Abstract
Background
The current prospective cohort study aimed to explore the potential associations between dietary sodium (Na), potassium (K), and sodium-to-potassium (Na-to-K) ratio with an incidence risk of cardiovascular disease (CVD) among Iranian adults.
Methods
The participants of the Tehran Lipid and Glucose Study (men and women aged 30–84 years, n = 2050), free of CVD at baseline (2006–2008) were included. Dietary intakes were assessed using a validated food frequency questionnaire (FFQ), and incident CVD (i.e., coronary heart disease, stroke, and CVD mortality) were documented up to March 2018. Cox proportional hazard models were used to estimate hazard ratios (HRs) and 95% confidence interval (CI) regarding the association between dietary Na, K, and Na-to-K ratio with CVD events.
Results
During a median follow-up of 10.6 years, 10.14% of participants experienced CVD outcomes. A 41% increased risk of CVD in relation to each increase in 1000 mg/d of Na intake. In the fully-adjusted model, higher Na intake (> 4143 versus < 3049 mg/d) was significantly related to the increased risk of CVD (HR = 1.99, 95% CI = 1.06–3.74). Independent of the well-known risk factors, a 56% reduced risk of CVD was observed in the participants with a higher dietary K intake (HR = 0.44, 95% CI = 0.20–0.94). A Higher Na-to-K ratio was associated with an increased risk of CVD (HR = 1.99, 95% CI = 1.13–3.52).
Conclusion
Our study showed that the Na-to-K ratio might independently predict future risk of CVD events in adults.
Similar content being viewed by others
Introduction
Cardiovascular disease (CVD) is a major cause of death globally [1]. Metabolic risk factors including hypertension (HTN), diabetes mellitus and dyslipidemia play an important role in the etiology of CVD, among which, HTN is considered as the major and preventable risk factor for CVD mortality and disability [1, 2]. The number of diet-attributable CVD deaths and disability-adjusted life years (DALYs) were 6.9 million and 153.2 million, in 2019, marking a 43.8 and 34.3% rise from 1990, respectively [3]. Dietary sodium (Na) and potassium (K) are the most important dietary components that are associated with HTN, CVD and other health outcomes [4]. High consumption of Na along with low dietary K, particularly in Western diets high in refined grain, added sugar, sweetened drinks and processed food, and low in fruit and vegetable, have a synergistic effect on development of HTN [5]. World Health Organization (WHO) recommends that consumption of less than 2000 mg of Na (2-gr Na ~ 5-gr salt) and at least 3510 mg of K per day to reduce HTN, incidence of CVD and coronary heart disease (CHD) [6,7,8]. In addition, the dietary guidelines for Americans (DGA) recommends that Na intake should be limited to less than 2.3 g/d in general population and 1.5 g/d in HTN and diabetes [7]. The global estimation of mean intake of salt is around 2- to 3-fold greater than the recommendations and almost 95% of people consume between 6–12 g/d [9]. Despite available evidence regarding harms of “excessive” sodium (Na) intake, the term is still remained undefined, and new evidence is going to critically change the conventional belief [10,11,12,13,14]. The cause-and-effect relationship between Na intake and risk of CVD still remains inconsistent; strong positive associations [15], U-shaped [16] and null association [10, 11] have been reported among different populations. There is an interaction between Na and K intakes in relation to the risk of CVD [17], and Na-to-K ratio may be a more accurate predictor of CVD risks than dietary Na or K per se [14]. Previous studies showed that dietary Na-to-K ratio that is strongly correlated with urinary Na and K concentrations, predicted the risk of developing CVD, HTN, ischemic strokes, and chronic kidney disease [2, 5, 7, 18, 19].
The present cohort study aimed to investigate the potential cumulative effects of Na, K and their ratio, obtained by repeated dietary assessments using food frequency questionnaires (FFQ), with CVD over 10.6 years follow-up.
Methods
Study design and population
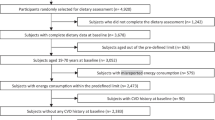
The present cohort study was conducted in the framework of the Tehran Lipid and Glucose Study (TLGS) [20]. In brief, TLGS is an ongoing population-based prospective study started from 1999 to 15,005 individuals aged ≥ 3 years old, resident in the Tehran, capital city of Iran. For the current analysis, CVD-free adult (≥ 30 years old) participants of the third phase of TLGS (2006–2008), with completed data on demographics, clinical, biochemical and dietary assessments were included (n = 2050), and followed-up to March 2018.
