Abstract
Background
Evidence for the association between social determinants of health (SDoH) and health-related quality of life (HRQoL) is largely based on single SDoH measures, with limited evaluation of cumulative social disadvantage. We examined the association between cumulative social disadvantage and the Health and Activity Limitation Index (HALex).
Methods
Using adult data from the National Health Interview Survey (2013–2017), we created a cumulative disadvantage index by aggregating 47 deprivations across 6 SDoH domains. Respondents were ranked using cumulative SDoH index quartiles (SDoH-Q1 to Q4), with higher quartile groups being more disadvantaged. We used two-part models for continuous HALex scores and logistic regression for poor HALex (< 20th percentile score) to examine HALex differences associated with cumulative disadvantage. Lower HALex scores implied poorer HRQoL performance.
Results
The study sample included 156,182 respondents, representing 232.8 million adults in the United States (mean age 46 years; 51.7% women). The mean HALex score was 0.85 and 17.7% had poor HALex. Higher SDoH quartile groups had poorer HALex performance (lower scores and increased prevalence of poor HALex). A unit increase in SDoH index was associated with − 0.010 (95% CI [-0.011, -0.010]) difference in HALex score and 20% higher odds of poor HALex (odds ratio, OR = 1.20; 95% CI [1.19, 1.21]). Relative to SDoH-Q1, SDoH-Q4 was associated with HALex score difference of -0.086 (95% CI [-0.089, -0.083]) and OR = 5.32 (95% CI [4.97, 5.70]) for poor HALex. Despite a higher burden of cumulative social disadvantage, Hispanics had a weaker SDoH-HALex association than their non-Hispanic White counterparts.
Conclusions
Cumulative social disadvantage was associated with poorer HALex performance in an incremental fashion. Innovations to incorporate SDoH-screening tools into clinical decision systems must continue in order to accurately identify socially vulnerable groups in need of both clinical risk mitigation and social support. To maximize health returns, policies can be tailored through community partnerships to address systemic barriers that exist within distinct sociodemographic groups, as well as demographic differences in health perception and healthcare experience.
Similar content being viewed by others
Introduction
Patient-reported outcomes have gained traction in the evaluation of healthcare quality and monitoring of population health in recent years [1, 2]. These subjective outcomes directly inform providers and health policy-makers about the experiences of patients (or groups) and their value systems, both of which influence therapeutic choices, disease management, and health policy. Health-related quality of life (HRQoL), a commonly assessed patient-reported outcome, captures symptoms and functional limitations associated with health conditions and is known to be influenced by an individual’s psychosocial environment and their value-based preferences [3].
Likewise, the role of social determinants of health (SDoH) in perpetuating disparities in health and healthcare delivery has garnered attention as well [4,5,6]. SDoH are observed to influence objective health outcomes such as morbidity and mortality [7,8,9], and patient-reported health and wellbeing [10, 11]. SDoH are intricately interconnected and adverse determinants often cluster in marginalized groups [12,13,14]. Additionally, these proximal determinants can have differential impact on health which calls for a multidimensional framework in their assessment. Yet, evidence on the association between SDoH and HRQoL is largely based on single assessments of SDoH [11, 15, 16]. Very few indices of cumulative social disadvantage capture burden across an exhaustive range of SDoH domains [17, 18]. A comprehensive multidimensional SDoH framework may afford a provider or health system nuanced information to better identify at-risk individuals or those likely amenable to social interventions.
Thus, in this study, we utilized an exhaustive SDoH framework [19] to evaluate the relationship between SDoH and HRQoL, the latter assessed with the Health and Activity Limitation Index (HALex) [20]. HALex is a generic HRQoL measure combining self-reported health with the performance of activities of daily living and instrumental activities of daily living [20]. It summarizes a person’s HRQoL into a global score ranging from 0 (dead) to 1.00 (perfect health and functioning). We used HALex for the following reasons. Originally developed from the National Health Interview Survey (NHIS), HALex can be adapted to other national datasets as perceived health and activity limitation information are readily available [21,22,23]. A quality-of-life instrument with a global score such as HALex also minimizes interpretational challenges that may be associated with offsetting or contradictory changes to domain scores for multidomain instruments. Third, the simultaneous consideration of perception with physical functioning provides an incremental validity to HALex, unlike other generic measures which separately assess physical functioning [20]. We assessed HALex scores across levels of cumulative social disadvantage for the total population and by age, sex, and race/ethnicity. We hypothesized that higher levels of cumulative social disadvantage would be associated with poorer performance on HALex.
Methods
Data source and study sample
The NHIS is a cross-sectional household interview survey conducted annually by the National Center for Health Statistics [24]. A complex multistage area probability design is used to sample the non-institutionalized civilian population of the United States. Survey items are organized into the following core components: Household Composition, Family, Sample Child, and Sample Adult. The Household Composition core collects basic demographic and relationship information about all persons in the household. The Family Core, administered separately for each family in the household, collects information on sociodemographic characteristics, indicators of health status, activity limitations, injuries, health insurance coverage, and access to and use of health services. From each family, one sample child and one sample adult are randomly selected to gather further information on them. Additionally, NHIS has imputed income datasets to complete missing information on family income and personal earnings for each year. In this study, we merged the Household Composition, Family, and Sample Adult data files with the imputed income files.
