 drink/day for women and ≥ 2 drinks/day for men. Physical activity level (< 150, 150–1800, > 1800 MET*min/week) was classified by the total MET-min per week, calculated by multiplying the standard metabolic equivalent (MET) value of each activity by its weekly minutes and then summing across activities. Dietary quality was assessed through 24-hour dietary recalls and HEI scores aligned with the 2015–2020 Dietary Guidelines for Americans were used for the 1999–2014 survey cycles. HEI scores were categorized as < 25%, 25-75%, > 75%, with higher HEI indicating healthier eating habits. Sleeping status included sleeping time (< 5, 5–9, > 9 h) and sleeping disorders (with/without sleeping disorder), obtained from the Sleep-Disorders questionnaire since NHANES 2005–2006. Additionally, depression status (with/without depression) was estimated as an indicator of the mental condition, defined using the nine-item depression screening instrument called the Patient Health Questionnaire (PHQ-9) [19], with a score ≥ 10 considered indicative of depression.
Demographic and physical variables
Demographic variables included age, gender (male/female), race/ethnicity (Mexican American, Other Hispanic, non-Hispanic White, non-Hispanic Black, other), family economic status (poverty-to-income ratio, ≤ 130%, 130–350%, > 350%), educational level (less than high school, high school or equivalent, college and more), health insurance status (uninsured, any health insurance), and marital status (married, unmarried, other status).
Body mass index (BMI) was calculated as the measured weight in kilograms divided by height in meters squared and categorized as normal (18.5–24.9), overweight (25.0–29.9), obese class I (30.0–34.9), obese class II (35.0–39.9), or obese class III (≥ 40.0). HbA1c level below 7% were considered well-controlled. Uncontrolled hypertension was defined as elevated blood pressure (≥ 140/90 mmHg) measured by a board-eligible physician after the participant had been in a seated position for 5 min of quiet rest. Dyslipidemia was determined by non-high-density lipoprotein (non-HDL) cholesterol, calculated as total measured cholesterol minus HDL cholesterol, with a non-HDL level < 130 mg/dL considered indicative of well-controlled plasma lipids [20].
Diabetic nephropathy (DN) was defined by either a urine albumin to creatinine ratio of 30 mg/g or higher, or estimated glomerular filtration rate less than 60 mL/min/1.73 m2. Cardiovascular diseases (CVD) were defined by self-reported any of the following diseases: self-reported congestive heart failure, coronary heart disease, heart attack, or stroke [21].
Statistical analysis
Data from the NHANES 1999–2000 to NHANES 2017–2018 cycles were analyzed. The distribution of demographic, clinical, and lifestyle characteristics among participants was examined. Lifestyle-related and mental factors were compared by using the student’s t test in a weighted linear regression model (for continuous variables) or the weighted Rao-Scott χ2 test (for categorical variables) between the two groups (DR and no DR). Given the well-established association between the duration of diabetes and the risk of DR, subgroup analyses were conducted, stratified by diabetic duration to assess differences and variations in lifestyle. A weighted univariable and multivariable logistic regression model was employed to explore the impact of lifestyle on DR, adjusting for potential confounders including gender, age, race/ethnicity, diabetic duration, DN, CVD, HbA1c, blood pressure, lipids control. Appropriate sample weights for the interview sample, examination sample and fasting subsample were utilized. If the level of missing data for primary analyses was less than 10%, a complete case analysis was applied. All statistical analyses were performed using Statistical Analysis Software procedures (SAS version 9.4). A two-sided p-value < 0.05 was considered statistically significant.
Results
Descriptive statistics
Of the 3482 patients included in the study, 1736 (51.2%) were male and 1746 (48.8%) were female (data was shown as unweighted number and weighted percent). The median age was 52.2 years (IQR: 44.7 to 58.4 years). Demographic and clinical characteristics were presented in Table 1. Participants with self-reported DR exhibited a longer diabetic duration (10.3 years vs. 5.1 years, p < 0.001), lower family income (family poverty-to-income ratio ≤ 1.3: 35.3% vs. 25.9%, p < 0.001) and lower educational level (less than high school: 27.1% vs. 22.7%, p = 0.013), worse HbA1c (< 7%: 53.3% vs. 38.3%, p < 0.001), blood pressure (< 140/90 mmHg: 79.1% vs. 71.5%, p = 0.004) control and higher prevalence of DN (26.9% vs. 41.4%, p < 0.001) and CVD (15.6% vs. 23.4%, p < 0.001).