This study was done according to the Helsinki declaration of ethics. Informed written consent was obtained from each participant, and the ethical committee of Shahid Beheshti University of Medical Sciences., Tehran, Iran, approved the study protocol.
Assessment of covariates
A pre-defined questionnaire was used to collect the participants’ information including sex, age, smoking habits, medications, and menopause status, personal and family history of diseases. A validated Modifiable Activity Questionnaire (MAQ) for Iranian was used to assess the physical activity status [21]. Weight was measured to the nearest 100 g using digital scales, while the subjects were minimally clothed, without shoes. Height was measured to the nearest 0.5 cm, in a standing position without shoes, using a tape meter. Body mass index (BMI) was calculated as weight (kg) divided by square of the height (m2). Waist circumference was measured to the nearest 0.1 cm, midway between the lower border of the ribs and the iliac crest at the widest portion, over light clothing, using a soft measuring tape, without any pressure to the body. Systolic blood pressure (SBP) and diastolic blood pressure (DBP) were determined after 15 min of rest in a seated position on the right arm using a calibrated mercury sphygmomanometer (Riester, Germany) [22]. Measurement was repeated twice with the interval of 30 s, and mean of them was considered as the participants’ blood pressure.
Details of biochemical measurements in the TLGS samples have been described elsewhere [23]. In brief, biochemical measurements [i.e., fasting serum glucose (FSG), triglyceride (TG), and high-density lipoprotein cholesterol (HDL-C] levels were all done after a 12-to 14-h overnight fast, at baseline and all subsequent examinations (with a 3-y follow-up intervals). The standard oral glucose tolerance test (OGTT) was performed for all adults (age ≥ 21 y) who were not on glucose-lowering medications. Serum glucose concentration was measured with an enzymatic colorimetric method using glucose oxidase (Pars Azmoon, Tehran, Iran). Serum Cr concentrations were determined by the Jaffe kinetic alkaline picrate method. Intra- and inter-assay coefficients of variation (CV) were less than 5.0% for all measurements.
Dietary assessment
Dietary intakes over the last year were collected using a validated 168-items food frequency questionnaire (FFQ) [24]. Participants were asked to report the frequency (e.g. daily, weekly, or monthly); portion size (e.g. cup, spoon, and ounce) of each consumed food were then converted to grams. Mean intake of Na and K during the last year was provided by FFQ. As the Iranian Food Composition Table (FCT) is incomplete and has limited data on the content of raw foods and beverages’ nutrient, the US Department of Agriculture (USDA) FCT was used. For national foods not listed in the USDA FCT, the Iranian FCT was used [25].
Definition of outcomes and terms
The data collection and definition of CVD outcome has been previously described by the TLGS research group [26, 27]. The international statistical classification of diseases and related health problems, 10th Revision CRITERIA, and the American heart association classification for cardiovascular events were used for definition of the outcomes. As described by Hadaegh et al. [26, 27], CVD was defined as any coronary heart disease (CHD)-related event, stroke, or CVD death [definite fatal myocardial infarction (MI), definite fatal CHD, and definite fatal stroke) (26, 28). The CHD-related events were considered as cases of definite MI (according to diagnostic ECG and biomarkers), probable MI (according to positive ECG findings, cardiac symptoms or signs, and missing biomarkers, or positive ECG findings and equivocal biomarkers), unstable angina pectoris (new cardiac symptoms or changing symptom patterns and positive ECG findings with normal biomarkers), angiographic-confirmed CHD, and CHD death. Death from CHD or stroke was confirmed by reviewing the death certificate or medical records. Stroke was considered as a new neurological deficit that lasted at least 24 h [26, 27].
Type 2 diabetes mellitus (T2DM) was defined as fasting serum glucose ≥ 126 mg/dL, 2-h serum glucose ≥ 200 mg/ dL, or using anti-diabetic medications [29]. Elevated systolic blood pressure (SBP) ≥ 140 mmHg, elevated diastolic blood pressure (DBP) ≥ 90 mmHg, or using antihypertensive drugs were considered as HTN [30].
