Abstract
Background
Pregnancy-induced hypertension is the new onset of high blood pressure after 20 weeks of gestation in women with previously normal blood pressure. To the best of our knowledge, no study has been conducted in our country to investigate the association between this pregnancy problem and iron-folic acid supplementation. The aim of this study was to determine the association between iron-folic acid supplementation and pregnancy-induced hypertension (PIH) in pregnant women at public hospitals in the Wolaita Sodo zone.
Methods
An institution-based case–control study was conducted among pregnant women who visited public hospitals in the Wolaita Sodo zone from March 3, 2022, to August 30, 2022. A consecutive sampling method was used to select the study participants. The total sample size was 492, of which 164 were cases and 328 were controls. The data were collected by conducting face-to-face interviews and measurements. The data were entered into EpiData version 4.6 and exported to STATA 14 for analysis. Those variables with a p-value less than 0.05 were considered statistically significant. Descriptive statistics and odds ratios were presented using texts, tables, and figures.
Results
A total of 471 women participated in this study, yielding a response rate of 96%. The cases had a mean age of 25 ± 4.43, while the controls had a mean age of 25 ± 3.99. The mean age at first pregnancy among cases was 20 ± 2.82 and among controls was 20 ± 2.97. The average number of deliveries for cases and controls was 1.97 ± 1.41 and 1.95 ± 1.38, respectively. There is no significant association between iron-folic acid supplementation and PIH. Pregnant women with high hemoglobin levels had higher odds of PIH as compared to those without it (AOR = 3.65; 95% CI: 1.0–12.9). Eating kocho (AOR = 14.4; 95% CI: 1.2–16.7) was positively associated with PIH.
Conclusions
There is no association between iron-folic acid supplementation during pregnancy and pregnancy-induced hypertension. Pregnant women with high hemoglobin levels had higher odds of PIH as compared to those without it. There is an association between kocho consumption and PIH. More research should be done using stronger designs.
Similar content being viewed by others
Background
Pregnancy-induced hypertension is the new onset of high blood pressure after 20 weeks of gestation in women with previously normal blood pressure [1]. According to a study conducted by the World Health Organization (WHO) from 2003–2009, the leading causes of maternal death were hemorrhage (27·1%), pregnancy-induced hypertension (14·0%) and sepsis (10·7%). These were responsible for more than half of all maternal deaths worldwide [2]. Pregnancy-induced hypertension (PIH) is the most common medical complication of pregnancy, with an incidence of between 5 and 10%. The WHO estimates that at least one woman dies every 7 min from a complication of PIH [3]. The incidence of PIH increased from 16.30 million to 18.08 million globally, with a total increase of 10.92% from 1990 to 2019 [4].
The burden of PIH is high in Africa, with one in 10 pregnancies affected. The burden is significantly higher in Central and Western Africa [5]. Sub-Saharan Africa accounted for approximately 86% of the estimated global maternal deaths in 2017 [6]. During the ten-year period (2006–2015), there was an increase in the number of maternal deaths due to direct causes of pregnancy [7].
The maternal mortality rate in Ethiopia declined from 5.51% to 4.98% from 2014 to 2017, respectively. Nevertheless, maternal mortality due to PIH has increased [8, 9]. Obstetric factors were directly responsible for 51 (86%) of all maternal deaths in Ethiopia. The primary direct causes of maternal mortality in Ethiopia were hemorrhage (45%), PIH (23%), and obstructed labor (18%). Among PIHs, preeclampsia was the most common type in the country [10,11,12,13].
Factors associated with PIH were: primigravida, extreme age, early gestational age, twin pregnancy, gravidity, long inter-pregnancy intervals, chronic hypertension, history of diabetes, multiple pregnancies, obesity, smoking, socioeconomic level, and diet [14,15,16,17,18,19]. Anemia and coffee intake during pregnancy are risk factors for the development of PIH [20]. Similarly, the level of iron was significantly higher in the PIH groups than in the control groups [21]. Antioxidant supplementation was associated with better maternal and perinatal outcomes than iron and folic acid supplementation alone [22]. The consumption of seafood was inversely associated with the odds of developing PIH [23]. PIH was less frequent in women who ate received iron and folic acid supplements [24]. Maternal ferritin concentration is primarily a reflection of maternal iron status, and a high level is associated with unfavorable outcomes. This indicates the need for further study of routine iron-folic acid supplementation in pregnant women [25].
