Abstract
Background
Obesity is a crucial risk factor for obstructive sleep apnea (OSA), but the association between adiposity deposition and OSA risk has not reached a consistent conclusion. This study sought to reveal the association of multiple adiposity indicators with OSA risk.
Methods
This cross-sectional study included 9,733 participants aged 35–74 years, recruited from an ongoing population-based cohort. OSA was assessed by the Berlin Questionnaire. Six adiposity indicators, including neck circumference (NC), body fat percentage (BF%), waist-to-hip ratio (WHR), visceral adiposity index (VAI), lipid accumulation product (LAP), and resting metabolic rate (RMR), were selected. Multivariate logistic regression models were used to examine the association of adiposity indicators with OSA risk.
Results
One thousand six hundred twenty-six participants (16.71%) were classified into the OSA group. NC, BF%, WHR, VAI, LAP, and RMR were all positively associated with the risk of OSA after adjusting for confounders, regardless of age, sex, and history of dyslipidemia. Every 1-unit increment of NC, BF%, and VAI was associated with a 13%, 9%, and 14% increased risk of OSA, respectively; every 0.01-unit increment of WHR was associated with a 3% increased risk of OSA; every 10-unit increment of LAP and RMR was associated with 2% and 4% increased risk of OSA, respectively.
Conclusions
NC, BF%, WHR, VAI, LAP, and RMR were all independently and positively associated with OSA risk, regardless of age, sex, history of dyslipidemia, and menopausal status. Application of these new indicators could help to more comprehensively reflect and predict the risk of OSA in the general population.
Similar content being viewed by others
Introduction
Obstructive sleep apnea (OSA) is a common and under-recognized sleep disorder, characterized by periodic reductions or cessations in ventilation caused by dependent complete or partial collapse of the upper airway, resulting in consequent hypoxia, hypercapnia, or arousals from sleep [1]. OSA has affected 9% to 38% of the general adult population in Europe and North America [2], 14.0% to 39.4% in Asia [3], and 8.8% to 24.2% in China [4]. It is estimated that only about 1 in 50 patients with symptoms suggestive of OSA syndrome is evaluated and treated [5], as quite a few OSA patients are under-diagnosed or asymptomatic [6]. When left untreated, individuals with OSA are at heightened risk of metabolic syndrome, cardiovascular diseases, reduced quality of life, premature death, etc. [1, 6].
Obesity is one of the most important risk factors for OSA [1], and weight change can influence OSA severity [7]. Body mass index (BMI) is a traditional indicator of general obesity and is widely used in predicting OSA [8]. However, BMI has been criticized for failing to distinguish the fat distribution [9], because OSA is mainly associated with the central distribution of body fat [10]. Waist-to-hip ratio (WHR), an indicator of abdominal obesity, has been demonstrated more strongly linked with OSA than BMI [11]. Most adult OSA patients have abdominal obesity and increased visceral fat, releasing more inflammatory cytokines than peripheral obesity with predominant subcutaneous fat accumulation [7, 11]. This could lead to neck adiposity, increased upper airway fat, and metabolic abnormalities, even in normal-weight subjects [7]. A cross-sectional study among 1,912 Turkish adults showed that neck circumference (NC) was significantly associated with OSA risk, and its ability to predict OSA was greater than that of waist circumference (WC) [12].
Visceral adiposity index (VAI) and lipid accumulation index (LAP) are newly proposed indicators combining anthropometric indicators with lipid levels. The former is a sensitive indicator to reflect visceral obesity, and the latter is derived from the combination of triglyceride level and waist circumference [13]. Zou and colleagues found that LAP and VAI were moderately correlated with OSA severity, and suggested that anthropometry combined with visceral fat markers could be a more effective diagnostic tool for OSA [13]. Besides, body fat percentage (BF%) is commonly used in obesity research, but there are few studies on its relationship with OSA. A study in Uppsala found that men with severe OSA had a higher BF% than those without OSA, even if the cases and controls were matched for age and BMI [14]. Also, considering that obesity is the result of energy imbalance and the resting metabolic rate (RMR) is correlated with daily energy expenditure, it would be more useful to combine RMR with adiposity indicators to explore the relationship between obesity and OSA.
However, the single utilization of the aforementioned indicators could not adequately reflect the effect of adiposity on OSA risk and current studies have not yielded consistent conclusions. Less is known about the association of novel indicators (such as VAI and LAP) with the risk of OSA. Therefore, this large-scale study was conducted by considering NC, WHR, VAI, LAP, BF%, and RMR to examine the association of adiposity with OSA risk based on Chinese adults.