Lifestyle and mental health factors in adults aged 18–64 years
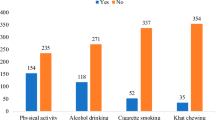
Lifestyle and mental health factors were examined including smoking, drinking, diet, physical activity, sleeping, and depression among participants with diabetes, both overall and stratified by self-reported DR status. In the general cohort, 22.5% of patients were current smokers and only 18.1% were non-drinkers. The median HEI score was 52.2 (IQR: 51.3–53.1). 37.6% of participants engaged in higher levels of physical activity (> 1800 MET-min/week) and the median sleeping time duration was 6.7 h. The prevalence of sleeping disorders and depression was 41.9% and 14.6%, respectively.
Considering the established link between diabetes duration and the risk of DR, we conducted a subgroup analysis of lifestyle and psychological status among 18-64-year-old diabetic patients, stratified by disease duration (see Table S1). Notably, with the extension of diabetic duration, the only significant change observed was in alcohol consumption patterns. The proportion of patients who did not drink increased from 16.9 to 20.8%, while excessive drinking decreased from 7.7 to 3.7%. However, no significant improvement were noted in smoking habits, diet quality, sleep status and exercise level, and, notably, the proportion of comorbid depression increased from 13.2 to 20.2% (p for trend < 0.001).
Association between lifestyle and mental health factors with DR
In comparison with patients without DR, self-reported patients exhibited a higher prevalence of heavy drinking habits (7.5% vs. 6.0%, p < 0.001), inadequate sleep duration (< 5 h: 12.6% vs. 5.3%, p < 0.001), lack of physical activity (< 150 MET-min /week: 16.0% vs. 10.0%, p = 0.038), and depression (with depression: 18.6% vs. 13.5%, p = 0.011) (see Table 1).
We further conducted a thorough exploration of the impact of lifestyle factors on diabetic retinopathy using univariable and multivariate logistic regression (Table 2). In the univariable logistics regression analysis, mild to moderate alcohol consumption (OR = 0.48, 95%CI: 0.33–0.71, p < 0.001) and adequate physical activity (OR = 0.54, 95%CI: 0.32–0.92, p = 0.023) were observed to be associated with a reduced risk of DR. In contrast, a sleeping duration of less than 5 h (OR = 2.64, 95%CI: 1.82–3.84, p < 0.001) and depression (OR = 1.47, 95%CI: 1.09–1.98, p = 0.012) increased the likelihood of developing DR for adults with diabetes. After adjusting for potential confounders including age, sex, race/ethnicity, diabetic duration, HbA1c, blood pressure, lipid control, DN and CVD, mild to moderate drinking (OR = 0.49, 95%CI: 0.32–0.75, p = 0.001) and greater physical activity (OR = 0.64, 95%CI: 0.35–0.92, p = 0.044) were associated with a lower risk of DR. Conversely, a sleeping duration of < 5 h (OR = 3.18, 95%CI: 2.04–4.95, p < 0.001) and the presence of depression (OR = 1.35, 95%CI: 1.06–1.64, p = 0.025) were found to elevate the chances of developing DR.
Discussion
In this large population-based study, we provided an initial description of the prevalence of diabetic retinopathy and lifestyle-related factors among 18 to 64-year-old adults with self-reported diagnosed diabetes. Moreover, we explored the impact of lifestyle and mental factors on DR. The findings highlighted that the individuals with DR tend to exhibit poorer lifestyle management and a more precarious mental condition. Specifically, mild to moderate alcohol consumption and greater physical activity were protective factors against DR, whereas sleep deprivation and depression were associated with an increased risk.
From our study, the overall prevalence of DR among working-age adults was found to be 20.8%, which is lower than the global prevalence reported in 2012 [22]. Several reasons might account for this difference. First, our study specifically included adults under 65 years old, suggesting a likely shorter diabetes duration than in the general patient population, potentially resulting in less progression of DR pathophysiology. Second, as a nationally cross-sectional research endeavor, data from NHANES may be limited in accurately representing global prevalence trends. Additionally, the prevalence estimates could be due to the reliance on self-reported diabetes instead of incorporating self-report and HbA1c, potentially resulting in an underestimation [23,24,25,26].