The potential harmful effects of iron-folic acid were not carefully debated in regards to its effectiveness. Even if iron-folic acid is beneficial for neonatal or maternal outcomes, it is associated with glucose impairment and pregnancy-induced hypertension in mid-pregnancy [26]. High hemoglobin levels in women who took iron-folic acid supplements were associated with an increased risk of PIH [27]. Its supplementation before 16 weeks of gestational age was significantly associated with an increased risk of developing PIH [28]. But there was no association between the occurrence of PIH and the timing of iron-folic acid (IFA) supplementation. Early (< 28 weeks) and late (≥ 28 weeks) onset or start of iron-folic acid supplementation, on the other hand, was found to be protective [29, 30]. Women in the lowest iron quartile had a 2.2-fold increase in PIH risk compared to women in the highest quartile [31]. In India, women who had a diet that was sufficiently varied and supplemented with iron and folic acid throughout pregnancy experienced fewer PIH symptoms. PIH symptoms were 36% lower in mothers who took iron-folic acid supplements for at least 90 days during their previous pregnancy [32].
A study showed that serum ferritin and serum iron were higher in PIH women [33]. The serum iron level has a direct correlation with the level of blood pressure, concentration of total hemoglobin, and serum iron [34, 35]. Iron supplementation during pregnancy may have resulted in iron overload, which may have resulted in oxidative stress and endothelial dysfunction in the patients [36]. In contrast, there was no significant difference between PIH and serum iron concentrations [37].
PIH can be avoided through early detection and eating vegetables and fruits during pregnancy. The WHO highly suggests that pregnant women take daily oral iron and folic acid supplements to prevent maternal anemia, puerperal sepsis, low birth weight, and premature birth. Iron-folic acid should be started as soon as possible to prevent neural tube defects [38]. It was found that iron-folic acid supplementation is recommended for the benefit of the fetus and herself, but the prevalence of PIH is increasing, unlike other complications during pregnancy. Most factors in PIH were assessed using cross-sectional studies, which could not show a cause-and-effect relationship. Hence, the association between iron-folic acid supplementation and pregnancy-induced hypertension is not clear yet. Furthermore, to the best of our knowledge, there is no study done in Ethiopia on this association. Therefore, the aim of this study was to determine the association between iron-folic acid supplementation and pregnancy-induced hypertension among pregnant women.
Methods
Study design and settings
The aim of this study was to determine the association between iron-folic acid supplementation and pregnancy-induced hypertension among pregnant women in the public hospitals of Wolaita Sodo zone. An institution-based, unmatched case–control study was conducted among pregnant women attending ANC and admitted for delivery in obstetrics and gynecology departments. The study was conducted in the four public hospitals of Wolaita Sodo zone in southern Ethiopia from March 3, 2022, to August 30, 2022. The Southern Nation Nationalities and Peoples Regional State (SNNPR) is one of the ten regions that has a wide variety of nations and nationalities with different cultures, languages, lifestyles, weather conditions, topography, habitats, and other natural phenomena. The region has 16 zones, of which the Wolaita Sodo zone is one. Wolaita Sodo town is found in the southern direction of Addis Ababa (the capital city of Ethiopia) and in the southwest direction of Hawassa, about 329 and 151 km apart, respectively.
There are about eight governmental hospitals and two private hospitals in the Wolita Sodo zone. They include Wolaita Sodo University Comprehensive Specialized Hospital (WSUCSH), Bodity, Bitena, Bele, Bombe, Gesuba, Humbo, and Kindo Halale primary hospitals. The Wolaita Sodo University College of Health Science and Medicine is located in Wolaita Sodo town, which is 151 km west of Hawassa and 329 km south of Addis Ababa.
Case definition
Cases are defined as pregnant women whose blood pressure was greater than or equal to 140/90 mmHg in two separate readings taken 4 h apart [1]. They were diagnosed and confirmed by obstetrics and gynecology physicians. Controls are defined as pregnant women in the same hospitals whose blood pressure is less than 140/90 mmHg after 20 weeks of gestation. During the study period, cases and controls were identified through record review and after physician diagnosis in ANC clinics and obstetrics and gynecology wards. The diagnosis includes history-taking, clinical manifestations, a physical examination, and laboratory tests.