Since the survey years 2013 to 2017 consistently featured items for our SDoH variables, we used data from these survey years to limit missingness. The data of respondents aged ≥ 18 years were merged and pooled over the five years. Survey weights were used to account for selection probability and non-response. NHIS data files are publicly available and deidentified, hence this study was exempt from the purview of the Houston Methodist’s Institutional Review Board.
Study measures
Independent variable
Our cumulative SDoH index was developed by adapting the Kaiser Family Foundation SDoH framework of six SDoH domains [19]. Using responses from 47 survey items related to social factors, we organized SDoH into the following domains: economic stability, neighborhood and physical environment, education, food security, community and social context, and healthcare system (Fig. 1). Economic stability included employment, household income level, and financial distress from bills and debt. Neighborhood and physical environment included housing type and tenure. Education comprised higher education and English language proficiency. Food security comprised food availability, adequacy, affordability, and access to balanced meals. Community and social context was assessed with neighborhood cohesion, and immigration status. Healthcare system domain comprised insurance coverage, existence of a usual source of care, provider availability, and health information technology use. Each SDoH was scored as “0” if favorable and “1” if unfavorable. Supplemental Table S1 displays the specific survey items used to develop the cumulative index. Then, we ranked individuals by quartiles of the cumulative index. The first quartile group (SDoH-Q1) had the least cumulative social disadvantage, and the fourth quartile group (SDoH-Q4) had the greatest cumulative social disadvantage. We performed our primary analyses with both the continuous cumulative SDoH index and the quartile groups.
Dependent variable
HALex is a validated generic HRQoL measure developed from two attributes: perceived health status and functional activity limitation. For perceived health, respondents rate their overall health as ‘poor’, ‘fair’, ‘good’, ‘very good’, or ‘excellent’. For activity limitation, each person is classified into one of six categories of activity limitation based on age and the ability to perform a major activity, as shown in Supplemental Table S2. Categories of physical activity limitation include, “not limited in activities”, “limited in performing other activities”, “limited in performing a major activity”, “unable to perform a major activity”, “limited in instrumental activities of daily living”, and “limited in performing activities of daily living (i.e., personal care needs)” [20]. Persons who are classified into more than one category are assigned to the category with the greater degree of dysfunction. The responses to the two items are combined in a matrix of 30 health states. The most favorable combined health state is excellent health and no activity limitation, and the worst state is poor health and an inability to perform activities of daily living. A dead state is included as the 31st health state. Full details of the mathematical derivation of HALex – via corresponding analysis and a general multiplicative model based on multi-attribute utility theory – have been described elsewhere [20]. HALex scores range from 0.10 (the worst combined health state) to 1.00 (the most favorable combined health state) for persons alive. Dead state has a value of 0 [20].
We evaluated HALex as both a continuous outcome (HALex score) and a binary outcome, poor HALex (yes/no). Poor HALex was defined as a HALex score less than the 20th percentile (0.79) for the analytic sample [25]. Respondents (8.0%) with missing HALex values (i.e., with incomplete responses to at least one of the two attribute items for HALex) were excluded from the analytic sample.
Covariates
Covariates included sex, age, race/ethnicity, region of residence (Northeast, Midwest, South and West), smoking status, psychological distress assessed with Kessler-6 score, and 11 self-reported comorbidities. Participants reported receiving a clinician diagnosis of arthritis, cancer, chronic liver disease, coronary heart disease (angina or myocardial infarction), chronic obstructive pulmonary disease, diabetes mellitus, hypertension, and stroke. They also reported a diagnosis of kidney failure in the twelve months before the interview. Finally, respondents recalled their weights and heights from which body-mass indices were estimated, and indices ≥ 30 kg/m2 were classified as obesity.
Statistical analysis
We summarized respondent demographic and health characteristics, as well as selected SDoH variables, across quartiles of the SDoH index. Continuous variables were summarized with mean (SD) or median (IQR), where appropriate, and categorical variables were summarized with frequency and weighted proportion. Our summary of HALex scores and poor HALex were adjusted for age and sex. We compared adjusted marginal HALex means and poor HALex prevalence across SDoH quartiles in the overall study population, and by age, sex, and racial/ethnic groups.
For primary analyses, we evaluated both HALex outcomes first using the original SDoH index and then quartile groups of the index as independent variables. HALex scores were converted onto a decrement utility scale using negative linear transformation (X = 1 – U, where U = utility index) before assessment with two-part modeling [26]. We first modelled the probability that a person had a non-zero transformed score with a logit model using the full sample (first part). In the second part, we used an ordinary least square regression model to estimate the predicted difference in the transformed scores using the subset of people with non-zero scores [26]. To obtain predicted estimates and confidence limits on the original HALex scale, we simply back-transformed (1 – X) the regression estimates [26]. Poor HALex was analyzed using odds ratio (OR) estimates from logistic regression. For both outcomes, three sequential models were tested: an unadjusted model; Model 1 adjusted for age (continuous), sex, and race/ethnicity; and Model 2 further adjusted for smoking, comorbidities, Kessler-6 score, and region of residence. Further, we stratified our analysis of the outcomes by sex, age, and race/ethnicity categories.