Demographic and socioeconomic factors have been shown to significantly influence diabetic complications, potentially mediated through lifestyle factors influencing healthcare tendentiousness and self-management engagement [27]. In our study, patients diagnosed with DR exhibited a worse socioeconomic status, including lower education and family income level, contributing to spatial heterogeneity and uneven health care. Although diabetic duration is a well-recognized risk factor for DR [23], our observation revealed that [28], only alcohol consumption significantly reduced over time. Few studies have explored the impact of diabetic duration on lifestyle. Longer diabetic duration was associated with poorer glycemic control, while early intervention could promote positive outcomes in pre-diabetes [29]. However, our data suggested that increased duration didn’t improve the patient’s self-management awareness or healthy lifestyle preferences, but rather increased the prevalence of depression. Therefore, it is critically important to deliver more robust health education to patients with long-term disease.
Numerous studies have consistently demonstrated that adopting healthy lifestyles can reduce the incidence of diabetes and its associated complications. In alignment with previous research, our research indicated that smoking tends to be a risk factor of DR. This conclusion Is supported by earlier investigations, including a meta-analysis involving 73 studies, which found a significant association between smoking and increased risk of DR in type 1 diabetes [30]. Notably, although smoking cessation le to a reduction of prevalence of DR, individuals who quit smoking still exhibited a higher prevalence of DR and CVD compared to non-smokers [31]. In our study, we observed that mild to moderate drinking appears to confer protection against diabetic retinopathy. Similar findings have been reported in prior studies. The EURODIAB Prospective Complications Study, which followed 1857 patients with diabetes, established a connection between moderate alcohol consumption and a decreased risk of proliferative retinopathy, neuropathy and macroalbuminuria. Furthermore, moderate alcohol consumption, defined as one to three drinks a day, has been associated with reduced cardiovascular disease risk in the general population [32, 33]. However, it’s important to note that some publications failed to identify a significant association between alcohol consumption and diabetic complications [34].
From our study, a higher HEI score reflecting better dietary quality did not show an association with a lower DR risk. However, emerging evidence suggests that improved diet quality is associated with other diabetes complications including DN and coronary heart disease. For instance, among 461 youth and young adults with type 1 diabetes, a borderline inverse association was observed between the HEI score and microalbuminuria (OR = 0.83, p = 0.07) [35]. Additionally, a cross-sectional study of 358 type 2 diabetic adults found that higher HEI scores were associated with a lower 10-year CVD risk through improved glucose/lipid control [36].
In contrast, our study indicated that greater physical activity is associated with reduced risk of DR, which was in line with previous studies. The inverse association between physical activity and the risk of diabetic complications may be attributed to reduced inflammation and elevated levels of 25-OH-Vitamin D3 [37].In this study, sleeping for less than 5 h was associated with an increased risk of DR. Sleep duration demonstrated a U-shaped association with diabetic vascular complications, with 6–8 h recommended for the lowest risk [38]. Beyond sleep deprivation, poor sleep quality has also been shown to impact the incidence of diabetic complication [39]. Potential mechanisms underlying these associations may involve sleep deprivation decreasing insulin sensitivity and enhancing systemic inflammatory responses due to circadian rhythm disruption [40].
Our observation establishes a link between depression and an increased risk of DR. This aligns with the findings reported in previous study where a meta-analysis of 27 studies revealed a significant correlation between depression and diabetes complications [41]. Depression influences diabetes control through biological and behavioral pathways, hypothalamic-pituitary-adrenal axis activation, sympathetic stimulation, inflammation, and subsequent poor glycemic control [42]. Additionally, depression can impair glycemic control by negatively affecting self-care practices, medication adherence, and preventive care. Notably, young patients with diabetes are more likely to experience depression [43], highlighting the need to address their psychological health as part of a comprehensive diabetes management approach.
Our study aimed to estimate the prevalence of DR and examine the impact of lifestyle and mental health factors among U.S. participants aged 18 to 64 years. However, there are several limitations that may hinder the generalizability of our findings. First, the reliance on self-reported diabetic retinopathy and lifestyle factors may introduce recall bias, as a proportion of individuals with DR may be unaware of their diagnosis [44]. A study by Kristen et al. reported that 10.6% of participants older than 40 years who underwent fundus photography in NHANES were unaware of their DR diagnosis, including 23.1% with self-reported diabetes and 6.8% reported without a diabetes diagnosis. Besides, the study is cross-sectional in nature, and further cohort studies are needed to confirm our findings. Nevertheless, it is important to note that this study included a large representative sample population selected through a complex, multistage sampling method. The use of objective statistical methods and adjustment for various interactions aimed to mitigate potential bias, thereby enhancing the reliability of the association between lifestyle factors and DR.