Population
The source populations were pregnant women, both cases and controls, who attended ANC and were admitted for delivery in public hospitals in the Wolaita Sodo zone. The study population consisted of pregnant women who fulfilled the eligibility criteria. These were both cases and controls who attended ANC and were admitted for delivery in the selected hospitals during the study period. Consecutively chosen pregnant women, both cases and controls, in the selected hospitals during that study period were the sampled populations.
Inclusion and exclusion criteria
Women who attended ANC and were admitted for delivery and had a blood pressure readings greater than or equal to 140/90 mmHg or had a blood pressure of less than 140/90 mmHg after 20 weeks of gestation were included in this study. The study excluded severely ill pregnant women.
Sample size determination
The sample size was calculated using OpenEpi version 2.3 statistical software by assuming a proportion of cases exposed of 13%, a minimum detectable odds ratio among controls of 2.14 [28], a case-to-control ratio of 1: 2, a significant level of 95%, and a power of 80%. The sample size was 447. With a 10% non-response rate, the total sample size was 492 people, with 164 cases and 328 controls.
Sampling procedures
The four hospitals—Wolaita Sodo University Comprehensive Specialty Hospital (WSUCSH), Bitena, Bodity, and Humbo Primary Hospitals—were selected due to their high patient flow rates. The cases that fulfilled the inclusion criteria were selected using the consecutive sampling method until the required sample size was attained. Then the next two immediate corresponding controls were also selected consecutively on the same day in the ANC unit and labor wards (Fig. 1).
Instruments (Questionnaire)
The data were collected through measurements, reviewing records, and face-to-face interviews using a pretested questionnaire. The measurements included blood pressure, weight, height, and urine from the women. The women were interviewed about their socio-demographic characteristics, obstetric factors, and behavioral factors by trained and experienced health professionals immediately before and after ANC and delivery services. A questionnaire was prepared by reviewing different pieces of literature that were similar to the current study [10, 20, 24, 25, 27, 35]. Then the questionnaire was changed from English to Amharic and back-translated to English to check its consistency. The questionnaire was pretested on 5% of the sampled pregnant women.
Laboratory measurements and individuals
Twelve health professionals—eight BSc midwives and four MPH health professionals—were recruited as data collectors and supervisors, respectively. There were two trained data collectors for each hospital. The trained laboratory technicians measured proteinuria and hemoglobin levels.
The proteinuria was measured using a dipstick test. Urine specimens for proteinuria assessment were obtained from spot urine samples collected from pregnant women who attended ANC. The health care providers used a dipstick test with a color-sensitive pad. The color changes on the dipstick indicated the women's levels of protein in the urine.
Hemoglobin levels were determined using an automated hematology analyzer machine. Blood samples were drawn from the women's veins on the inside of their elbows. Needles were inserted into the veins, and the blood samples were collected using airtight vials. The blood samples were put into the automated hematology analyzer machine. Then hemoglobin levels were determined from these blood samples using this machine. The hemoglobin levels were categorized as > = 11.0 mg/dl and < 11.0 mg/dl [39].
Data quality management
Training was given for data collectors and supervisors about two days before data collection. A clear explanation of the purpose of the study was provided to the respondents at the beginning of the interview. Supervisors and the principal investigator provided close supervision. The data from each respondent was checked for completeness, clarity, consistency, and accuracy by the data collectors, supervisors, and the principal investigator.
Statistical analysis
After data collection, the data were coded and entered using Epidata version 4.6 software. The entered data was then transformed into the STATA 14 version. Descriptive statistics like frequencies, percentages, means, and standard deviations were done. The association between pregnancy-induced hypertension and each variable was checked using bivariate logistic regression. Variables with a p-value less than 0.25 in the bivariate logistic regression were entered into multivariable logistic analysis, and those variables with a p-value of less than 0.05 in multivariate logistic regression were considered statistically associated factors. Text, tables, and figures were used to present the findings.
