Abstract
Background and aims
Developing accessible, affordable, and effective approaches to smoking cessation is crucial for tobacco control. Mobile health (mHealth) based interventions have the potential to aid smokers in quitting, and integrating treatments from multiple sources may further enhance their accessibility and effectiveness. As part of our efforts in smoking cessation, we developed a novel behavioral intervention delivery modality for smoking cessation that integrated three interventions using the WeChat app, called the “Way to Quit” modality (WQ modality). It is presented here the protocol for a randomized controlled trial evaluating the effectiveness, feasibility, and cost-effectiveness of the WQ modality in Chinese smokers.
Methods
Eligible participants (n = 460) will be recruited via online advertisement in Beijing, China. They will be randomly assigned to receive either quitline-based treatment (QT, n = 230) or WQ modality-based treatment (WQ, n = 230) using a block randomization method. Participants in the QT group will receive telephone-assisted treatment over a four-week period (multi-call quitline protocol), while those in the WQ group will receive integrated interventions based on the WQ modality for four weeks. A four-week supply of nicotine replacement therapy (gums) will be provided to all participants. Participants will be asked to complete phone or online follow-up at 1, 3, 6, and 12-months. At 1-month follow-up, individuals with self-reported smoking abstinence for more than 7 days will be invited to receive an exhaled carbon monoxide (CO) test for biochemical validation. The primary aim is to determine whether the WQ modality is effective in assisting smokers in quitting smoking. The secondary aims are to evaluate the acceptability, satisfaction, and cost-effectiveness of the WQ modality.
Discussion
If the WQ modality is determined to be effective, acceptable, and affordable, it will be relatively easy to reach and provide professional cessation treatments to the communities, thus helping to reduce the disparities in smoking cessation services between different regions and socioeconomic groups.
Trial registration
Chinese Clinical Trial Registry: ChiCTR2200066427, Registered December 5, 2022.
Similar content being viewed by others
Background
Tobacco use is the leading cause of preventable morbidity and mortality in the world, and quitting smoking is known as the best approach to reduce these hazards [1]. Due to nicotine dependence, it is necessary to offer help to quit tobacco use [1]. Although many evidence-based treatment approaches to smoking cessation have been developed, the financing of smoking cessation services, such as tobacco quitlines, is a significant challenge for many governments, particularly those in low- and middle-income countries [2]. Consequently, many smokers do not have access to cessation services, and their quit rates are low [2].
This situation also exists in China, where more than 300 million Chinese people continue to use tobacco [3]. Among these smokers, 36.4% had tried to quit in the past 12 months, whereas more than 90% of them had never received any professional help [3]. A nationwide survey showed that there are only 366 smoking cessation clinics in mainland China [4], which leaded to smokers in most parts of China did not access to face-to-face treatment from smoking cessation specialists. Evidence has shown that the tobacco quitline is effective and could remove barriers that may hinder face-to-face service delivery [5]. However, there are only three quitlines in mainland China, all of which are facing the challenges of low awareness and utilization due to a lack of financial and policy support [6,7,8]. In addition, more and more people prefer to communicate via chat applications on their smartphones rather than by telephone [9]. Therefore, it needs to develop novel accessible, affordable, and effective approaches to assist smokers in quitting.
The rapid development of mobile health (mHealth) technology makes it possible to address the above concerns [10]. Moreover, during the COVID-19 pandemic, people preferred to obtain medical and health services through mHealth [11, 12], and this trend may continue in the post-epidemic era [13]. A variety of mHealth approaches, such as short message service (SMS) texting, web, social media, and mobile applications (apps), have existed to deliver smoking cessation behavioral interventions [10] and demonstrated the potential to assist smokers in quitting smoking [14, 15]. But at the same time, some limitations were also found that may reduce their availability and effectiveness, such as non-tailored contents, lack of interactivity, etc. Given the dynamic, quickly evolving nature of the technology, a possible strategy to overcome these limitations has been proposed that is to integrate interventions from multiple sources [10], but it still needs high-quality evidence to support it.