The CKD Epidemiology Collaboration (EPI) equation was used to calculate estimated glomerular filtration rate (eGFR). As a single equation CKD-EPI has been expressed as follows: eGFR = 141×min (Scr/κ, 1)α × max (Scr/κ, 1)−1.209 × 0.993age × 1.018 [if female] × 1.159 [if black]. In this equation, Scr is serum creatinine in mg/dL; κ is 0.7 and 0.9 for men and women, respectively, α is -0.329 and − 0.411 for men and women; min indicates the minimum of Scr/κ or 1, and max indicates a maximum of Scr/κ or 1 [31].
Statistical analysis
Baseline characteristics of the study participants were described by mean ± standard deviation (SD) values, frequency (%), or median (inter-quartile range, IQR) across CVD status. Continuous variables with normal distribution were compared between the groups using independent t-test whereas dichotomous variables were compared using chi-square test. To compare variables with non-normal distributions, the Mann-Whitney U test was used.
The hazard ratio (HR) and 95% CI for the incidence of CVD were reported as continuous (per 1000 mg/d increase in Na and K) and categorized (tertiles). Time to event was defined as time from baseline (2006–2008) to end of follow-up (censored cases) or time to having an event, whichever occurred first. The proportional hazards assumption was tested. We censored participants at the time of death due to non-CVD causes, at the time of leaving the district, or study follow-up end time of March 2018.
Two Cox models were planned; model 1 was controlled for CVD risk score, which was included a sex-specific general CVD algorithms incorporating age, total cholesterol, HDL-C, SBP, treatment for HTN, smoking and T2DM [32]. The accuracy and clinical importance of this score for our population was also reported in detail [33]. Model 2 was additionally adjusted for physical activity, energy intake, dietary fat and fibers. Model 3 was additionally adjusted for eGFR and menopause status (yes/no).
The receiver operator characteristic (ROC) curve analysis was used with an estimation of the variable sensitivity and specificity to determine the cut-off point of dietary Na-to-K ratio for risk of developing CVD. The cut-off point was assessed by pre-defined value of sensitivity (i.e., 0.7). To obtain the optimal cut-off dietary Na-to-K ratio for risk of developing CVD, Youden index (the maximum value of sensitivity + specificity–1) was used, the index is preferable for the finding of the optimal cut-off point because it is clinically translated to maximizing correct classification and minimizing misclassification rates [34].
We used Statistical Package for Social Science (SPSS) (version 22; IBM Corp., Armonk, NY, USA) for all analyses. P values < 0.05 were considered as statistical significance.
Results
A total of 2050 participants (46% male) with the mean (SD) age of 46.28 (11.34) were included to the analysis. Median (interquartile range) of follow-up duration was 10.6 (9.9–11.1) years, the incidence rate of CVD events during that time was 10.14%. In comparison with non-CVDs, participants who developed CVD outcomes were more likely to be older, male and had higher WC, SBP, DBP, TG to HDL-C ratio, serum total cholesterol and fasting serum glucose levels (P for all < 0.001). No significant differences in other sociodemographic characteristics, including BMI, current smoking, physical activity and Na-to-K ratio were observed (Table 1).
The mean dietary Na and K intake were 4200 and 3790 mg/d, respectively. The baseline dietary intakes of participants across tertiles of the Na intakes are presented in Table 2. Dietary intakes of energy, protein, carbohydrates, fats, cholesterols, saturated fats, fibers, fruits and vegetables, potassium, refined grains, whole grains, dairy products, salty snacks, fast foods, red meats and poultry were higher in the third versus first tertiles (P for all < 0.001).
The risk of CVD across tertile categories of Na, K, and Na-to-K ratio are shown in Table 3. Continuous variable analysis showed a 41% increased incidence of CVD associated with each 1000 mg/d increase in dietary Na (HR = 1.41, 95% CI = 1.11–1.79) in the full model. After adjustment of CVD risk score, higher Na intake (> 4143 versus < 3049 mg/d) was significantly related to the increased risk of CVD (HR = 1.41, 05% CI = 1.03–1.94). Further adjustment for physical activity, energy intake, dietary fiber and fats, eGFR and menopause status, the association was potentiated (HR = 1.99, 95% CI = 1.06–3.74). In addition, fully-adjusted model showed a lower risk of CVD for 56% in participants with the highest K intake in compared to lowest tertile (HR = 0.44, 95% CI = 0.20–0.94) (Table 3). Continuous variable analysis showed a 29% decreased incidence of CVD associated with each 1000 mg/d increase in dietary K (HR = 0.71, 95% CI = 0.52–0.97) in the full model.