Results
Socio-demographic characteristics
A total of 471 women participated in this study. The response rate was 96%. The cases had a mean age of 25 ± 4.43, while the controls had a mean age of 25 ± 3.99. The mean age at first pregnancy among cases was 20 ± 2.82 and among controls was 20 ± 2.97. The average number of deliveries for cases and controls was 1.97 ± 1.41 and 1.95 ± 1.38, respectively. About 153 (97.5%) of cases and 304 (96.8%) of controls were married. Regarding educational status, 12 (7.6%) of the cases and 11 (3.5%) of the controls could not read or write. Similarly, 79 (50.3%) of cases and 136 (43.3%) of controls were housewives. More than half (51.0%) of the cases and 198 (63.0%) of the controls were from urban residences (Table 1).
Obstetric and gynecological factors
About 100 (63.7%) of the cases and 225 (71.7%) of the controls were multigravida, and 9 (5.7%) of the cases and 11 (3.5%) controls had multiple pregnancies (Table 2).
Iron-folic acid supplementation related factors
One hundred thirty (82.8%) of the cases and 252 (80.2%) of the controls had taken iron during their pregnancies. After 16 weeks of gestation, 113 (86.9%) of the cases and 228 (90.5%) of the controls began their first dose of iron and folic acid supplementation. In terms of hemoglobin levels, 115 (73.2%) cases and 211 (67.2%) controls had levels greater than or equal to 11.0 g/dl. Only 42 (26.8%) cases and 103 (32.8%) controls had hemoglobin levels below the normal range (Table 3).
Behavioral factors
We have also assessed the behavioral and nutritional histories of the women who consumed during their pregnancies. Most of these nutrients are sources of iron. Among the commonly known sources of iron are teff (Eragrostis tef), animal products, cereals, and fish. Based on this study, 45 (28.7%) of cases and 112 (35.6%) of the controls ate injera that was prepared from teff [40]. Similarly, 78 (49.7%) of cases and 138 (43.9%) of controls consumed sweet foods or soft drinks [41] during this pregnancy (Table 4).
Association of iron supplementation and PIH
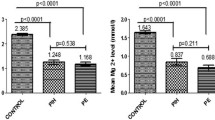
We used logistic regression analysis to identify factors associated with pregnancy-induced hypertension. Based on this, about nine variables were eligible for multivariable logistic regression analysis. There is no association between iron-folic acid supplementation and PIH. Even if our main objective was to determine the association between iron-folic acid supplementation and pregnancy-induced hypertension, we also assessed other dietary factors that could be sources of iron. Hence, hemoglobin levels and the consumption of kocho or bulla were found to be significantly associated with pregnancy-induced hypertension. The odds of PIH was 3.65 (1.0–12.9) times higher among women with a hemoglobin level > = 11.0 mg/dl as compared with women whose hemoglobin level was less than 11.0 mg/dl (Table 5).
Discussion
Pregnant women with high hemoglobin levels had higher odds of pregnancy-induced hypertension as compared to controls. The finding of this study was in line with a study conducted in Iran [27]. According to the study conducted in Iran, a high hemoglobin level in the first trimester was a risk factor for pregnancy-induced hypertension. The current finding is also consistent with the study conducted in Arba Minch and Abbottabad [34, 42]. Hemoglobin determines the viscosity of blood. According to various studies, systolic and diastolic blood pressure both increase as hemoglobin levels rise [43, 44]. An increase in free hemoglobin contents results in vasoconstriction, which leads to the development of PIH [45]. In pregnancy-induced hypertension, a drop in intravascular volume and a rise in tissue edema were caused by the loss of serum protein and an increase in capillary endothelial permeability [46]. Any organ, including the liver, brain, and lungs, could be affected. The blood volume reduction can cause the maternal hemoglobin concentration to rise [47]. However, the current finding contradicts a study conducted in India [32]. The possible explanation might be that the sample size in the Indian study was very small, which might have had a small effect. In addition to this, the study design was cross-sectional as compared with the current study, which did not show the cause-effect relationship as a case–control study. Furthermore, the study populations were different between the two studies. The current study's population included all pregnant women of any age and gestational age. It is found that pregnant women who later develop PIH have considerably higher levels of hemoglobin, hematocrit, serum iron, serum ferritin, and transferrin saturation [48]. In the PIH group, the platelet indices were lower, and the serum iron levels were higher [49].