We performed two supplementary analyses. First, we reanalyzed the cumulative SDoH-HALex association after multiply imputing by chained equations the missing values for variables with more than 5% missingness – close-knit neighborhood (5.2%), helpful neighbors (5.4%), trusting neighbors (5.6%), sick leave provision (6.4%), difficulty paying medical bills (7.6%), and English language proficiency (10.2%). Independent variables for the imputation models included all covariates in the full model of the primary analyses, HALex score, and the following SDoH-related variables selected with subject-matter knowledge ̶ employment status, household income level, education, and insurance status. Twenty complete datasets were created. Secondly, we evaluated the independent associations between each SDoH and the HALex outcomes, adjusting for sex, age, race/ethnicity, and all the clinical characteristics.
All statistical analyses incorporated the complex survey design and weighting for selection probabilities and non-response. Variance estimation for the entire pooled cohort was obtained from the Integrated Public Use Microdata Series (https://nhis.ipums.org/nhis/). Statistical significance was assessed with a two-tailed significance level of 5%. We used Stata version 16 software (Stata Corp, College Station, Texas) for all analyses.
Results
Table 1 describes the characteristics of the study population. The analytic sample included 156,182 NHIS adult participants with no missing HALex scores, representing 232.8 million adults in the U.S. (mean age 46 [SD 17] years, 51.7% women, 65.2% non-Hispanic White, and 15.8% Hispanic). The median cumulative SDoH index of the overall population was 3 (IQR, 2–6), with 37.7% in SDoH-Q1, 23.3% in SDoH-Q2, 16.6% in SDoH-Q3, and 22.5% in SDoH-Q4. Individuals in the more disadvantaged groups (higher quartiles of cumulative SDoH index) were younger, had higher representations of females, Hispanics and non-Hispanic Blacks, and a greater burden of comorbidities than their counterparts in the lower quartile groups. Supplemental Table S3 shows the distribution of the 47 SDoH across the quartile groups of the cumulative SDoH index. Supplemental Tables S4-S6 show the distributions of the combined health states across sex, age, and race/ethnicity groups.
Summary of HALex scores and poor HALex
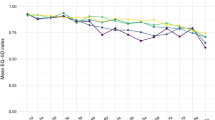
The mean HALex score of the overall population was 0.85 (SE 0.00), and 17.7% (95% CI [17.4, 18.0]) performed poorly on HALex. Adult males (0.85, SE [0.00]), adults aged 18–39 years (0.90, SE [0.00]), and non-Hispanic Asians (0.87, SE [0.00]) had the highest HALex scores (Supplemental Table S7). On the other hand, adults aged ≥ 65 years (0.79, SE [0.00]) and those of other racial/ethnic origins (0.78, SE [0.01]) had the lowest HALex scores. Figure 2 illustrates the mean HALex scores across SDoH quartile groups in the total population as well as across sex, age, and race/ethnicity categories. Overall, higher SDoH quartile groups had lower mean HALex scores. Men and women had similar HALex score profiles across cumulative SDoH quartiles. Adults aged ≥ 65 years had the lowest HALex scores across all cumulative SDoH quartiles, while those aged 18–39 years had the highest scores. Generally, non-Hispanic Asians had higher HALex scores than all other individuals at all levels of cumulative social disadvantage. At the highest level of cumulative disadvantage (SDoH-Q4), Hispanics had higher HALex scores than non-Hispanic Whites. Persons of Other origin, on the other hand, had the lowest HALex scores across all cumulative SDoH quartiles. Poor HALex was most prevalent in women (18.3%), individuals aged 55–64 years (27.3%), and adults of Other racial/ethnic origins (30.3%) (Supplemental Table S8). The prevalence of poor HALex increased with cumulative SDoH quartiles in the total population and across sex, age, and race/ethnicity groups (Fig. 3).
Age- and sex-adjusted prevalence of poor HALex. [Caption] Poor HALex score defined by HALex < 20th percentile score (0.79). (A) Total population. (B) Sex (C) Age group (D) Race/ethnicity. Abbreviations: HALex – Health and Activity Limitation Index; and SDoH-Q – quartile group of cumulative social determinants of health index
Primary multivariable analyses
The results of our multivariable regression analysis are shown in Table 2. A unit increase in the cumulative SDoH index (i.e., an additional deprivation) was associated with 0.02 decrease in HALex score (β = -0.020; 95% CI [-0.021, -0.020]) after accounting for age, sex, and race/ethnicity. This difference was moderately attenuated in the full model (β = -0.010; 95% CI [-0.011, -0.010]). Using SDoH quartile groupings, predicted HALex scores decreased incrementally with higher cumulative SDoH quartiles, relative SDoH-Q1 in the minimally adjusted model (SDoH-Q4: β = -0.167; 95% CI [-0.171, -0.163]). This manner of HALex score decrements with quartiles of the cumulative SDoH index, persisted in the full model, albeit with some attenuation (SDoH-Q4: β = -0.086; 95% CI [-0.089, -0.083]).