After adjustment of CVD risk factors, an increased risk of CVD events was observed in the participants with highest compared to lowest dietary Na-to-K ratio (HR = 1.73, 95% CI = 1.24–2.40). Additional adjustment for physical activity, energy intake, dietary fiber and fats, eGFR and menopause status increased the CVD incident risk in relation to higher dietary Na-to-K ratio (HR = 1.99, 95% CI = 1.13–3.52) (Table 3).
The critical cut-off point of Na-to-K ratio for predicting CVD events was 0.80 (at a fixed sensitivity of 70%, AUC = 0.56, 95% CI 0.54–0.58, P value = 0.001) (Supplementary Fig. 1). In the presence of traditional CVD risk factors, dietary Na-to-K ratio higher than the cut-off value (≥ 0.80) was related to increased risk of CVD by % (HR = 1.63, 95% CI = 1.17–2.27). The calculated Youden index addressed a cut-off point of 1.26 for Na-to-K ratio (sensitivity = 29.3 and specificity = 84.3); participants with a ratio higher than the 1.26 were estimated to have an elevated risk of CVD by2.62-fold (95% CI = 1.91–3.59).
Discussion
The present study investigated the prospective association between dietary intakes of Na, K, and Na-to-K ratio with the incident risk of CVD over 10.6 years of follow-up among an Iranian population. Our results showed that an increased risk of CVD events in relation to high intake of Na and Na-to-K ratio, and lower risks in people with higher intake of dietary K. The associations were independent of the well-established risk factors of CVD in our population. Moreover, our results showed that a 41% increased risk of CVD in relation with each increase in 1000 mg/d of Na intake. Using a ROC curve analysis with a fixed sensitivity of 70% and calculating Youden index, a cut-off value of 0.80 and 1.26 was determined as a critical and optimal cut-off points of dietary Na-to-K ratio, respectively; higher dietary Na-to-K ratio higher than the cut-off values was associated with an elevated risk of CVD by 63 and 162%, respectively.
Our findings are in agreement with previous reports of some nation-wide cohorts. The Prospective Urban Rural Epidemiology study reported an increased risk of CVD and stroke only in communities whose daily Na intake was greater than five g/d [35]. A high-Na diet (> 6 g/d) in diabetics with poorly controlled blood glucose in Japan Diabetes Complications Study (JDCS) cohort was associated with an elevated risk of CVD by ~ 10-fold, while in good-controlled patients Na intake was not related with developing CVD [15]. Recent studies suggested that direct relation between Na intake and CVD risk independent of HTN-related mechanism [36,37,38]. A high-Na diet causes endothelial dysfunction by reducing nitric oxide (NO) bioavailability, leading to development of atherosclerosis and cardiovascular events [37, 39]. Excessive Na intakes decreases arterial compliance and induces arterial fibrosis by increasing transforming of growth factor (TGF)-β and overproduction of reactive oxygen species [40, 41].
Mean intake of K in our population (i.e., 3790 mg/d) meets the WHO recommendation [8]. The protective effect of dietary K against development of CVD in our population was similar to previous reports linking higher dietary and urinary K with a reduced risk of CVD [42] [43], especially in those who had a high-Na intake [44, 45]. Interestingly, the Northern Manhattan study found a positive association between potassium intake and stroke among those with < 2300 mg/day sodium intake; and an inverse association among those with ≥ 2300 mg/day sodium intake [17].