In this study, there was no association between pregnancy-induced hypertension and iron-folic acid supplementation. It is consistent with a Thailand study, which found that taking iron and folic acid supplements late in pregnancy has no effect on pregnancy-induced hypertension. But, according to this study, early initiation of iron-folic acid supplementation before 16 weeks of pregnancy dramatically raised the risk of developing PIH [27]. This variation may be due to the fact that the time of initiation was early in pregnancy, whereas in the current study we assessed the association among pregnant women irrespective of gestational age or anemia status. Similarly, women who use high-dose folic acid supplements before pregnancy and through mid-pregnancy may be at increased risk for high blood pressure [50]. This finding is contrary to a study conducted in Poland. According to that study, PIH risk was 2.19 times higher in women in the lowest iron quartile (801.20 g/L) compared to those in the highest (> 1211.75 g/L) iron quartile [31]. The rise in blood iron observed in patients with PIH appears to be caused by a persistent, clinically undetectable hemolytic response. In addition to this, supplementing women with folic acid and multivitamins that contain folic acid rather than folic acid alone throughout pregnancy considerably lowers the incidence of PIH [29, 30]. The possible reasons might be due to the differences between the study designs.
We have also assessed the association between pregnancy-induced hypertension and other nutritional-related factors. Based on this, there was another nutrient that was assessed in this study called kocho or bulla, which is one of the well-known and commonly consumed cultural foods in the study area. According to previous studies, iron is one of its constituents. There is a strong association between kocho or bulla consumption and PIH. Pregnant women who consumed bulla or kocho were 14.4 (1.2–16.7) times more likely to develop pregnancy-induced hypertension as compared with control groups [51]. This result seems to recommend that pregnant women avoid using bulla or kocho during their pregnancy, but this is the first study assessing this food and PIH. We were unable to obtain studies on the preceding or their mechanisms of action. We could not get any justification from previous literature about the effects of kocho or bulla on pregnancy-induced hypertension. Hence, it needs further study with regard to this association. We have also assessed nutrients that are rich in iron, like teff, but they had no association with pregnancy-induced hypertension.
The strength of this study is that, to the best of our knowledge, it is the first study of its kind in our country. But this study did not differentiate between the specific type of pregnancy-induced hypertension and its association with iron-folic acid supplementation. As a limitation, there might be respondents' recall biases regarding the number of iron and folic acid tablets they took, the total number of weeks they took the tablets, and the starting time of tablet taking. Some women might also hide the truth about whether they took the tablets completely or left them after taking them from the health institutions. This intern might affect the true association.
Conclusions
There is no association between iron-folic acid supplementation and pregnancy-induced hypertension. However, pregnant women with high hemoglobin levels had a higher risk of developing pregnancy-induced hypertension than those who did not. The concentration of iron-folic acid has a direct relationship with hemoglobin levels. Before giving iron and folic acid supplements to pregnant women, it is crucial to assess their iron status because they may have more detrimental consequences than good ones. All pregnant women should have their hemoglobin levels measured as a routine task during their first visit. Strong designs like randomized clinical trials or meta-analyses should be carried out with a large sample size.
Availability of data and materials
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- ANC:
-
Antenatal Care
- AOR:
-
Adjusted Odds Ratio
- BMI:
-
Body Mass Index
- BP:
-
Blood Pressure
- DBP:
-
Diastolic Blood Pressure
- DM:
-
Diabetes Mellitus
- EDHS:
-
Ethiopian Demographic and Health Statistics
- FA:
-
Folic Acid
- GA:
-
Gestational Age
- HDP:
-
Hypertension Disorder of Pregnancy
- HEPI:
-
Health Professionals Education Partnership Initiative
- HTN:
-
Hypertension
- IFA:
-
Iron Folic Acid
- MNM:
-
Multiple Micronutrients
- OR:
-
Odds Ratio
- PIH:
-
Pregnancy Induced Hypertension
- SBP:
-
Systolic Blood Pressure
- SNNP:
-
Southern Nation Nationalities
- WSUTCSH:
-
Wolaita Sodo University Teaching and Comprehensive Specialized Hospital
References
American College of Obstetricians and Gynecologists. Clinical Management Guidelines for Obstetrician-Gynecologists: ACOG Practice Bulletin No. 202. Obstet Gynecol. 2018;99:159–67.
Say L, Chou D, Gemmill A, Tunçalp Ö, Moller AB, Daniels J, et al. Global causes of maternal death: a WHO systematic analysis. Lancet Glob Health. 2014;2(6):e323–33.