Methods
Setting and subjects
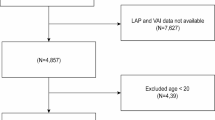
This cross-sectional study was based on the Guangzhou Heart Study, an ongoing population-based prospective cohort. The baseline survey was accomplished from 2015 to 2017 in Guangzhou permanent residents by multistage sampling method. The details have been described elsewhere [15,16,17,18]. In brief, a total of 12,013 participants aged ≥ 35 years were recruited in the baseline survey, and 2,280 subjects were excluded due to the following exclusion criteria: age older than 74 years (n = 1,043), lack of OSA-related data (n = 5), suffering from the chronic obstructive pulmonary disease (COPD, n = 678) or cardiovascular disease (CVD, n = 554). Recent studies have demonstrated that COPD characterized by a chronic bronchitis phenotype could promote OSA, while lung hyperinflation could protect against OSA [19]. OSA patients tend to be comorbid with CVD [20], which may affect the reliability of our results. Therefore, participants with COPD or CVD were excluded to avoid potential bias. Ultimately, 9,733 participants were selected for further analyses. This study was approved by the Ethical Review Committee for Biomedical Research, School of Public Health, Sun Yat-sen University. The study was performed following the Declaration of Helsinki and written informed consent was obtained from each participant.
OSA ascertainment
OSA was determined by the Berlin Questionnaire (BQ), which was widely used to screen for OSA [21]. The Chinese versions of BQ have been proven to have superior predictive validity and reliability [22, 23]. BQ is a commonly used questionnaire in epidemiological and clinical settings and consists of ten questions in three categories: snoring and breathing cessation (Category 1), excessive daytime sleepiness (Category 2), and BMI and hypertension (Category 3). Category 1 and Category 2 are considered positive with a persistent report of corresponding symptoms (frequency more than three times per week), and Category 3 is considered positive with the report of a history of hypertension or with a BMI of more than 30 kg/m2. Positive scores in two or more categories suggest that the respondent is at high risk for OSA, otherwise at low risk [24]. Then the participants judged to be at high risk of OSA by BQ were assigned to the OSA group and those at low risk of OSA were assigned to the non-OSA group.
Adiposity indicators and anthropometric measurements
Six adiposity indicators were assessed, including NC, BF%, WHR, RMR, VAI, and LAP. Each participant was asked to wear light clothes and step barefoot on the uniformed device to undergo a physical measurement by trained staff. Height and weight were measured to the nearest 0.1 cm (cm) and 0.1 kg (kg), respectively. NC, WC, and hip circumference (HC) were measured to the nearest 0.1 cm through a portable measuring tape. Subjects were asked to stand upright and look straight ahead with shoulders down, and NC was measured by putting the measuring tape midway around the neck, at the level of the laryngeal prominence. WC was gauged at the midpoint between the iliac crest and the lower end of the rib cage, and HC was measured at the maximum extension of the buttocks. Height, weight, NC, WC, and HC were all measured three consecutive times and the mean of each parameter was calculated. BMI was calculated as the mean of body weight in kilograms divided by the mean of height in meters squared (kg/m2) and WHR was calculated by dividing the mean measurement of WC by that of HC.
BF%, VAI, and RMR were measured by the bioelectrical impedance device (OMRON-HBF-371-SH: OMRON Corporation, Yangzhou, China) [25]. BF% was calculated by dividing total fat mass by total mass (including fat mass and fat-free mass) and then multiplying by 100. LAP is based on a combination of waist circumference and the fasting concentration of circulating triglycerides and is defined to describe the extent to which an individual has traveled the route of both increasing waist and increasing triglycerides [26]. LAP is calculated depending on gender: LAP for men = (WC [cm]—65) × (triglycerides concentration [mmol/L]), LAP for women = (WC [cm]—58) × (triglycerides concentration [mmol/L]). To avoid having nonpositive values for LAP, any waist values for men that were 65 cm or less were revised upward to 66.0 cm and for women that were 58 cm or less were revised upward to 59.0 cm [26].