In agreement with our result, NOMAS cohort reported that higher Na-to-K ratio increased risk of developing ischemic stroke by 60% [17]. A recent meta-analysis of population-based studies reported a pooled estimated relative risk of stroke by 1.22 (95% CI = 1.04, 1.41) for a one-unit increased in dietary Na-to-K ratio (mmol/mmol) [46]. The authors also reported that a urinary Na-to-K ratio of ≤ 1 may be associated with a clinically relevant reduction in stroke risk and represents a potential target for health interventions [47]. It is highly recommended to lower Na-to-K ratio and improve cardiometabolic health following greater consumption of dietary K sources such as whole grains, low-fat dairy products, fruits and vegetables [48, 49]. K intakes also reduce blood pressure by relaxing blood vessels and excreting Na through urine [38, 50].
Mean intake of salt among different populations was reported between 0.5 and 55 g/d [51, 52]. A recent national report of estimated Iranian salt intake indicated a mean population of 9.52 g/d (95% CI = 9.48–9.56) and ~ 97.7% overconsumption of salt among the population [53]. Although it can be different among populations, about 75% of dietary Na is attributed to processed foods, 10–12% is naturally occurring in foods, and the rest of 10–15% is discretionary salt intake (salt used in home-cooking or at the table) [54, 55]. In our population, Na intakes has been estimated to be originated mainly from salty-snacks and processed foods, and urinary Na was strongly correlated with the Wester dietary pattern [18].
The prospective design of our study, relatively large sample size and long-term follow-up are considered as the strength of current study. Using CVD risk score allowed us to account for major traditional CVD confounders without adding many variables and improving our models’ instability [33]. In addition, we used a validated FFQ to estimate usual dietary intakes of the Iranian population. The present study has potential limitations. We used dietary intakes for variables of interest rather than urine collections, which more accurately reflect the status of Na-to-K ratio. Additionally, we were unable to capture table salt intake, as the important source of dietary Na. As inherent in any prospective study, some degree of misclassification might have occurred during study follow-up due to possible changes in an individual’s diet as well as changes in confounders. Finally, as the questionnaires we used were self-repots, potential measurement errors in estimated of food and nutrient intakes might accrues.
To sum up, our results provided further support for previous studies reported adverse effect of high-Na intake and favorable effect of dietary K on the cardiovascular function. We also showed that Na-to-K ratio may independently predict future risk of CVD events in adult population. A balanced Na-to-K ratio needs to be considered to prevent diet-induced secondary hypertension as a risk factor of cardiovascular disease.
Data Availability
Data will be presented upon forwarding the request to the corresponding author (z.bahadoran@endocrine.ac.ir) and confirmation of the director of RIES (azizi@endocrine.ac.ir).
References
Roth GA, Mensah GA, Johnson CO, Addolorato G, Ammirati E, Baddour LM et al. Global burden of cardiovascular diseases and risk factors, 1990–2019: update from the GBD 2019 study. 2020;76(25):2982–3021.
Gonçalves C, Abreu S. Sodium and potassium intake and cardiovascular disease in older people: a systematic review. Nutrients. 2020;12(11):3447.
Dong C, Bu X, Liu J, Wei L, Ma A, Wang T. Cardiovascular disease burden attributable to dietary risk factors from 1990 to 2019: A systematic analysis of the Global Burden of Disease study. Nutrition, metabolism, and cardiovascular diseases : NMCD. 2022;32(4):897–907. Epub 2022/01/25. 10.1016/j.numecd.2021.11.012. PubMed PMID: 35067445.
Whelton PK. Sodium, potassium, blood pressure, and cardiovascular disease in humans. Curr Hypertens Rep. 2014;16(8):1–8.
Okayama A, Okuda N, Miura K, Okamura T, Hayakawa T, Akasaka H, et al. Dietary sodium-to-potassium ratio as a risk factor for stroke, cardiovascular disease and all-cause mortality in Japan: the NIPPON DATA80 cohort study. BMJ open. 2016;6(7):e011632.
Organization WH. Guideline: sodium intake for adults and children. World Health Organization; 2012.
Bahadoran Z, Mirmiran P, Norouzirad R, Ghasemi A, Azizi FJN. Metabolism. Monitoring population salt intake using casual urinary sodium: Tehran Lipid and Glucose Study. 2022;19(1):1–9.
Organization WH. Guideline: potassium intake for adults and children. World Health Organization; 2012.
McCarron DA, Kazaks AG, Geerling JC, Stern JS, Graudal NAJAJoH. Normal range of human dietary sodium intake: a perspective based on 24-hour urinary sodium excretion worldwide. 2013;26(10):1218–23.