Regarding poor HALex, a unit increase in SDoH index was associated with 31% higher odds of poor HALex (OR = 1.31; 95% CI [1.30, 1.32]) in the minimally adjusted model. This association remained with minimal attenuation in the full model (OR = 1.20; 95% CI [1.19, 1.21]). Assessing with SDoH quartiles, we observed incrementally higher odds of poor HALex with higher cumulative SDoH quartiles, in both adjusted models, with some attenuation in the full model. For example, the odds of poor HALex performance in SDoH-Q4 was more than 11-fold the odds in SDoH-Q1 (OR = 11.11; 95% CI [10.47, 11.79]) in Model 1. In the full model, SDoH-Q4 was associated with at least 5-fold higher odds of poor HALex than SDoH-Q1 (OR = 5.32; 95% CI [4.97, 5.70]).
Table 3 shows the results of our stratified analyses for HALex scores and poor HALex. The graded negative association between cumulative social disadvantage and HALex was found to be consistent across sex, age, and race/ethnicity subgroups. The associations were stronger in the older age groups, non-Hispanic Whites, and non-Hispanic Blacks. On the other hand, the decrement in HALex scores at higher levels of cumulative disadvantage were lower in non-Hispanic Asian and Hispanic individuals. Men and women had similar gradients in the negative association between cumulative social disadvantage and HALex scores. The trends in the associations between cumulative disadvantage and poor HALex across the demographic groups were similar to those described for HALex scores, except the age groups, wherein the odds ratio of poor HALex associated with SDoH-Q4 in adults aged ≥ 65 years was lower than that of the younger adults 40–64 years.
Supplementary analyses
After multiply imputing missing values of the covariates, we found cumulative disadvantage-HALex associations similar to those described in the primary analysis (Supplemental Table S9). Evaluating individual SDoH, employment status, household income level, cost-related barriers to medical care, housing, food security, literacy, and transportation barrier to medical care were most strongly associated with poorer HALex performance (Supplemental Table S10).
Discussion
In this nationally representative study of US adults, we demonstrated that the simultaneous accumulation of adverse socioeconomic factors across 6 domains of SDoH – economic stability, neighborhood quality, education, food security, social cohesion, and healthcare system – was associated with lower HALex performance in a graded fashion. This negative association persisted across sex, age, racial and ethnic groups, even after accounting for demographic and clinical characteristics. Hispanics, despite having a higher burden of cumulative social disadvantage, had a weaker SDoH-HALex association than non-Hispanic Whites. Employment status, household income level, cost-related barriers to medical care, housing, food security, literacy, and transportation barrier to medical care were most strongly associated with poorer HALex performance.
Functional activity level and perceived health status are important determinants of quality of life and health care resource utilization [27, 28]. Existing literature assessing the association between cumulative social disadvantage and HRQoL have two shortfalls the present study addresses. First, indices used to evaluate the association of cumulative disadvantage with health status have mostly been limited to a few SDoH items and domains [17, 29, 30]. Here, we utilized a more nuanced construct of cumulative social disadvantage encompassing objective and perceived measures of economic stability, language and literacy, housing, community and social environment, and the healthcare system access and experience. Secondly, health status studies have more often than not assessed perceived health status and functional activity limitation separately as they capture different aspects of health status [17, 31]. Yet, these two measures are correlated [32, 33], and using HALex maximizes this complex relationship in providing a global quality of life score that offers an opportunity to track changes in HRQoL.
We highlight the lower decrement in HALex scores and the increase in the odds of poor HALex relative to cumulative disadvantage found in elderly persons compared to younger adults. Similar age-related trends have been observed in studies that assessed HALex differences for chronic conditions like arthritis [22]. A potential explanation is the phenomenon that vulnerable or aging populations tend to report better subjective health due to lowered expectations rather than ‘actual’ better health [34]. This observation could also be due to the selective participation of healthier elderly individuals in the survey and/or the lower participation of healthier persons in the younger age groups.
Generally, historically marginalized non-Hispanic Black and Hispanic groups experience worse health outcomes than non-Hispanic White persons [35]. Despite greater Hispanic presence in the more disadvantaged groups, we found that Hispanics had better HALex performance than their non-Hispanic White counterparts at comparable levels of cumulative social disadvantage (Fig. 1). Furthermore, we found that the negative association between cumulative disadvantage and HALex was relatively weaker in Hispanics than the non-Hispanic White population (Table 3). These findings point to diminished health returns for Hispanics and concur with the theory of diminished returns for marginalized groups, including racial and ethnic minorities [36]. The theory posits that the health effects of socioeconomic and psychological resources may be differentially weaker in marginalized populations. Such unequal health returns of resources (or lack thereof) across racial/ethnic groups may stem from structural and environmental barriers, in particular the pervasive effects of structural racism and discrimination experienced by Hispanic, non-Hispanic Black, Asian, American Indian, and other underserved communities, which may mitigate the effects of socioeconomic resources and other SDoH on the health and wellbeing of these vulnerable subgroups.