China is regarded as the fastest growing smartphone market, and has the largest smartphone user group (n > 850 million people) in the world [16]. WeChat is the most popular app in China, which has become a major tool for communication, entertainment, and payment for Chinese smartphone users [17]. In 2021, an innovative mHealth-based integrated modality for smoking cessation including three interventions using the WeChat app, called the “Way to Quit” modality (WQ modality), was developed in a large urban general hospital in Beijing, China. This hospital is the first hospital to set up a smoking cessation clinic and a free national quitline in mainland China, which makes it has outstanding clinical and research capability in smoking cessation services [7, 8]. A preliminary evaluation of the WQ modality conducted in 12 provinces and cities in western China indicated that it has been effective in assisting Chinese smokers in quitting [18].
This paper describes the protocol for a randomized controlled trial (RCT) that will evaluate the effectiveness, feasibility, and cost-effectiveness of the WQ modality in Chinese smokers. The primary aim of this study is to evaluate the effectiveness of the WQ modality in assisting smoking cessation. Secondary aims are: 1) to evaluate the acceptability and satisfaction of the WQ modality, and 2) to evaluate its cost-effectiveness. We hypothesize that the WQ modality would increase the smoking abstinence rate compared to quitline.
Methods
Study design
This study will be a two-group, open label, randomized, parallel controlled trial among Chinese smokers in Beijing, China. Eligible participants will be randomized in a 1:1 ratio into the control group (quitline-based treatment, QT) or the intervention group (WQ modality-based treatment, WQ). All participants will complete baseline, and 1-, 3-, 6-, and 12-month follow-up assessments. The flow chart is shown in Fig. 1. This protocol has been designed in accordance with the Standardized Protocol Items: Recommendations for Interventional Trials (SPIRIT) guidelines and checklist (Additional file 1).
Participants and recruitment
Participants will be Chinese smokers. The inclusion criteria are: 1) 18–65 years of age; 2) smoked at least 100 cigarettes in lifetime; 3) currently smoke at least 5 cigarettes per day; 4) willing to make a quit attempt within 1 month; 5) possess a smartphone and a WeChat account, and could operate WeChat app skillfully; 6) promise to complete follow-up on time. Exclusion criteria include: 1) difficulty in understanding the questionnaire; 2) acute exacerbation of chronic cardiopulmonary disease; 3) having received coronary surgical interventions (such as coronary angiography, percutaneous coronary interventions, etc.) in the last month or will receive these interventions in the next month; 4) discharge within 1 month; 5) medical conditions that preclude the use of nicotine replacement therapy (NRT); 6) currently using smoking cessation medications or other aids, including NRT, bupropion, varenicline, electronic cigarettes and other mHealth-based tools, such as web, SMS texting, apps, etc.; 7) being enrolled in another smoking cessation study.
Eligible participants will be recruited through online advertisement. Individuals who are interested in participating will screen a Quick Response (QR) cord to complete a screening questionnaire to ascertain their eligibility for the study. Eligible individuals will be provided an electronic consent (e-consent) form online. After reading the e-consent, they should click the agree button at the end of the form to indicate their consent to participate in the study. The participant recruitment has been started since December 15, 2022, and is ongoing.
Randomization and allocation
Participants will be randomized in a 1:1 ratio to the QT group or the WQ group using a block randomization method with randomly selected block sizes (4 or 6). This study is an open-label trial. A non-participating staff member will generate a randomization sequence via SAS 9.4 software which will be concealed using sealed opaque envelopes.
Interventions
Quitline-based treatment (QT group)
Participants in the QT group will receive standard proactive quitline services from China Professional Quitline (400–888-5531) for 4 weeks using a multi-call quitline protocol [19]. The first session will focus on quitting history, motivation, self-efficacy, social support, and planning in advance of the quitting date. Smokers will be reminded in the second session to quit smoking completely from the quitting date, be provided with the practical information on how to quit. The sessions followed the quitting date (up to three sessions) will focus on building cessation skills and relapse prevention. Three Chinese-speaking counselors will provide the quitline services. All of them possess at least a bachelor’s degree in medicine or nursing, and have received more than 60 h of training in smoking cessation counseling.
To improve the participants’ adherence of the quitline intervention protocol, the counselors will call them at three time points for three consecutive days during each session, arrange the next session time for the participants, and contact the participants’ relatives and friends whenever necessary.