Kalogeropoulos AP, Georgiopoulou VV, Murphy RA, Newman AB, Bauer DC, Harris TB et al. Dietary sodium content, mortality, and risk for cardiovascular events in older adults: the Health, Aging, and Body Composition (Health ABC) Study. 2015;175(3):410–9.
Singer P, Cohen H, Alderman MJAjoh. Assessing the associations of sodium intake with long-term all-cause and cardiovascular mortality in a hypertensive cohort. 2015;28(3):335–42.
Alderman MH. Dietary sodium: where Science and Policy Diverge. Am J Hypertens. 2016;29(4):424–7. https://doi.org/10.1093/ajh/hpu256.
McGuire S, Institute of Medicine. 2013. Sodium Intake in Populations: Assessment of Evidence. Washington, DC: The National Academies Press, 2013. Advances in Nutrition. 2014;5(1):19–20. https://doi.org/10.3945/an.113.005033.
Mirmiran P, Bahadoran Z, Nazeri P, Azizi F. Dietary sodium to potassium ratio and the incidence of hypertension and cardiovascular disease: a population-based longitudinal study. Clin Exp Hypertens. 2018;40(8):772–9. https://doi.org/10.1080/10641963.2018.1431261.
Horikawa C, Yoshimura Y, Kamada C, Tanaka S, Tanaka S, Hanyu O et al. Dietary sodium intake and incidence of diabetes complications in Japanese patients with type 2 diabetes: analysis of the Japan Diabetes Complications Study (JDCS). 2014;99(10):3635–43.
van Dieren S, van der Uiterwaal CSPM, Spijkerman JMA. Coffee and tea consumption and risk of type 2 diabetes. Diabetologia. 2009;52(12):2561–9. https://doi.org/10.1007/s00125-009-1516-3.
Willey J, Gardener H, Cespedes S, Cheung YK, Sacco RL, Elkind MSV. Dietary Sodium to Potassium Ratio and Risk of Stroke in a Multiethnic Urban Population. 2017;48(11):2979–83. https://doi.org/10.1161/STROKEAHA.117.017963.
Mirmiran P, Gaeini Z, Bahadoran Z, Ghasemi A, Norouzirad R, Tohidi M et al. Urinary sodium-to-potassium ratio: a simple and useful indicator of diet quality in population-based studies. 2021;26(1):1–8.
Mirmiran P, Nazeri P, Bahadoran Z, Khalili-Moghadam S, Azizi F. Dietary sodium to potassium ratio and the incidence of chronic kidney disease in adults: a Longitudinal Follow-Up study. Prev Nutr Food Sci. 2018;23(2):87–93. PubMed PMID: 30018885; PubMed Central PMCID: PMCPMC6047877.
Azizi F, Zadeh-Vakili A. Takyar MJIjoe, metabolism. Review of rationale, design, and initial findings: Tehran Lipid and Glucose Study. 2018;16(4 Suppl).
Delshad M, Ghanbarian A, Ghaleh NR, Amirshekari G, Askari S. Azizi FJIjopm. Reliability and validity of the modifiable activity questionnaire for an Iranian urban adolescent population. 2015;6.
Askari S, Asghari G, Ghanbarian A, Khazan M, Alamdari S, Azizi FJAoIm. Seasonal variations of blood pressure in adults: Tehran lipid and glucose study. 2014;17(6):0-.
Tohidi M, Ghasemi A, Hadaegh F, Derakhshan A, Chary A, Azizi F. Age- and sex-specific reference values for fasting serum insulin levels and insulin resistance/sensitivity indices in healthy iranian adults: Tehran lipid and glucose study. Clin Biochem. 2014;47(6):432–8. https://doi.org/10.1016/j.clinbiochem.2014.02.007. Epub 2014/02/18.
Mirmiran P, Hosseini Esfahani F, Mehrabi Y, Hedayati M, Azizi F. Reliability and relative validity of an FFQ for nutrients in the Tehran lipid and glucose study. Public Health Nutr. 2010;13(5):654–62. https://doi.org/10.1017/S1368980009991698. Epub 2009/10/07.