An alternative reason for the apparently better HALex performance of Hispanic individuals at higher levels of social disadvantage could be the different perception of health across sociodemographic characteristics. The notion of health is influenced by experiences and expectations that differ across racial, ethnic, and socioeconomic characteristics [37,38,39]. Therefore, the impact of cumulative social disadvantage on perceived health status may be lower in individuals with a more resilient construct of health, emanating from cultural- and self-efficacy. Hispanic communities are particularly noted to have such resilience [40], which may play a role in their lower odds of reporting fair/poor health compared to non-Hispanic White counterparts [41]. In our study population, despite more non-Hispanic Whites (64.7%) reporting excellent/very good health than Hispanics (57.3%), there was a greater proportion of non-Hispanic Whites (5.5%) with at least limitation in a major activity who reported fair/poor health, than Hispanics (4.3%) with similar health states (Supplemental Table S6). This draws attention to the concept of community resilience and the need for population “wellness” interventions to be responsive to such phenomenon.
Implications
There are several implications for our study results. First, the socioeconomic gradient found with such a patient-reported outcome that influences healthcare utilization and the value of care adds to the growing calls to integrate social care with clinical workflow [42]. This begins with improving patient-provider communication on health-related social needs and standardized screening and documentation of SDoH data. Despite the willingness of most patients to engage their providers on their socioeconomic circumstances during clinical encounters, such discussions often do not happen at all [43,44,45]. Clinicians can strategize to include concise conversations about SDoH in clinical encounters to better place patients’ expectations of their clinical care.
Regarding SDoH documentation, several screening tools [46, 47] and diagnostic coding [48] have been developed to enable hospitals capture the social needs data of patient groups. Software developers are also optimizing electronic medical records with user-friendly SDoH wheel to facilitate screening and referral to available community resources [49]. Standardizing screening and documentation of SDoH data will enable health systems to: track social needs more effectively in order to personalize care: identify population health trends: and guide community partnerships [48]. Unfortunately, adoption has been limited by a lack of clarity on who could document SDoH data, disincentivizing fee-for-service payment systems, and generally low prioritization by health systems.
Given the unequal return of socioeconomic and psychological resources across demographic divides, community-wide coalitions between health systems, public health agencies, and community partners present the most effective tool for addressing SDoH at both the community and individual patient level [50]. Such partnerships could better crystallize the unique social assets and risks of patient populations and inform the adjustment of clinical and social services to accommodate identified systemic social barriers.
Study Limitations
We note some limitations of this study. HALex as a generic HRQoL measure is not without limitations. First, with the omission of emotional, mental, and social functioning from its derivation, HALex is limited in discriminating the levels of wellbeing, especially for populations who at the highest level of health [51]. Secondly, the reliance of HALex on subjective health status raises the issue of how much HALex score differences associated with socioeconomic disadvantage are related to differential reporting behaviors and health expectations. Beyond latent health, a person’s rating of their health is influenced by the interplay between their sociocultural environment and biology [39, 52]. Such heterogeneity may explain away some of the differences we observed with the concomitant HALex. Regardless, the manner in which people account for the many dimensions of health when rating their overall health is relatively stable across specific populations [37], assuring that the incremental negative changes in HRQoL associated with increasing cumulative social disadvantage are less likely to be artificial. Third, there is no established clinical minimally important difference for HALex, though a difference of 0.03 has been suggested as a threshold of clinical significance for health utility indices on which HALex derivation is based [40]. Nevertheless, HALex is strongly congruent with more widely used HRQoL measures [23, 53], and our results are consistent with studies that used other HRQoL measures [16, 54, 55].
Our cumulative SDoH index had some limitations as well. First, the self-report of all SDoH information without objectively assessed information may misclassify the social disadvantage profile of the study respondents. Nonetheless, previous studies have found a strong correlation between the self-reported information in NHIS, and the verified information found in other national datasets [56]. Secondly, simply adding up the SDoH items to quantify the overall burden of social disadvantage experienced across six domains may not sufficiently capture the potentially varying effects of individual SDoH on study outcomes. However, unweighted summation of individual social factors into an aggregate index is widely accepted [17, 30], and our approach for capturing SDoH burden in an aggregate index has been used to predict outcomes in diverse settings and patient populations [57,58,59]. A more thorough assessment of the relative effects of individual SDoH – within and across domains – on HRQoL and other patient-reported outcomes need to be explored in future studies. Finally, NHIS lacks objective measures of community-level disadvantage such as the area deprivation index or the social vulnerability index, for which reason our estimates may suffer some unmeasured confounding by community-level disadvantage. However, we have other community-context factors in our SDoH framework to provide some proxy for community disadvantage in our SDoH index.
Conclusions
In a nationally representative study of US adults, cumulative social disadvantage was associated with poorer HALex performance in a graded fashion. In order to accurately identify socially vulnerable groups in need of both clinical risk mitigation and social support, innovations to incorporate SDoH-screening tools into clinical decision systems must continue. Policies can be tailored through community partnerships to address systemic barriers that exist within distinct sociodemographic groups, as well as demographic differences in health perception and healthcare experience, in order to maximize returns.
Data Availability
The datasets analyzed in the current study are available from the corresponding author on reasonable request.
Abbreviations
- HALex:
-
Health and Activity Limitation Index
- HRQoL:
-
health-related quality of life
- NHIS:
-
National Health Interview Survey
- OR:
-
odds ratio
- SDoH:
-
social determinant of health
- SDoH-Q:
-
quartile groups of cumulative social determinant of health index
References
Frosch DL. Patient-reported outcomes as a measure of Healthcare Quality. J Gen Intern Med. 2015 Oct;30(10):1383–4.