Bharti CS. Perinatal outcome in hypertensive disorder of pregnancy in a rural community of Haryana. Int J Community Med Public Health. 2019;6(10):4267–70.
Wang W, Xie X, Yuan T, Wang Y, Zhao F, Zhou Z, Zhang H. Epidemiological trends of maternal hypertensive disorders of pregnancy at the global, regional, and national levels: a population-based study. BMC Pregnancy Childbirth. 2021;21(1):364.
Jeyabalan A. Epidemiology of preeclampsia: impact of obesity. Nutr Rev. 2013;1(01):S18-25.
Ullah MB, Mridha MK, Arnold CD, et al. Newborn physical condition and breastfeeding behaviors: Secondary outcomes of a cluster-randomized trial of prenatal lipid-based nutrient supplements in Bangladesh. Matern Child Nutr. 2019;15(4): e12844.
Bwana VM, Rumisha SF, Mremi IR, Lyimo EP, Mboera LEG. Patterns and causes of hospital maternal mortality in Tanzania: A 10-year retrospective analysis. PLoS ONE. 2019;14(4):e0214807.
Berhan Y, Berhan A. Causes of maternal mortality in Ethiopia: a significant decline in abortion related death. Ethiop J Health Sci. 2014;24:15–28.
Mersha AG, Abegaz TM, Seid MA. Maternal and perinatal outcomes of hypertensive disorders of pregnancy in Ethiopia: systematic review and meta-analysis. BMC Pregnancy Childbirth. 2019;19:458.
Sara J, Haji Y, Gebretsadik A. Determinants of Maternal Death in a Pastoralist Area of Borena Zone, Oromia Region, Ethiopia: Unmatched Case-Control Study. Obstet Gynecol Int. 2019;2019:5698436.
Geleto A, Chojenta C, Taddele T, et al. Magnitude and determinants of obstetric case fatality rate among women with the direct causes of maternal deaths in Ethiopia: a national cross-sectional study. BMC Pregnancy Childbirth. 2020;20:130.
Tesfaye G, Loxton D, Chojenta C, et al. Magnitude, trends and causes of maternal mortality among reproductive aged women in Kersa health and demographic surveillance system, eastern Ethiopia. BMC Women’s Health. 2018;18:198.
Mekonnen Wubegzier, Hailemariam Damen, Gebremariam Alem. Causes of maternal death in Ethiopia between 1990 and 2016: A systematic review with meta-analysis. Ethiop J Health Dev. 2018;32(4):225–42.
Ness RB, Markovic N, Harger G, Day R. Barrier methods, length of preconception intercourse, and preeclampsia. Hypertens Pregnancy. 2004;23(3):227–35.
Singh S, Ahmed EB, Egondu SC, Ikechukwu NE. Hypertensive disorders in pregnancy among pregnant women in a Nigerian Teaching Hospital. Niger Med J. 2014;55(5):384-8.
Dekker G, Sibai B. Primary, Secondary, and tertiary prevention of preeclampsia. Lancet. 2001;357(2):209–15.
Osungbade KO, Ige OK. Public health perspectives of preeclampsia in developing countries: Implication for health system strengthening. J Pregnancy. 2011;2011:481095.
Das S, Das R, Bajracharya R, Baral G, Jabegu B, Odland JØ, Odland ML. Incidence and Risk Factors of Pre-Eclampsia in the Paropakar Maternity and Women’s Hospital, Nepal: A Retrospective Study. Int J Environ Res Public Health. 2019;16(19):3571.
Bergen NE, Schalekamp-Timmermans S, Roos-Hesselink J, Roeters van Lennep JE, Jaddoe VVW, Steegers EAP. Hypertensive disorders of pregnancy and subsequent maternal cardiovascular health. Eur J Epidemiol. 2018;33(8):763–71.
Endeshaw M, Ambaw F, Aragaw A, Ayalew A. Effect of Maternal Nutrition and Dietary Habits on Preeclampsia: A Case-Control Study. Int J Clin Med. 2014;5:1405–16.
Kolusari A, Kurdoglu M, Yildizhan R, Adali E, Edirne T, Cebi A, Demir H, Yoruk IH. Catalase activity, serum trace element and heavy metal concentrations, and vitamin A, D and E levels in pre-eclampsia. J Int Med Res. 2008;36(6):1335–41.