Conclusions
Collectively, adults aged 18–64 years with DR demonstrated a higher prevalence of lifestyle risk factors, including excessive drinking, sleep disorders, unhealthy diet, and a more precarious mental status including the presence of depression. Beyond the management of metabolic risk factors, there is a clear need to promote healthy lifestyles within this population to effectively prevent the onset and progression of diabetic retinopathy.
Data Availability
The code and data generated during the study were available from the corresponding author on reasonable request. And the original data was public on the NHANES website (https://www.cdc.gov/nchs/nhanes/index.htm).
Abbreviations
- DR:
-
Diabetic Retinopathy
- NHANES:
-
National Health and Nutrition Examination Survey
- HbA1c:
-
Glycosylated hemoglobin A1c
- SBP:
-
Systolic Blood Pressure
- DBP:
-
Diastolic Blood Pressure
- Non-HDL:
-
Non-high-Density Lipoprotein
- DN:
-
Diabetic Nephropathy
- HEI:
-
Healthy Eating Index
- MET:
-
Metabolic Equivalent
- PHQ:
-
Patient Health Questionnaire
- CVD:
-
Cardiovascular Diseases
- BMI:
-
Body Mass Index
- OR:
-
Odds Ratio
- CI:
-
Confidential Interval
- IQR:
-
Inter-quartile Range
References
Dal Canto E, Ceriello A, Rydén L, Ferrini M, Hansen TB, Schnell O, et al. Diabetes as a cardiovascular risk factor: an overview of global trends of macro and micro vascular Complications. Eur J Prev Cardiol. 2019;26(2suppl):25–32.
Flaxel CJ, Adelman RA, Bailey ST, Fawzi A, Lim JI, Vemulakonda GA, et al. Diabet Retinopathy Preferred Pract Pattern® Ophthalmol. 2020;127(1):P66–p145.
Flaxman SR, Bourne RRA, Resnikoff S, Ackland P, Braithwaite T, Cicinelli MV, et al. Global causes of blindness and distance vision impairment 1990–2020: a systematic review and meta-analysis. Lancet Glob Health. 2017;5(12):e1221–e34.
Zhang Y, Pan XF, Chen J, Xia L, Cao A, Zhang Y, et al. Combined lifestyle factors and risk of incident type 2 Diabetes and prognosis among individuals with type 2 Diabetes: a systematic review and meta-analysis of prospective cohort studies. Diabetologia. 2020;63(1):21–33.
Bryl A, Mrugacz M, Falkowski M, Zorena K. The Effect of Diet and Lifestyle on the Course of Diabetic Retinopathy-A Review of the literature. Nutrients. 2022;14(6).
Tanaka S, Tanaka S, Iimuro S, Yamashita H, Katayama S, Ohashi Y, et al. Cohort profile: the Japan Diabetes Complications study: a long-term follow-up of a randomised lifestyle intervention study of type 2 Diabetes. Int J Epidemiol. 2014;43(4):1054–62.
Liu G, Li Y, Pan A, Hu Y, Chen S, Qian F, et al. Adherence to a healthy Lifestyle in Association with Microvascular Complications among adults with type 2 Diabetes. JAMA Netw Open. 2023;6(1):e2252239.
Ward BW, Schiller JS, Freeman G, Clarke TC. Early Release of Selected Estimates Based on Data From the January–June 2015 National Health Interview Survey United States: National Center for Health Statistics; 2015 [updated 11/2015. Available from: http://www.cdc.gov/nchs/nhis.htm.
Williams R, Colagiuri S, Chan J, Gregg E, Ke C, Lim L-L et al. IDF Atlas 9th Edition 20192019.
Schulze MB, Hoffmann K, Boeing H, Linseisen J, Rohrmann S, Möhlig M, et al. An accurate risk score based on anthropometric, dietary, and lifestyle factors to predict the development of type 2 Diabetes. Diabetes Care. 2007;30(3):510–5.
Schulze MB, Weikert C, Pischon T, Bergmann MM, Al-Hasani H, Schleicher E, et al. Use of multiple metabolic and genetic markers to improve the prediction of type 2 Diabetes: the EPIC-Potsdam Study. Diabetes Care. 2009;32(11):2116–9.
Fain JA. NHANES Diabetes Educ. 2017;43(2):151.