Potential confounding factors
Structured questionnaires were applied to acquire information on demographic characteristics, lifestyle factors, and history of diseases at the face-to-face interview. The modified Global Physical Activity Questionnaire was used to assess leisure-time physical activity (LTPA, MET-h/week) for each participant as we reported previously [15]. Blood pressure was measured and serum cholesterol, low-density lipoprotein cholesterol, and triglyceride were detected. The participant who self-reported physician-diagnosed dyslipidemia or with serum cholesterol of ≥ 5.2 mmol/L, or low-density lipoprotein cholesterol of ≥ 3.4 mmol/L or triglyceride of ≥ 1.7 mmol/L was defined as having dyslipidemia [27]. The subject who self-reported physician-diagnosed hypertension or whose systolic blood pressure was ≥ 140 mmHg or diastolic blood pressure ≥ 90 mmHg was considered as having hypertension. The confounders included age (years), sex (male, female), marital status (married, others), educational status (primary school and lower, junior high school, senior high school, and college or above), work intensity (light, moderate, vigorous, and retirement), smoking (never, occasion or frequent smoking), alcohol drinking (never, occasion or frequent drinking), vegetable intake (< once/day, ≥ once/day), fruit intake (< once/day, ≥ once/day), hypertension (yes, no), and dyslipidemia (yes, no).
Statistical analysis
All statistical analyses were performed using R software (version 3.6.3). Data were expressed as mean (standard deviation, SD), median (interquartile range, IQR), or frequency (proportion, %), in accordance with the normal, skewed, or categorical distribution. Differences in the baseline characteristics among the non-OSA and OSA groups were computed by t-test, Wilcoxon rank-sum test, or chi-square test. The Pearson correlation test was used for the normally distributed data, and the Spearman correlation test was used for the non-normally distributed data. Each adiposity indicator was converted to a categorical variable based on the quartiles.
The odds ratio (OR) with a 95% confidence interval (CI) was calculated using logistic regression models to demonstrate the association between each indicator and OSA risk. Three models were considered: model 1 was without any adjustment; model 2 was adjusted for age, sex, marital status, education, smoking, alcohol drinking, fruit intake, vegetable intake, work intensity, and LTPA; model 3 was further mutually adjusted for adiposity indicators, aiming to examine the independent association of each indicator with OSA. The multicollinearity was also considered among all variables in the models and variance inflation factors (VIFs) were calculated. The results showed that BMI was not suitable for the adjusted models (VIF > 10) because BMI was closely correlated with adiposity indicators.
Stratified analysis was conducted by age (< 65 years, ≥ 65 years), sex (male or female), and dyslipidemia (yes, no). The multiplicative interaction of adiposity indicators with age, sex, and dyslipidemia was calculated, with the likelihood ratio test by comparing the likelihood scores of the two models with or without the interaction items. A sensitivity analysis was conducted by adjusting the upper and lower 2.5% of the adiposity indicators to the means of which, aiming to exclude the influence of possible outliers. Besides, considering that women's menopausal status plays an important role in OSA occurrence [28], we divided all women into premenopausal group and postmenopausal group based on their self-reported information. Then, we repeated analyses to estimate whether there were differences in the associations between adiposity indicators and OSA risk in women with different menopausal status. All P values were two-tailed and a P value < 0.05 was considered statistically significant.
Results
A total of 9,733 participants were enrolled in this study and 1626 participants (16.71%) were classified into the OSA group. Relative to the participants in the non-OSA group, subjects in the OSA group were more likely to be older, male, and married, to smoke and drink alcohol, to be retirees or take up a vigorous occupation, to have a higher level of education, to eat vegetables or fruit at least once per day, to actively take up LTPA, to have hypertension or dyslipidemia, to have a higher value of BMI, NC, BF%, WHR, VAI, LAP, and RMR (Table 1).
Regarding subjects in the lowest quartile of each indicator, ORs (95%CIs) for those in the highest quartile were 2.29 (1.78, 2.97), 2.65 (2.01, 3.48), 2.15 (1.73, 2.71), 4.58 (3.49, 6.02), 2.24 (1.81, 2.77) and 7.43 (5.75, 9.64) for NC, BF%, WHR, VAI, LAP, and RMR respectively after adjusting for all covariates (Table 2). The exposure–response trend of OSA with six indicators was observed (all P -trend < 0.05). Every 1-unit increment of NC, BF%, and VAI was associated with a 13%, 9%, and 14% increased risk of OSA, respectively; every 0.01-unit increment of WHR was associated with a 3% increased risk of OSA; every 10-unit increment of LAP and RMR was associated with a 2% and 4% increased risk of OSA, respectively. The sensitivity analysis yielded consistent results that six indicators were positively associated with an increased OSA risk, and the positive association was independent of a woman's menopausal status (supplementary table S 1 and S2).