WQ modality-based treatment (WQ group)
Conceptual framework of the WQ modality
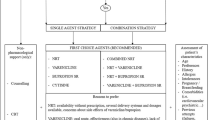
By integrating three behavior change theories, including the COM-B model (C-capability, O-opportunity, M-motivation, and B-behavior) [20], Ecological Systems Theory (EST) [21] and Transtheoretical Model (TTM) [22], we constructed the conceptual framework of the WQ modality, named Integrated COM-B/ Ecological systems/ Transtheoretical model (I-CET model), as shown in Fig. 2. In the I-CET model, three potential factors (Capability, Motivation, and Environment) could influence individual behaviors. Motivation is all those brain processes that energize and direct behavior, including habitual processes, emotional response, and analytical decision-making [23]. Capability is the individual’s psychological and physical capacity to engage in the activity concerned, such as having the necessary knowledge and skills [20]. Environment is defined as all the factors or resources that lie outside the individual that make behavior possible or prompt it (including microsystem, mesosystem, exosystem, macrosystem, and chronosystem) [21]. To promote the occurrence of behavioral change, nine intervention functions are determined based on the behavior change wheel (BCW) [20], as shown in Fig. 2. This is not a linear model in that components within the behavior system interact with each other as do the functions within the intervention layer. The definitions of these intervention functions have been described by Michie et al. [20]. In addition, the interventions need to match the stages of behavior change (including precontemplation, contemplation, preparation, action, maintenance, and relapse [22]), in order to provide tailored interventions.
WQ modality-based treatment
The WQ modality integrated three WeChat app-based cessation services, including the QUIT WeChat mini program (WMP), the QUIT WeChat group (WG), and the QUIT WeChat official account (WOA). Screenshots of these services have been shown in our previous study [18]. They are developed based on the I-CET model and connected with each other [18]. Since the target population is smokers who plan to quit in the next month, the WQ modality is mainly aimed at the stages of preparation, action, and maintenance, which are focus on motivating to quit, building skills for coping with smoking cravings, and preventing relapse, respectively.
The QUIT WMP is an application function module embedded in the WeChat app [24] (similar to cessation apps [25,26,27]), which could assist smokers in developing the capacity to quitting smoking and motivating them to do so. It was developed based on the behavior change techniques (BCTs) [28], and smoking cessation treatment guidelines [10, 29]. The detailed description of its functions is shown in Table 1. In addition, the QUIT WMP will automatically send stage-matched short messages, articles or videos via the WeChat app to the users once a day for 4 weeks from the quit date. A list of weekly topics and examples of messages is shown in Table 2. It can be accessed by scanning a QR code (Additional file 2) or searching Chinese ‘Jie Yan You Dao’ in the WeChat app without downloading or installing [24]. The QR code and introduction video of the QUIT WMP will be sent to the participants via the WeChat app after allocation. To increase the participants’ adherence of using the QUIT WMP, daily usage reminders messages will be sent in the WeChat group. Moreover, we will download the participants’ usage data of the QUIT WMP every week, and remind those who use the program less than 5 days per week by telephone.
The QUIT WG is an online group chat function embedded in the WeChat app (up to 500 persons), which could offer real-time online cessation counseling, group interventions, and facilitate the formation of mutually reciprocated, strong, and long-lasting social bounds that support smoking cessation in a similar manner to that of Twitter and Facebook [30,31,32]. Three counselors are arranged to provide online counseling, and a group administrator is arranged to manage the group and encourage participant to send message. Stage-matched online group interventions will be provided via two manners, including video courses on smoking cessation by clinical expert, and Q&A session after the courses and at 12:00 a.m.-13:00 p.m. from Monday to Friday. We also conducted interactive activities, including topic discussion and quit tasks. Table 2 shows a list of weekly topics and examples of group interventions and interactive activities. Moreover, we introduce the "herd effect" model (opinion leader model) into the WQ modality. Herding is a phenomenon by which individuals follow the behavior of others [33]. It has been reported that people tend to emulate opinion leaders (leading sheep) who could influence others’ opinions, attitudes, beliefs, motivations, and behaviors of others [34], and thus can effectively change many behaviors [35,36,37].Ten self-selected opinion leaders from the participants will be enrolled as the “Quitting Pioneer” to set an example of successful quitter, share experience of smoking cessation, and promote other smokers to participate in interaction activities.
The QUIT WOA could provide electronic self-help materials for smoking cessation, which could be the material library of the WQ modality. The materials are classified according to the stage of smokers and updated weekly. In addition, the information of online group interventions and interactivities will be also uploaded in the QUIT WOA (named Smoking Cessation College) for smokers to download at any time.