Anker SD, Agewall S, Borggrefe M, Calvert M, Jaime Caro J, Cowie MR et al. The importance of patient-reported outcomes: a call for their comprehensive integration in cardiovascular clinical trials. Eur Heart J 2014 Aug 7;35(30):2001–9.
Valderas JM, Alonso J. Patient reported outcome measures: a model-based classification system for research and clinical practice. Qual Life Res Int J Qual Life Asp Treat Care Rehabil 2008 Nov;17(9):1125–35.
Braveman P, Egerter S, Williams DR. The social determinants of health: coming of age. Annu Rev Public Health. 2011;32:381–98.
Marmot M, Friel S, Bell R, Houweling TAJ, Taylor S, Commission on Social Determinants of Health. Closing the gap in a generation: health equity through action on the social determinants of health. Lancet Lond Engl 2008 Nov 8;372(9650):1661–9.
Marmot M, Allen J, Bell R, Bloomer E, Goldblatt P. Consortium for the european review of Social Determinants of Health and the health divide. WHO European review of social determinants of health and the health divide. Lancet Lond Engl. 2012 Sep;15(9846):1011–29.
King JB, Pinheiro LC, Bryan Ringel J, Bress AP, Shimbo D, Muntner P et al. Multiple social vulnerabilities to Health Disparities and Hypertension and Death in the REGARDS Study. Hypertens Dallas Tex 1979. 2022 Jan;79(1):196–206.
Berman AN, Biery DW, Ginder C, Singh A, Baek J, Wadhera RK et al. Association of Socioeconomic Disadvantage With Long-term Mortality After Myocardial Infarction: The Mass General Brigham YOUNG-MI Registry. JAMA Cardiol. 2021 Aug 1;6(8):880–8.
Canterbury A, Echouffo-Tcheugui JB, Shpilsky D, Aiyer A, Reis SE, Erqou S. Association between cumulative social risk, particulate matter environmental pollutant exposure, and cardiovascular disease risk. BMC Cardiovasc Disord. 2020 Feb;11(1):76.
Bonner WIA, Weiler R, Orisatoki R, Lu X, Andkhoie M, Ramsay D et al. Determinants of self-perceived health for Canadians aged 40 and older and policy implications. Int J Equity Health 2017 Jun 6;16(1):94.
Tan JJX, Kraus MW, Carpenter NC, Adler NE. The association between objective and subjective socioeconomic status and subjective well-being: a meta-analytic review. Psychol Bull. 2020 Nov;146(11):970–1020.
Figueroa JF, Frakt AB, Jha AK. Addressing Social Determinants of Health: time for a polysocial risk score. JAMA. 2020 Apr;28(16):1553–4.
Committee on the Recommended Social and Behavioral Domains and Measures for Electronic Health Records, Board on Population Health and Public Health Practice, Institute of Medicine. Capturing Social and Behavioral Domains and Measures in Electronic Health Records: Phase 2 [Internet]. Washington (DC): National Academies Press (US). ; 2015 [cited 2022 Mar 1]. Available from: http://www.ncbi.nlm.nih.gov/books/NBK268995/.
Javed Z, Jilani H, Yahya T, Khan SU, Dubey P, Hyder A et al. Social Determinants. In: Martin SS, editor. Precision Medicine in Cardiovascular Disease Prevention [Internet]. Cham: Springer International Publishing; 2021 [cited 2022 Mar 11]. p. 1–29. Available from: https://doi.org/10.1007/978-3-030-75055-8_1.
Hanmer J. Cross-sectional validation of the PROMIS-Preference scoring system by its association with social determinants of health. Qual Life Res Int J Qual Life Asp Treat Care Rehabil. 2021 Mar;30(3):881–9.
Hanmer J. Measuring population health: association of self-rated health and PROMIS measures with social determinants of health in a cross-sectional survey of the US population. Health Qual Life Outcomes. 2021 Sep;22:19:221.
Rhee TG, Marottoli RA, Cooney LM Jr, Fortinsky RH. Associations of Social and behavioral determinants of Health Index with Self-Rated Health, Functional Limitations, and Health Services Use in older adults. J Am Geriatr Soc. 2020;68(8):1731–8.
Javed Z, Valero-Elizondo J, Dudum R, Khan SU, Dubey P, Hyder AA, et al. Development and validation of a polysocial risk score for atherosclerotic cardiovascular disease. Am J Prev Cardiol. 2021 Dec;1:8:100251.
Artiga S, Hinton E. Beyond Health Care: The Role of Social Determinants in Promoting Health and Health Equity [Internet]. Henry J Kaiser Family Foundation (KFF); 2018 May p. 13. (Racial Equity and Health Policy). Available from: https://files.kff.org/attachment/issue-brief-beyond-health-care.
Erickson P. Evaluation of a population-based measure of quality of life: the Health and Activity Limitation Index (HALex). Qual Life Res Int J Qual Life Asp Treat Care Rehabil. 1998 Feb;7(2):101–14.
Kaplan RM, Hays RD. Health-Related Quality of Life Measurement in Public Health. Annu Rev Public Health. 2022;43(1):355–73.