Curtin LR, Mohadjer LK, Dohrmann SM, Montaquila JM, Kruszan-Moran D, Mirel LB, et al. The National Health and Nutrition Examination Survey: Sample Design, 1999–2006. Vital Health Stat. 2012;2(155):1–39.
Wang L, Li X, Wang Z, Bancks MP, Carnethon MR, Greenland P, et al. Trends in Prevalence of Diabetes and control of risk factors in Diabetes among US adults, 1999–2018. JAMA. 2021;326(8):1–13.
Jiang W, Zhang J, Yang R, Sun X, Wu H, Zhang J, et al. Association of urinary nitrate with Diabetes complication and disease-specific mortality among adults with hyperglycemia. J Clin Endocrinol Metab. 2023;108(6):1318–29.
Atkin SL, Butler AE, Hunt SC, Kilpatrick ES. The retinopathy-derived HbA1c threshold of 6.5% for type 2 Diabetes also captures the risk of diabetic Nephropathy in NHANES. Diabetes Obes Metab. 2021;23(9):2109–15.
Sun XJ, Zhang GH, Guo CM, Zhou ZY, Niu YL, Wang L, et al. Associations between psycho-behavioral risk factors and diabetic retinopathy: NHANES (2005–2018). Front Public Health. 2022;10:966714.
Phillips JA. Dietary guidelines for americans, 2020–2025. Workplace Health Saf. 2021;69(8):395.
Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16(9):606–13.
Fang M, Wang D, Coresh J, Selvin E. Trends in Diabetes Treatment and Control in U.S. adults, 1999–2018. N Engl J Med. 2021;384(23):2219–28.
Ali MK, Bullard KM, Saaddine JB, Cowie CC, Imperatore G, Gregg EW. Achievement of goals in U.S. Diabetes care, 1999–2010. N Engl J Med. 2013;368(17):1613–24.
Yau JW, Rogers SL, Kawasaki R, Lamoureux EL, Kowalski JW, Bek T, et al. Global prevalence and major risk factors of diabetic retinopathy. Diabetes Care. 2012;35(3):556–64.
Yin L, Zhang D, Ren Q, Su X, Sun Z. Prevalence and risk factors of diabetic retinopathy in diabetic patients: a community based cross-sectional study. Med (Baltim). 2020;99(9):e19236.
Mendenhall E, Kohrt BA, Norris SA, Ndetei D, Prabhakaran D. Non-communicable Disease syndemics: poverty, depression, and Diabetes among low-income populations. Lancet. 2017;389(10072):951–63.
Yusuf S, Joseph P, Rangarajan S, Islam S, Mente A, Hystad P, et al. Modifiable risk factors, Cardiovascular Disease, and mortality in 155 722 individuals from 21 high-income, middle-income, and low-income countries (PURE): a prospective cohort study. Lancet. 2020;395(10226):795–808.
Thomas CG, Channa R, Prichett L, Liu TYA, Abramoff MD, Wolf RM. Racial/Ethnic disparities and barriers to Diabetic Retinopathy Screening in youths. JAMA Ophthalmol. 2021;139(7):791–5.
Sundquist K, Chaikiat A, León VR, Johansson SE, Sundquist J. Country of birth, socioeconomic factors, and risk factor control in patients with type 2 Diabetes: a Swedish study from 25 primary health-care centres. Diabetes Metab Res Rev. 2011;27(3):244–54.
Salk RH, Hyde JS, Abramson LY. Gender differences in depression in representative national samples: Meta-analyses of diagnoses and symptoms. Psychol Bull. 2017;143(8):783–822.
Hu FB, Manson JE, Stampfer MJ, Colditz G, Liu S, Solomon CG, et al. Diet, lifestyle, and the risk of type 2 Diabetes Mellitus in women. N Engl J Med. 2001;345(11):790–7.
Cai X, Chen Y, Yang W, Gao X, Han X, Ji L. The association of Smoking and risk of diabetic retinopathy in patients with type 1 and type 2 Diabetes: a meta-analysis. Endocrine. 2018;62(2):299–306.
Qin R, Chen T, Lou Q, Yu D. Excess risk of mortality and cardiovascular events associated with Smoking among patients with Diabetes: meta-analysis of observational prospective studies. Int J Cardiol. 2013;167(2):342–50.
Liu G, Li Y, Hu Y, Zong G, Li S, Rimm EB, et al. Influence of Lifestyle on Incident Cardiovascular Disease and Mortality in patients with Diabetes Mellitus. J Am Coll Cardiol. 2018;71(25):2867–76.