Rad AH, Esmaeili M, Abdollahi M, Azar M, editors. Compiling and validation of iranian food composition tables. ANNALS OF NUTRITION AND METABOLISM; 2007. KARGER ALLSCHWILERSTRASSE 10, CH-4009 BASEL, SWITZERLAND.
Hadaegh F, Harati H, Ghanbarian A, Azizi F. Association of total cholesterol versus other serum lipid parameters with the short-term prediction of cardiovascular outcomes: Tehran lipid and glucose study. Eur J Cardiovasc Prev Rehabil. 2006;13(4):571–7. https://doi.org/10.1097/01.hjr.0000216552.81882.ca. Epub 2006/07/29. 00149831-200608000-00014 [pii]. PubMed PMID: 16874147.
Barkhordari M, Padyab M, Sardarinia M, Hadaegh F, Azizi F, Bozorgmanesh M. Survival regression modeling strategies in CVD prediction. Int J Endocrinol Metab. 2016;14(2):e32156. https://doi.org/10.5812/ijem.32156. Epub 2016-03-23.
Nejat A, Mirbolouk M, Mohebi R, Hasheminia M, Tohidi M, Saadat N, et al. Changes in lipid measures and incident coronary heart disease: Tehran lipid & glucose study. Clin Biochem. 2014;47(13–14):1239–44. Epub 2014/03/25. S0009-9120(14)00117-9 [pii]. https://doi.org/10.1016/j.clinbiochem.2014.03.004. PubMed PMID: 24657509.
American Diabetes A. Standards of medical care in diabetes–2014. 2014;37:S14-S80.
Mancia G, De Backer G, Dominiczak A, Cifkova R, Fagard R, Germano G et al. Guidelines for the management of arterial hypertension: The Task Force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). 2007;28(12):1462–536.
Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF 3rd, Feldman HI, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150(9):604–12. Epub 2009/05/06. 150/9/604 [pii]. PubMed PMID: 19414839; PubMed Central PMCID: PMC2763564.
D’Agostino Sr RB, Vasan RS, Pencina MJ, Wolf PA, Cobain M, Massaro JM et al. General cardiovascular risk profile for use in primary care: the Framingham Heart Study. 2008;117(6):743–53.
Khalili D, Hadaegh F, Soori H, Steyerberg EW, Bozorgmanesh M, Azizi FJAjoe. Clinical usefulness of the Framingham cardiovascular risk profile beyond its statistical performance: the Tehran Lipid and Glucose Study. 2012;176(3):177–86.
Perkins NJ, Schisterman EF. The inconsistency of “optimal” cutpoints obtained using two criteria based on the receiver operating characteristic curve. Am J Epidemiol. 2006;163(7):670–5. https://doi.org/10.1093/aje/kwj063. Epub 2006/01/18.
Mente A, O’Donnell M, Rangarajan S, McQueen M, Dagenais G, Wielgosz A, et al. Urinary sodium excretion, blood pressure, cardiovascular disease, and mortality: a community-level prospective epidemiological cohort study. The Lancet. 2018;392(10146):496–506. https://doi.org/10.1016/S0140-6736(18)31376-X.
Appel LJ, Frohlich ED, Hall JE, Pearson TA, Sacco RL, Seals DR, et al. The importance of population-wide sodium reduction as a means to prevent cardiovascular disease and stroke: a call to action from the. Am Heart Association. 2011;123(10):1138–43.
Edwards DG. Farquhar WBJCoin, hypertension. Vascular Eff Diet salt. 2015;24(1):8.
Aburto NJ, Hanson S, Gutierrez H, Hooper L, Elliott P, Cappuccio FP. Effect of increased potassium intake on cardiovascular risk factors and disease: systematic review and meta-analyses.Bmj. 2013;346.
Lukaszewicz KM, Falck JR, Manthati VL, Lombard JHJCS. Introgression of Brown Norway CYP4A genes on to the Dahl salt-sensitive background restores vascular function in SS-5BN consomic rats. 2013;124(5):333–42.
Zimmerman MC, Lazartigues E, Sharma RV, Davisson RLJCr. Hypertension caused by angiotensin II infusion involves increased superoxide production in the central nervous system. 2004;95(2):210–6.