Rumiris D, Purwosunu Y, Wibowo N, Farina A, Sekizawa A. Lower rate of preeclampsia after antioxidant supplementation in pregnant women with low antioxidant status. Hypertens Pregnancy. 2006;25(3):241–53.
Ikem E, Halldorsson TI, Birgisdóttir BE, Rasmussen MA, Olsen SF, Maslova E. Dietary patterns and the risk of pregnancy-associated hypertension in the Danish National Birth Cohort: a prospective longitudinal study. BJOG. 2019;126(5):663–73.
Agrawal S, et al. Adequately diversified dietary intake and iron and folic acid supplementation during pregnancy is associated with reduced occurrence of symptoms suggestive of pre-eclampsia or eclampsia in Indian women. PloS one. 2015;10:3e0119120.
Lao TT, Tam KF, Chan LY. Third trimester iron status and pregnancy outcome in non-anaemic women; pregnancy unfavourably affected by maternal iron excess. Hum Reprod. 2000;15(8):1843–8.
Bo S, Menato G, Villois P, Gambino R, Cassader M, Cotrino I, Cavallo-Perin P. Iron supplementation and gestational diabetes in midpregnancy. Am J Obstet Gynecol. 2009;201(2):158.e1-6.
Imam MU, Zhang S, Ma J, Wang H, Wang F. Antioxidants Mediate Both Iron Homeostasis and Oxidative Stress. Nutrients. 2017;9(7):671.
Jirakittidul P, Sirichotiyakul S, Ruengorn C, Techatraisak K, Wiriyasirivaj B. Effect of iron supplementation during early pregnancy on the development of gestational hypertension and pre-eclampsia. Arch Gynecol Obstet. 2018;298(3):545–50.
Shim SM, Yun YU, Kim YS. Folic acid alone or multivitamin containing folic acid intake during pregnancy and the risk of gestational hypertension and preeclampsia through meta-analyses. Obstet Gynecol Sci. 2016;59(2):110–5.
Chen S, Li N, Mei Z, Ye R, Li Z, Liu J, Serdula MK. Micronutrient supplementation during pregnancy and the risk of pregnancy-induced hypertension: A randomized clinical trial. Clin Nutr. 2019;38(1):146–51.
Lewandowska M, Sajdak S, Lubiński J. Can Serum Iron Concentrations in Early Healthy Pregnancy Be Risk Marker of Pregnancy-Induced Hypertension? Nutrients. 2019;11(5):1086.
Basutkar RS, Chauhan BS. A Cross-Sectional Study Investigating the Association of Serum Iron Concentration and Platelet Count as a Risk Biomarker among the Pregnancy-Induced Hypertensive Women in the Highlands Western Ghats of Nilgiris. Indian J Community Med. 2022;47(1):125–9.
Gutierrez-Aguirre CH, García-Lozano JA, Treviño-Montemayor OR, Iglesias-Benavides JL, Cantú-Rodríguez OG, González-Llano O, et al. Comparative analysis of iron status and other hematological parameters in preeclampsia. Hematology. 2017;22(1):36–40.
Sendeku FW, Azeze GG, Fenta SL. Adherence to iron-folic acid supplementation among pregnant women in Ethiopia: a systematic review and meta-analysis. BMC Pregnancy Childbirth. 2020;20:138.
Adam B, Malatyalioğlu E, Alvur M, Talu C. Magnesium, zinc and iron levels in preeclampsia. J Matern Fetal Med. 2001;10(4):246–50.
Serdar Z, Gür E, Develioğlu O. Serum iron and copper status and oxidative stress in severe and mild preeclampsia. Cell Biochem Funct. 2006;24(3):209–15.
Shaji Geetha N, Bobby Z, Dorairajan G, Jacob SE. Increased hepcidin levels in preeclampsia: a protective mechanism against iron overload mediated oxidative stress? J Matern Fetal Neonatal Med. 2020;20:1–6.
WHO. Guideline: Daily iron and folic acid supplementation in pregnant women. Geneva: World Health Organization; 2012.