Lu C, Weng R, Wu W, Wang Y, Gu X. Moderate alcohol consumption and carotid intima-media thickness in type 2 Diabetes. Asia Pac J Clin Nutr. 2021;30(3):497–503.
Lee CC, Stolk RP, Adler AI, Patel A, Chalmers J, Neal B, et al. Association between alcohol consumption and diabetic retinopathy and visual acuity-the AdRem Study. Diabet Med. 2010;27(10):1130–7.
Costacou T, Crandell J, Kahkoska AR, Liese AD, Dabelea D, Lawrence JM, et al. Dietary patterns over Time and Microalbuminuria in Youth and Young adults with type 1 Diabetes: the SEARCH Nutrition Ancillary Study. Diabetes Care. 2018;41(8):1615–22.
Huffman FG, Zarini GG, McNamara E, Nagarajan A. The healthy eating Index and the alternate healthy eating index as predictors of 10-year CHD risk in Cuban americans with and without type 2 Diabetes. Public Health Nutr. 2011;14(11):2006–14.
Klenk J, Rapp K, Denkinger M, Nagel G, Nikolaus T, Peter R, et al. Objectively measured physical activity and vitamin D status in older people from Germany. J Epidemiol Community Health. 2015;69(4):388–92.
Jee D, Keum N, Kang S, Arroyo JG. Sleep and diabetic retinopathy. Acta Ophthalmol. 2017;95(1):41–7.
Zheng Z, Wang C, Li C, Wu Q, Chen X, Chen H, et al. Meta-analysis of relationship of Sleep Quality and Duration with Risk of Diabetic Retinopathy. Front Endocrinol (Lausanne). 2022;13:922886.
Buxton OM, Pavlova M, Reid EW, Wang W, Simonson DC, Adler GK. Sleep restriction for 1 week reduces insulin sensitivity in healthy men. Diabetes. 2010;59(9):2126–33.
de Groot M, Anderson R, Freedland KE, Clouse RE, Lustman PJ. Association of depression and Diabetes Complications: a meta-analysis. Psychosom Med. 2001;63(4):619–30.
Golden SH. A review of the evidence for a neuroendocrine link between stress, depression and Diabetes Mellitus. Curr Diabetes Rev. 2007;3(4):252–9.
Dibato J, Montvida O, Ling J, Koye D, Polonsky WH, Paul SK. Temporal trends in the prevalence and incidence of depression and the interplay of comorbidities in patients with young- and usual-onset type 2 Diabetes from the USA and the UK. Diabetologia. 2022;65(12):2066–77.
Nwanyanwu K, Nunez-Smith M, Gardner TW, Desai MM. Awareness of Diabetic Retinopathy: insight from the National Health and Nutrition Examination Survey. Am J Prev Med. 2021;61(6):900–9.
Acknowledgements
The authors would like to express their gratitude to EditSprings (https://www.editsprings.cn) for the expert linguistic services provided.
Funding
This study was supported by grants from the National Natural Science Foundation of China (No. 82271111), Clinical Research Innovation Plan of Shanghai General Hospital (No. CTCCR-2021C01), Shanghai Science and Technology Development Foundation (No. 22QA1407500), Shanghai Rising Stars of Medical Talent Youth Development Program (No. SHWSRS [2022-65]), and Shanghai Key Clinical Specialty Project. The funder has no any role in the conceptualization, design, data collection, analysis, decision to publish, or preparation of the manuscript.
Author information
Authors and Affiliations
Contributions
Conceptualization: BL, CZ and ZZ; Methodology and Formal analysis: BL and CZ; Writing - Original Draft: BL and CZ; Writing - Review & Editing: XC, CG, CL, YW and MM; Supervision and Project administration: YF, XX, HC and ZZ; Funding acquisition: CZ and ZZ. All authors approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
All participants provided informed written consent and the study received ethics approval from the National Center for Health Statistics. The research has followed the Tenets of the Declaration of Helsinki. Shanghai General Hospital Ethics Committee waived for additional ethics approvals as for this committee ethics approval from the National Center for Health Statistics is enough.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Li, B., Zhou, C., Gu, C. et al. Modifiable lifestyle, mental health status and diabetic retinopathy in U.S. adults aged 18–64 years with diabetes: a population-based cross-sectional study from NHANES 1999–2018. BMC Public Health 24, 11 (2024). https://doi.org/10.1186/s12889-023-17512-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12889-023-17512-8