In stratified analyses by age, sex, and dyslipidemia, the associations of NC, BF%, WHR, VAI, LAP, and RMR with OSA were not significantly changed (Tables 3, 4 and 5). The associations of NC, BF%, WHR, VAI, and RMR with OSA risk were stronger in the middle-aged than in the elderly (P -interaction < 0.001); the associations of VAI and RMR with OSA were slightly stronger in women than in men (P -interaction = 0.020 and 0.024, respectively); and the associations of BF%, WHR, and LAP with OSA were stronger in the non-dyslipidemia group than in the dyslipidemia group (all P -interaction < 0.05).
Discussion
To our knowledge, this is the first study to comprehensively examine the effects of common and novel adiposity indicators on the risk of OSA. This large population-based study found that NC, BF%, WHR, VAI, LAP, and RMR were all independently and positively associated with the OSA risk. The stratified and sensitivity analysis yielded similar results, indicating the robustness of the results.
This study found that the OSA risk increased with NC increment, which was consistent with previous studies [13, 29]. Increased NC implies more adipose tissue adjacent to the upper airway, with consequent reduced upper airway caliber and predisposes to OSA [29]. By contrast, BF% has received little attention in the etiology of OSA. We found that every 1-unit increment of BF% was associated with a 9% increased risk of OSA, indicating excessive fat accumulation was a risk factor for OSA regardless of fat distribution.-T-he risk of tissue hypoxia develops as adipocyte hypertrophy continues, with subsequent inflammatory activation, oxidative stress, and increased sympathetic activity, which eventually leads to the occurrence of OSA [7].
Indicators of abdominal adiposity including WHR, VAI, and LAP were all found to be independent risk factors for OSA, which was consistent with previous studies [13, 30]. Two separate observational and longitudinal studies concluded that abdominal obesity characterized by WC and HC was more strongly correlated with OSA than general obesity in China [11]. A cross-sectional study suggested that VAI was significantly associated with OSA risk, with all significantly correlated with an apnea–hypopnea index (AHI), and mean and lowest oxygen saturation [31]. LAP was initially developed for recognizing cardiovascular risk and then applied in the identification of metabolic diseases and OSA. Zou et al. suggested that LAP might be one key exponent in screening for OSA [13]. Abdominal adiposity accumulation may reduce pharyngeal lumen size, decrease upper airway muscle protective force and size, and affect restrictive respiratory dysfunction, finally leading to daytime hypoxemia and the development of OSA [30, 32]. RMR was positively associated with the OSA risk in this study. A university-based cross-sectional study showed that increased resting energy expenditure was independently associated with AHI, resulting in greater severity of sleep-disordered breathing [33]. Another study conducted a three-month continuous positive airway pressure therapy for OSA patients and found that the basal metabolic rate (equal to the RMR) was reduced in the absence of changes in physical activity, thus favoring a positive energy balance in terms of energy expenditure [34].
The stratified analysis by age showed that the associations of NC, BF%, WHR, VAI, LAP, and RMR with OSA risk were stronger in the middle-aged than in the elderly. This disparity could be explained by the contradictory effect of adipose tissue distribution on the elderly. Many elderly obese may exhibit late-onset obesity, health risks, and comorbidities not manifest due to its short duration [35]. Besides, Tung and colleagues followed 4,000 older adults for 5 years and found that older men were resistant to hazards of overweight and adiposity; mild-grade overweight or obesity might be protective [36]. The aging process is indeed characterized by an increase in total body fat mass and a concomitant decrease in lean mass and bone density, independent of general and physiological fluctuations in weight and BMI [37]. A systematic review concluded that five-year increases in the visceral adipose tissue (VAT) area declined with the advanced age group in both men and women, regardless of race [38].