WHO. Haemoglobin concentrations for the diagnosis of anaemia and assessment of severity. Vitamin and Mineral Nutrition Information System. Geneva, World Health Organization. 2011. (WHO/NMH/NHD/MNM/11.1) (http://www.who.int/vmnis/indicators/haemoglobin.pdf.
Abebe Y, Bogale AA, Hambidge KM, Stoecker BJ, Bailey KB, Gibson RS. Phytate, zinc, iron and calcium content of selected raw and prepared foods consumed in rural Sidama, Southern Ethiopia, and implications for bioavailability. J Food Compos Anal. 2007;20:161–8.
Health Canada. Canadian Nutrient File, 2010. www.hc-sc.gc.ca/fn-an/nutrition/fiche-nutri-data/index-eng.php.
Zafar T, Iqbal Z. Iron status in preeclampsia. Professional Med J. 2008;15(1):74–80.
Gobel BO, Schulte-Gobel A, Weisser B, Glanzer K, Vetter H, Dusing R. Arterial blood pressure, correlation with erythrocyte count, hematocrit, and hemoglobin concentration. Am J Hypertens. 1991;4:14–9.
Atsma F, Veldhuizen I, De Kort W, Van Kraaij M, Pasker-de Jong P, Deinum J. Hemoglobin level is positively associated with blood pressure in a large cohort of healthy individuals. Hypertension. 2012;60:936–41.
Sarrel PM, Lindsay DC, Poole-Wilson PA, Collins P. Hypothesis: inhibition of endothelium-derived relaxing factor by haemoglobin in the pathogenesis of pre-eclampsia. Lancet. 1990;336:1030–2.
Chappel LL, Bewley S. Pre-eclamptic toxaemia: the role of uterine artery Doppler. Br J Obstet Gynaecol. 1998;105:379–82.
Walker JJ. Pre-eclampsia Lancet. 2000;356:1260–5.
Gudeta TA, Regassa TM. Pregnancy Induced Hypertension and Associated Factors among Women Attending Delivery Service at Mizan-Tepi University Teaching Hospital, Tepi General Hospital and Gebretsadik Shawo Hospital, Southwest. Ethiopia Ethiop J Health Sci. 2019;29(1):831–40.
Tesfay Y, Berhe S, Aregay A. Risk factors of pregnancy related hypertension among women attending maternal health care service in selected public hospitals of Tigray. Ethiopia. 2016;06:8904–11.
Panchal AR, Bartos JA, Cabañas JG, Donnino MW, Drennan IR, Hirsch KG, et al. Adult Basic and Advanced Life Support Writing Group. Part 3: Adult Basic and Advanced Life Support: 2020 American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation. 2020;142(16_suppl_2):S366–468.
Lakew A, Assefa T, Woldeyohannes M, Megersa N, Chandravanshi BS. Development and validation of liquid chromatography method for simultaneous determination of multiclass seven antibiotic residues in chicken tissues. BMC Chem. 2022;16(1):5.
Acknowledgements
First of all, we thank you the study participants and data collectors. We would like to thank Health Professionals Education Partnership Initiative and Wolaita Sodo University.
Funding
This research work was supported by the Health Professionals Education Partnership Initiative Ethiopia with grant number R25TW011214.
Author information
Authors and Affiliations
Contributions
AWA, AT, SBW, SS, AT & AA: participated in conception, data curation, formal analysis, investigation, funding acquisition, methodology, project administration, software, supervision, validation, visualization, writing-original draft preparation, writing -review & editing. AWA, WBD, SS, MA, HM, contributed to supervision, validation, visualization, writing -review & editing. All authors read and approved this manuscript submission.
Corresponding author
Ethics declarations
Ethics approval and consent for participants
The study was approved by Wolaita Sodo University Institution Review Board with ethical reference number of CRCSD 49/02/2014. Informed consent was obtained from all study participants and the study was carried out in accordance with relevant guidelines and regulations. Confidentiality and privacy of the respondents’ responses were maintained during data collection, analysis, and reporting of the findings.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Asres, A.W., Samuel, S., Daga, W.B. et al. Association between iron-folic acid supplementation and pregnancy-induced hypertension among pregnant women in public hospitals, Wolaita Sodo, Ethiopia 2021: a case- control study. BMC Public Health 23, 843 (2023). https://doi.org/10.1186/s12889-023-15794-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12889-023-15794-6