Khanna D, Maranian P, Palta M, Kaplan RM, Hays RD, Cherepanov D, et al. Health-related quality of life in adults reporting arthritis: analysis from the National Health Measurement Study. Qual Life Res Int J Qual Life Asp Treat Care Rehabil. 2011 Sep;20(7):1131–40.
Stewart ST, Cutler DM, Rosen AB. Comparison of trends in US health-related quality of life over the 2000s using the SF-6D, HALex, EQ-5D, and EQ-5D visual analog scale versus a broader set of symptoms and impairments. Med Care. 2014 Dec;52(12):1010–6.
National Center for Health Statistics. NHIS - Data, Questionnaires and Related Documentation [Internet]. Centers for Disease Control and Prevention. 2021 [cited 2021 Nov 17]. Available from: https://www.cdc.gov/nchs/nhis/data-questionnaires-documentation.htm.
Kachan D, Fleming LE, Christ S, Muennig P, Prado G, Tannenbaum SL, et al. Health Status of older US Workers and Nonworkers, National Health interview Survey, 1997–2011. Prev Chronic Dis. 2015 Sep;24:12:E162.
Wolowacz SE, Briggs A, Belozeroff V, Clarke P, Doward L, Goeree R et al. Estimating Health-State Utility for Economic Models in Clinical Studies: an ISPOR Good Research Practices Task Force Report. Value Health 2016 Sep 1;19(6):704–19.
Baptista FM, Rodrigues AM, Gregório MJ, de Sousa R, Cruz E, Canhão H. Functional status and quality of Life determinants of a Group of Elderly People with Food Insecurity. Front Nutr. 2018 Oct;25:5:99.
Alonso MAM, Barajas MES, Ordóñez JAG, Ávila Alpirez H, Fhon JRS, Duran-Badillo T. Quality of life related to functional dependence, family functioning and social support in older adults. Rev Esc Enferm U P. 2022;56:e20210482.
Lui F, Finik J, Leng J, Gany F. Social determinants and health-related quality of life in a sample of diverse, low socioeconomic status cancer patients. Psychooncology. 2022 Nov;31(11):1922–32.
Rhee TG, Lee K, Schensul JJ. Black-white Disparities in Social and behavioral determinants of Health Index and their Associations with Self-rated Health and Functional Limitations in older adults. J Gerontol A Biol Sci Med Sci. 2021 Mar;31(4):735–40.
Cain CL, Wallace SP, Ponce NA. Helpfulness, Trust, and safety of neighborhoods: Social Capital, Household Income, and self-reported health of older adults. The Gerontologist 2018 Jan 18;58(1):4–14.
Idler EL, Kasl SV. Self-ratings of health: do they also predict change in functional ability? J Gerontol B Psychol Sci Soc Sci. 1995 Nov;50(6):344–53.
Wu S, Wang R, Zhao Y, Ma X, Wu M, Yan X, et al. The relationship between self-rated health and objective health status: a population-based study. BMC Public Health. 2013 Apr;9(1):320.
Sarkisian CA, Prohaska TR, Wong MD, Hirsch S, Mangione CM. The relationship between expectations for aging and physical activity among older adults. J Gen Intern Med. 2005 Oct;20(10):911–5.
Watkinson RE, Sutton M, Turner AJ. Ethnic inequalities in health-related quality of life among older adults in England: secondary analysis of a national cross-sectional survey. Lancet Public Health. 2021 Mar 1;6(3):e145–54.
Assari S. Unequal gain of Equal Resources across racial groups. Int J Health Policy Manag 2018 Jan 1;7(1):1–9.
Erving CL, Zajdel R. Assessing the Validity of Self-rated Health Across Ethnic Groups: Implications for Health Disparities Research. J Racial Ethn Health Disparities [Internet]. 2021 Feb 5 [cited 2022 Mar 2]; Available from: https://doi.org/10.1007/s40615-021-00977-x.
Farmer MM, Ferraro KF. Are racial disparities in health conditional on socioeconomic status? Soc sci Med 1982. 2005 Jan;60(1):191–204.
Layes A, Asada Y, Kephart G. Whiners and deniers – what does self-rated health measure? Soc Sci Med. 2012 Jul;75(1):1–9.
Gallo LC, Penedo FJ, Espinosa de los Monteros K, Arguelles W. Resiliency in the Face of disadvantage: do hispanic cultural characteristics protect Health Outcomes? J Pers. 2009;77(6):1707–46.
Santos-Lozada AR. Self-rated mental health and race/ethnicity in the United States: support for the epidemiological paradox. PeerJ. 2016;4:e2508.
Chen M, Tan X, Padman R. Social determinants of health in electronic health records and their impact on analysis and risk prediction: a systematic review. J Am Med Inform Assoc JAMIA. 2020 Nov;7(11):1764–73.
Hunter WG, Zhang CZ, Hesson A, Davis JK, Kirby C, Williamson LD, et al. What strategies do Physicians and Patients discuss to reduce out-of-Pocket costs? Analysis of cost-saving strategies in 1,755 Outpatient Clinic visits. Med Decis Mak Int J Soc Med Decis Mak. 2016 Oct;36(7):900–10.
Chang C, Ceci C, Uberoi M, Waselewski M, Chang T. Youth Perspectives on their Medical Team’s role in screening for and addressing Social Determinants of Health. J Adolesc Health. 2022 Jun;70(1):928–33.