In the stratified analysis by sex, the negative associations of VAI and RMR with OSA were stronger in women than in men. Studies have shown that women tend to have higher percent body fat throughout the entire life span with relatively more adipose tissue deposited in the hips and thighs, while men tend to have a greater degree of visceral obesity with excess fat more concentrated in the abdomen and neck [32]. These yielded consistent results that women had higher BF% (33.6% vs. 24.7%), lower VAI (6 vs. 11), and lower WHR (0.86 vs. 0.91) than men. However, it is reported that menopause is followed by redistribution of adipose tissue towards a more central phenotype and raised visceral adiposity in women during the peri-menopausal transition presumably due to the fall in estrogen levels [32, 39]. 67.3% of the women in this study were menopausal. Sensitivity analysis showed that the association between adiposity indicators and OSA was independent of menopausal status, which indicated that even premenopausal women should pay more attention to OSA prevention. Moreover, the energy expenditure in women was lower than in men, and women were more susceptible to accumulating fat tissue, especially old-age women.
In addition, among non-dyslipidemia subjects, BF%, WHR, LAP, and RMR were more strongly associated with OSA risk than those with dyslipidemia. There are complex interactions between obesity, dyslipidemia, and OSA, and in many cases, they coexist. Studies have reported that dyslipidemia predisposes to excess fatty deposition in the neck, thorax, and abdomen, impacts the pulmonary system and thereby increases OSA susceptibility [40]. Participants not suffering from dyslipidemia may be more sensitive to visceral fat accumulation, leading to a higher risk of OSA, compared to those with dyslipidemia.
Study strengths and limitations
There are some strengths. First, the multi-stage sampling method was applied to recruit participants from the general population in Guangzhou communities, which greatly reduced the selection bias and enhanced the representativeness of the sample. Second, the large sample size improved the statistical power and allowed for comparisons by age, sex, and history of dyslipidemia. Third, this study considered the effect of regional fat distribution on OSA and combined traditional and novel parameters of adiposity. Finally, we performed several stratified and sensitivity analyses and the results of which showed consistent associations, indicating the robustness of our results to a certain degree.
Some limitations also exist. First, the cross-sectional design could not provide causal inference according to our report. However, the dose–response relationship between adiposity indicators and OSA enhanced the existence of causation. Second, OSA was determined by the Berlin Questionnaire due to the lack of polysomnography during data collection, which is a commonly used validated tool in epidemiological and clinical research [24]. Compared with many other screening questionnaires that are lengthy and complicated, the Berlin questionnaire has been widely adopted and validated in various populations because of its ease of use, efficiency, and good sensitivity. Third, adiposity indicators were measured by Omron body composition monitor, which may not provide measurements as accurate as other advanced methods, such as Dual-energy X-ray absorptiometry [41]. The accuracy of the measurements was susceptible to being affected by body temperature, food ingestion, ambient temperature, and humidity. However, the portable Omron device has been applied in several studies and could provide a rapid, non-invasive, and reasonably accurate measurement of body composition [42,43,44]. But considering the cost and convenience, it was more practical to use portable protocols in this large-scale population study.
Conclusion
NC, BF%, WHR, VAI, LAP, and RMR were all independently and positively associated with OSA risk, regardless of age, sex, history of dyslipidemia, and menopausal status. Application of these new indicators could help to more comprehensively reflect and predict the risk of OSA in the general population. More attention should be paid to the middle-aged, women, or non-dyslipidemia population.
Availability of data and materials
The data used to support the findings of this study are available from the corresponding author upon request. A proposal with a detailed description of study objectives and a statistical analysis plan will be needed for the evaluation of the reasonability of requests if someone requests data sharing.
Abbreviations
- AHI:
-
Apnea–hypopnea index
- BF%:
-
Body fat percentage
- BMI:
-
Body mass index
- BQ:
-
Berlin Questionnaire
- CI:
-
Confidence interval
- IL:
-
Interleukin
- IQR:
-
Interquartile range
- LAP:
-
Lipid accumulation product
- LTPA:
-
Leisure-time physical activity
- NC:
-
Neck circumference
- OR:
-
Odds ratio
- OSA:
-
Obstructive sleep apnea
- RMR:
-
Resting metabolic rate
- SD:
-
Standard deviation
- VAI:
-
Visceral adiposity index
- VAT:
-
Visceral adipose tissue
- VIF:
-
Variance inflation factors
- WC:
-
Waist circumference
- WHR:
-
Waist-to-hip ratio
References
Veasey SC, Rosen IM. Obstructive Sleep Apnea in Adults. New Engl J Med. 2019;380(15):1442–9.
Senaratna CV, Perret JL, Lodge CJ, Lowe AJ, Campbell BE, Matheson MC, Hamilton GS, Dharmage SC. Prevalence of obstructive sleep apnea in the general population: A systematic review. Sleep Med Rev. 2017;34:70–81.