Jung S, Kim MK, Shin J, Choi BY, Lee Y-H, Shin DH, et al. High sodium intake and sodium to potassium ratio may be linked to subsequent increase in vascular damage in adults aged 40 years and older: the korean multi-rural communities cohort (MRCohort). Eur J Nutr. 2019;58(4):1659–71. https://doi.org/10.1007/s00394-018-1712-3.
Ma Y, He FJ, Sun Q, Yuan C, Kieneker LM, Curhan GC et al. 24-hour urinary sodium and potassium excretion and cardiovascular risk. 2022;386(3):252–63.
Umesawa M, Iso H, Date C, Yamamoto A, Toyoshima H, Watanabe Y, et al. Relations between dietary sodium and potassium intakes and mortality from cardiovascular disease: the Japan Collaborative Cohort Study for evaluation of. Cancer Risks. 2008;88(1):195–202.
Morris RC Jr, Sebastian A, Forman A, Tanaka M, Schmidlin OJH. Normotensive salt sensitivity: effects of race and dietary potassium. 1999;33(1):18–23.
Krishna GG, Miller E, Kapoor SJNEJoM. Increased blood pressure during potassium depletion in normotensive men. 1989;320(18):1177–82.
Jayedi A, Ghomashi F, Zargar MS, Shab-Bidar S. Dietary sodium, sodium-to-potassium ratio, and risk of stroke: a systematic review and nonlinear dose-response meta-analysis. Clin Nutr. 2019;38(3):1092–100. https://doi.org/10.1016/j.clnu.2018.05.017.
Averill MM, Young RL, Wood AC, Kurlak EO, Kramer H, Steffen L, et al. Spot urine sodium-to-potassium ratio is a predictor of stroke. Stroke. 2019;50(2):321–7. https://doi.org/10.1161/strokeaha.118.023099. Epub 2019/01/22.
Mozaffarian D, Appel LJ, Van Horn LJC. Components of a cardioprotective diet: new insights. 2011;123(24):2870–91.
Folsom AR, Parker ED, Harnack LJJAjoh. Degree of concordance with DASH diet guidelines and incidence of hypertension and fatal cardiovascular disease. 2007;20(3):225–32.
Reducing sodium and increasing potassium may lower risk of cardiovascular disease 13 novamber 2021. Available from: https://www.hsph.harvard.edu/news/press-releases/reducing-sodium-and-increasing-potassium-may-lower-risk-of-cardiovascular-disease/.
Oliver WJ, Cohen EL, Neel JVJC. Blood pressure, sodium intake, and sodium related hormones in the Yanomamo Indians, a” no-salt” culture. 1975;52(1):146–51.
Dahl LKJIjoe. Possible role of salt intake in the development of essential hypertension. 2005;34(5):967–72.
Rezaei S, Mahmoudi Z, Sheidaei A, Aryan Z, Mahmoudi N, Gohari K, et al. Salt intake among iranian population: the first national report on salt intake in Iran. J Hypertens. 2018;36(12):2380–9. https://doi.org/10.1097/hjh.0000000000001836. Epub 2018/07/14.
Elliott P, Brown I. Sodium intakes around the world. 2007.
Brown IJ, Tzoulaki I, Candeias V, Elliott P. Salt intakes around the world: implications for public health. Int J Epidemiol. 2009;38(3):791–813. https://doi.org/10.1093/ije/dyp139. Epub 2009/04/09.
Acknowledgements
We thank the Tehran Lipid and Glucose Study participants and the field investigators of the Tehran Lipid and Glucose Study for their cooperation and assistance in physical examinations, biochemical evaluation and database management. This study was supported by Shahid Beheshti University of Medical Sciences.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
Z.B, P.M, and F.A designed and conducted research; Z.B, Z.M, and M.J analyzed data and wrote the paper. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
We obtained written informed consent from all participants. Based on the ethical guidelines of the 1975 Declaration of Helsinki, the study protocol was approved by the Ethics Research Council of the Research Institute for Endocrine Sciences, Shahid Beheshti University of Medical Sciences.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Mosallanezhad, Z., Jalali, M., Bahadoran, Z. et al. Dietary sodium to potassium ratio is an independent predictor of cardiovascular events: a longitudinal follow-up study. BMC Public Health 23, 705 (2023). https://doi.org/10.1186/s12889-023-15618-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12889-023-15618-7