Long M, Frederiksen B, Ranji U, Diep K. 2023. Women’s Experiences with Provider Communication and Interactions in Health Care Settings: Findings from the 2022 KFF Women’s Health Survey - Issue Brief [Internet]. KFF. 2023 [cited 2023 Jun 7]. Available from: https://www.kff.org/report-section/womens-experiences-with-provider-communication-and-interactions-in-health-care-settings-findings-from-the-2022-kff-womens-health-survey-issue-brief/.
Billioux A, Verlander K, Anthony S, Alley D. Standardized Screening for Health-Related Social Needs in Clinical Settings: The Accountable Health Communities Screening Tool. NAM Perspect [Internet]. 2017 May 30 [cited 2023 Jun 5]; Available from: https://nam.edu/standardized-screening-for-health-related-social-needs-in-clinical-settings-the-accountable-health-communities-screening-tool/.
Centers for Medicare & Medicaid Services. The Accountable Health Communities Health-Related Social Needs Screening Tool [Internet]. 2017 [cited 2023 Jun 5]. Available from: https://innovation.cms.gov/files/worksheets/ahcm-screeningtool.pdf.
American Hospital Association. ICD-10-CM coding for social determinants of health [Internet]. 2022. Available from: https://www.aha.org/system/files/2018-04/value-initiative-icd-10-code-social-determinants-of-health.pdf.
Rogers CK, Parulekar M, Malik F, Torres CA. A Local Perspective into Electronic Health Record Design, Integration, and Implementation of Screening and Referral for Social Determinants of Health. Perspect Health Inf Manag. 2022 Mar 15;19(Spring):1 g.
Nehme E, Castedo de Martell S, Matthews H, Lakey D. Addressing Social Needs through Integrated Healthcare and Social Care: Case Studies, Key Issues, and Recommendations to Advance Practice in Texas [Internet]. Austin, TX: Texas Health Improvement Network/UT System Population Health; 2020 p. 1–49. Available from: https://utsystem.edu/sites/default/files/sites/texas-health-journal/THIN_Healthcare%2BSocial-Care-October2020.pdf.
Borrell LN, Lancet EA. Race/ethnicity and all-cause mortality in US adults: revisiting the hispanic paradox. Am J Public Health. 2012 May;102(5):836–43.
Jylhä M. What is self-rated health and why does it predict mortality? Towards a unified conceptual model. Soc Sci Med. 2009 Aug 1;69(3):307–16.
Fryback DG, Palta M, Cherepanov D, Bolt D, Kim JS. Comparison of 5 health-related quality-of-life indexes using item response theory analysis. Med Decis Mak Int J Soc Med Decis Mak. 2010 Feb;30(1):5–15.
Miravitlles M, Naberan K, Cantoni J, Azpeitia A. Socioeconomic status and health-related quality of life of patients with chronic obstructive pulmonary disease. Respir Int Rev Thorac Dis. 2011;82(5):402–8.
Delpierre C, Kelly-Irving M, Munch-Petersen M, Lauwers-Cances V, Datta GD, Lepage B et al. SRH and HrQOL: does social position impact differently on their link with health status? BMC Public Health 2012 Jan 10;12:19.
Nelson DE, Powell-Griner E, Town M, Kovar MG. A comparison of national estimates from the National Health interview Survey and the behavioral risk factor Surveillance System. Am J Public Health. 2003 Aug;93(8):1335–41.
Acquah I, Hagan K, Javed Z, Taha MB, Valero-Elizondo J, Nwana N et al. Social Determinants of Cardiovascular Risk, Subclinical Cardiovascular Disease, and Cardiovascular Events. J Am Heart Assoc. 2023 Mar 21;12(6):e025581.
Khan SU, Acquah I, Javed Z, Valero-Elizondo J, Yahya T, Blankstein R et al. Social Determinants of Health Among Non-Elderly Adults With Stroke in the United States. Mayo Clin Proc. 2022 Feb;97(2):238–49.
Javed Z, Valero-Elizondo J, Maqsood MH, Mahajan S, Taha MB, Patel KV, et al. Social determinants of health and obesity: findings from a national study of US adults. Obes Silver Spring Md. 2022 Feb;30(2):491–502.
Acknowledgements
Not applicable.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
K.H., K.N., and Z.J. contributed to the study’s conception and design. Material preparation and data analysis were performed by K.H. and J.VE. The first draft of the manuscript was prepared by K.H. and Z.J. K.H. prepared all figures. All authors reviewed the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics Approval and Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Competing Interests
KN is on the advisory board of Amgen and Novartis, and his research is partly supported by the Jerold B. Katz Academy of Translational Research. KN and MCA are on the Steering Committee of the PAK-SEHAT Study, partially funded by an unrestricted research grant from Getz Pharma. AAH declares current funding from the United States National Institutes of Health, the World Bank, and the World Health Organization. The other authors report no conflicts of interest relevant to this work.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Hagan, K., Javed, Z., Cainzos-Achirica, M. et al. Cumulative social disadvantage and health-related quality of life: national health interview survey 2013–2017. BMC Public Health 23, 1710 (2023). https://doi.org/10.1186/s12889-023-16168-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12889-023-16168-8