Hnin K, Mukherjee S, Antic NA, Catcheside P, Chai-Coetzer CL, McEvoy D, Vakulin A. The impact of ethnicity on the prevalence and severity of obstructive sleep apnea. Sleep Med Rev. 2018;41:78–86.
Benjafield AV, Ayas NT, Eastwood PR, Heinzer R, Ip MSM, Morrell MJ, Nunez CM, Patel SR, Penzel T, Pepin JL, et al. Estimation of the global prevalence and burden of obstructive sleep apnoea: a literature-based analysis. Lancet Respir Med. 2019;7(8):687–98.
Patel SR. Obstructive Sleep Apnea. Ann Inter Med. 2019;171(11):ITC81–96.
Natsky AN, Vakulin A, Coetzer CLC, McEvoy RD, Adams RJ, Kaambwa B. Economic evaluation of diagnostic sleep studies for obstructive sleep apnoea: a systematic review protocol. Syst Rev. 2021;10(1):104.
Bonsignore MR, McNicholas WT, Montserrat JM, Eckel J. Adipose tissue in obesity and obstructive sleep apnoea. Eur Respir J. 2012;39(3):746.
Gasa M, López-Padrós C, Monasterio C, Salord N, Mayos M, Vilarrasa N, Fernandez-Aranda F, Montserrat JM, Dorca J. Anthropometrical phenotypes are important when explaining obstructive sleep apnea in female bariatric cohorts. J Sleep Res. 2019;28(5):e12830.
Yu T, Bo Y. Chang L-y, Liu X, Tam T, Lao XQ: Adiposity and risk of death: A prospective cohort study of 463,002 adults. Clin Nutr. 2021;40(4):1932–41.
Carneiro G, Zanella MT. Obesity metabolic and hormonal disorders associated with obstructive sleep apnea and their impact on the risk of cardiovascular events. Metabolism. 2018;84:76–84.
Zhao X, Xu H, Qian Y, Liu Y, Zou J, Yi H, Guan J, Yin S. Abdominal Obesity Is More Strongly Correlated with Obstructive Sleep Apnea than General Obesity in China: Results from Two Separated Observational and Longitudinal Studies. Obes Surg. 2019;29(8):2535–47.
Onat A, Hergenç G, Yüksel H, Can G, Ayhan E, Kaya Z, Dursunoğlu D. Neck circumference as a measure of central obesity: Associations with metabolic syndrome and obstructive sleep apnea syndrome beyond waist circumference. Clin Nutr. 2009;28(1):46–51.
Zou J, Wang Y, Xu H, Xia Y, Qian Y, Zou J, Guan J, Chen B, Yi H, Yin S. The use of visceral adiposity variables in the prediction of obstructive sleep apnea: evidence from a large cross-sectional study. Sleep Breathing. 2020;24(4):1373–82.
Kamble PG, Theorell-Haglöw J, Wiklund U, Franklin KA, Hammar U, Lindberg E, Eriksson JW. Sleep apnea in men is associated with altered lipid metabolism, glucose tolerance, insulin sensitivity, and body fat percentage. Endocrine. 2020;70(1):48–57.
Duan X, Zheng M, He S, Lao L, Huang J, Zhao W, Lao XQ, Deng H, Liu X. Association between physical activity and risk of obstructive sleep apnea. Sleep breathing. 2021;25(4):1925–34.
Deng H, Guo P, Zheng M, Huang J, Xue Y, Zhan X, Wang F, Liu Y, Fang X, Liao H, et al. Epidemiological Characteristics of Atrial Fibrillation in Southern China: Results from the Guangzhou Heart Study. Sci Rep. 2018;8(1):17829.
Duan X, Huang J, Zheng M, Zhao W, Lao L, Li H, Wang Z, Lu J, Chen W, Deng H, et al. Association of healthy lifestyle with risk of obstructive sleep apnea: a cross-sectional study. BMC Pulm Med. 2022;22(1):33.
Deng H, Hu P, Li H, Zhou H, Wu X, Yuan M, Duan X, Lao M, Wu C, Zheng M, et al. Novel lipid indicators and the risk of type 2 diabetes mellitus among Chinese hypertensive patients: findings from the Guangzhou Heart Study. Cardiovasc Diabetol. 2022;21(1):212.
McNicholas WT. COPD-OSA Overlap Syndrome: Evolving Evidence Regarding Epidemiology, Clinical Consequences, and Management. Chest. 2017;152(6):1318–26.
Yeghiazarians Y, Jneid H, Tietjens JR, Redline S, Brown DL, El-Sherif N, Mehra R, Bozkurt B, Ndumele CE, Somers VK. Obstructive Sleep Apnea and Cardiovascular Disease: A Scientific Statement From the American Heart Association. Circulation. 2021;144(3):e56–67.
Netzer NC, Stoohs RA, Netzer CM, Clark K, Strohl KP. Using the Berlin Questionnaire to identify patients at risk for the sleep apnea syndrome. Ann Intern Med. 1999;131(7):485–91.
Chiu HY, Chen PY, Chuang LP, Chen NH, Tu YK, Hsieh YJ, Wang YC, Guilleminault C. Diagnostic accuracy of the Berlin questionnaire, STOP-BANG, STOP, and Epworth sleepiness scale in detecting obstructive sleep apnea: A bivariate meta-analysis. Sleep Med Rev. 2017;36:57–70.
Ha SC, Lee DL, Abdullah VJ, van Hasselt CA. Evaluation and validation of four translated Chinese questionnaires for obstructive sleep apnea patients in Hong Kong. Sleep breathing. 2014;18(4):715–21.
Senaratna CV, Perret JL, Matheson MC, Lodge CJ, Lowe AJ, Cassim R, Russell MA, Burgess JA, Hamilton GS, Dharmage SC. Validity of the Berlin questionnaire in detecting obstructive sleep apnea: A systematic review and meta-analysis. Sleep Med Rev. 2017;36:116–24.
Vasold KL, Parks AC, Phelan DML, Pontifex MB, Pivarnik JM. Reliability and Validity of Commercially Available Low-Cost Bioelectrical Impedance Analysis. Int J Sport Nutr Exerc Metab. 2019;29(4):406–10.
Kahn HS. The “lipid accumulation product” performs better than the body mass index for recognizing cardiovascular risk: a population-based comparison. BMC Cardiovasc Disord. 2005;5(1):26.
Joint Committee on the Chinese Guidelines for Lipid Management. [Chinese guidelines for lipid management (2023)]. Zhonghua Xin Xue Guan Bing Za Zhi. 2023;51(3):221–55. https://pubmed.ncbi.nlm.nih.gov/36925135/.
Jehan S, Auguste E, Zizi F, Pandi-Perumal SR, Gupta R, Attarian H, Jean-Louis G, McFarlane SI. Obstructive Sleep Apnea: Women’s Perspective. J Sleep Med Disorders. 2016;3(6):1064.
Brown LK. The effects of body fat distribution on obstructive sleep apnea: are older and younger adults the same? Sleep. 2013;36(4):457–9.
Seidell JC. Waist circumference and waist/hip ratio in relation to all-cause mortality, cancer and sleep apnea. Eur J Clin Nutr. 2010;64(1):35–41.
Chen G-P, Qi J-C, Wang B-Y, Lin X, Zhang X-B, Zhao J-M, Chen XF, Lin T, Chen D-D, Lin Q-C. Applicability of visceral adiposity index in predicting metabolic syndrome in adults with obstructive sleep apnea: a cross-sectional study. BMC Pulm Med. 2016;16(1):37.
Harada Y, Oga T, Chihara Y, Azuma M, Murase K, Toyama Y, Aihara K, Tanizawa K, Yoshimura C, Hitomi T, et al. Differences in Associations between Visceral Fat Accumulation and Obstructive Sleep Apnea by Sex. Ann Am Thorac Soc. 2014;11(3):383–91.
Kezirian EJ, Kirisoglu CE, Riley RW, Chang E, Guilleminault C, Powell NB. Resting Energy Expenditure in Adults With Sleep Disordered Breathing. Arch Otolaryngology-Head Neck Surg. 2008;134(12):1270–5.
Tachikawa R, Ikeda K, Minami T, Matsumoto T, Hamada S, Murase K, Tanizawa K, Inouchi M, Oga T, Akamizu T, et al. Changes in Energy Metabolism after Continuous Positive Airway Pressure for Obstructive Sleep Apnea. Am J Respir Crit Care Med. 2016;194(6):729–38.
Hainer V, Aldhoon-Hainerová I. Obesity Paradox Does Exist Diabetes care. 2013;36(Supplement 2):S276.
Auyeung TW, Lee JSW, Leung J, Kwok T, Leung PC, Woo J. Survival in older men may benefit from being slightly overweight and centrally obese–a 5-year follow-up study in 4,000 older adults using DXA. J Gerontol A Biol Sci Med Sci. 2010;65(1):99–104.
Ponti F, Santoro A, Mercatelli D, Gasperini C, Conte M, Martucci M, Sangiorgi L, Franceschi C, Bazzocchi A. Aging and Imaging Assessment of Body Composition: From Fat to Facts. Front Endocrinol. 2020;10:861.
Hainer V, Aldhoon-Hainerová I. Obesity paradox does exist. Diabetes care. 2013;36(Suppl 2):S276-281.
Mazzuca E, Battaglia S, Marrone O, Marotta AM, Castrogiovanni A, Esquinas C, Barcelò A, Barbé F, Bonsignore MR. Gender-specific anthropometric markers of adiposity, metabolic syndrome and visceral adiposity index (VAI) in patients with obstructive sleep apnea. J Sleep Res. 2014;23(1):13–21.
Xu H, Guan J, Yi H, Zou J, Meng L, Tang X, Zhu H, Yu D, Zhou H, Su K, et al. Elevated low-density lipoprotein cholesterol is independently associated with obstructive sleep apnea: evidence from a large-scale cross-sectional study. Sleep Breathing. 2016;20(2):627–34.
Stagi S, Irurtia A, Rosales Rafel J, Cabras S, Buffa R, Carrasco-Marginet M, Castizo-Olier J, Marini E. Segmental body composition estimated by specific BIVA and dual-energy X-ray absorptiometry. Clin Nutr. 2021;40(4):1621–7.
Dong Y, Wang Z, Chen Z, Wang X, Zhang L, Nie J, Zheng C, Wang J, Shao L, Tian Y, et al. Comparison of visceral, body fat indices and anthropometric measures in relation to chronic kidney disease among Chinese adults from a large scale cross-sectional study. BMC Nephrol. 2018;19(1):40.
Martín Moreno V, Gómez Gandoy B, Antoranz González M, Fernández Herranz S. Gómez De La Cámara A, de Oya Otero M: [Validation of the OMRON BF 300 monitor for measuring body fat by bioelectric impedance]. Aten Primaria. 2001;28(3):174–81.
Ballesteros-Pomar MD, Calleja-Fernández A, Diez-Rodríguez R, Vidal-Casariego A, Blanco-Suárez MD, Cano-Rodríguez I. Comparison of different body composition measurements in severely obese patients in the clinical setting. Nutr Hosp. 2012;27(5):1626–30.
Acknowledgements
The author would like to thank epidemiologists, nurses, and doctors in Guangdong Provincial People’s Hospital, in Guangzhou Center for Disease Control and Prevention, and in community healthcare centers in data collection, and thank all study subjects for their participation.
Funding
This work was supported by the Guangdong Basic and Applied Basic Research Foundation (No. 2022A1515010686), the Medical Science and Technology Research Foundation of Guangdong Province (No. A2023408), and the Guangdong Provincial Key R&D Program (No.2019B020230004), the National Key R&D Program of China (No.2018YFC1312502), and innovation team of ordinary universities in Guangdong Province (No.2020KCXTD022).
Author information
Authors and Affiliations
Contributions
XL conceived the study; XL and WZ and supervised the study; HD, MZ, JH, XD, FW, QS, ZZ, YM and LH collected the data, XD analyzed the data, XD and HD drafted the manuscript, XM, MZ, XX, WY, ML, WZ and XL reviewed and edited the manuscript. All co-authors provided comments and approved the final version.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
This study was approved by the Ethical Review Committee for Biomedical Research, School of Public Health, Sun Yat-sen University. The study was performed in accordance with the Declaration of Helsinki and written informed consent was obtained from each participant before they joined in the study.
Consent for publication
No identifying information of patients was contained. Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Supplementary table S1.
Sensitivity analysis on the association between adjusted adiposity indicators and obstructive sleep apnea. Supplementary table S2. Association between adiposity indicators and obstructive sleep apnea by menopause status among women.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Deng, H., Duan, X., Huang, J. et al. Association of adiposity with risk of obstructive sleep apnea: a population-based study. BMC Public Health 23, 1835 (2023). https://doi.org/10.1186/s12889-023-16695-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12889-023-16695-4