Discontinuing or modifying allocated interventions
Participants who experience severe withdrawal symptoms (determined by smoking cessation specialist who does not participate in the study) during the intervention period will stop receiving allocated interventions and be referred to a smoking cessation clinic in a large general hospital in Beijing for intensive treatment.
Other interventions
All participants will receive a 4-week supply of NRT gum by mail (300 pieces). The gum is FDA-approved Nicorette brand 2 mg nicotine mint gum (McNeil Sweden AB, Inc., Helsingborg, Sweden). Participants will be directed to use the gum as needed when they encounter situations or cues that tempt them to smoke, or in anticipation of such situations [38]. A smoking cessation material with a video link to the instruction of medication use will be mailed with the gum.
After receiving the allocation interventions for 4 weeks, participants in the QT group will receive reactive smoking cessation counseling via telephone for one year, and participants in the WQ group will also receive the reactive counseling via the WeChat group. Smoking cessation interventions other than the allocated intervention are prohibited during the study, such as bupropion, varenicline, electronic cigarettes and other mHealth-based tools, such as web, SMS texting, apps, etc.
Measures
Baseline measures
Baseline data will be collected by an online questionnaire using the Tencent Questionnaire platform, including demographic information, smoking history, previous quit attempts, and comorbidities, etc. Nicotine dependence is measured by the Fagerstrӧm test for nicotine dependence (FTND) [39].
Follow-up measures
All participants will complete 1-, 3-, 6-, and 12-month follow-up assessments over the phone or online using the Tencent Questionnaire platform at participants’ convenience. Three non-participating staff will conduct the telephone follow-ups. The follow-up assessment information includes smoking status, quitting attempts, acceptability and satisfaction of the cessation services, and the usage of smoking cessation services, etc. A scale of 0 to 10 (0: not at all; 10: very high) for acceptability and satisfaction with each service will be evaluated. Participants who report quitting smoking or reducing their consumption of cigarettes will fill out the Minnesota Nicotine Withdrawal Scale (MNWS) [40] and the Brief- Questionnaire of Smoking Urges (QSU Brief) [40]. Adverse events and other unintended effects of interventions during the intervention period will be spontaneously reported. At 1-month follow-up, individuals with self-reported smoking abstinence for more than 7 days will be invited to participate in an exhaled carbon monoxide (CO) test for biochemical validation. To increase follow-up compliance, participants will receive 100 RMB (approximately US $ 14.95) in the form of the WeChat red packet (Hongbao, which is similar to an electronic cash reward [41]). The full list of measurements is presented in Table 3.
Service usage data
Service usage data will also be downloaded from the WeChat app and quitline platforms on a weekly basis during the intervention period. The QUIT WMP utilization metrics (registration, logins) will be extracted from host servers; participation in the QUIT WG (e.g., retention time and the number of messages sent) will be extracted. Telephone counsellors used a web-based tool to record the number, duration and content of calls.
Cost data
The cost of each service will be recorded through questionnaire, staff recording, and interview, including direct operating expenses (e.g., salary of staff members and materials used for the interactive activities and training of counselors), costs for hosting the quitline and WeChat-based services, technical support and recruitment costs (which consist of both advertising costs in web-based and offline media, as well as the costs of printing promotional material), and costs for promoting intervention delivery (e.g., rewards for participating in the interactive activities in the QUIT WG).
Outcomes
The primary outcome is biochemically validated 7-day point prevalence of abstinence (PPA) at the 1-month follow-up [42], defined as the proportion of smokers who self-report not even a puff of smoke in the past 7 days at the 1-month follow-up and are confirmed by a carbon monoxide level in expired air of less than 3 parts per million (ppm) [43].
Secondary outcomes include self-reported 7-day PPA [42] at the 1-, 3-, 6-, 12-month follow-up, self-reported continuous smoking abstinence rate (defined as the proportion of participants who are smoking less than 5 cigarettes [42]) from the 1-month to 12-month follow-up, self-reported quit attempts rate (defined as the proportion of participants who are continuously smoking but have tried to quit smoking for at least 24 h [44]) at the 1-, 3-, 6-, 12-month follow-up, acceptability and satisfaction scores of the services, incremental cost-effectiveness ratio (ICER) of the services.
Sample size
The sample size calculation is based on the primary aim of comparing biologically verified 7-day PPA between the QT group and the WQ group at the 1-month follow-up. We used the following assumptions: (1) significance level of 0.05 for a two-sided test; (2) statistical power of 90%; (3) biologically verified 7-day PPA at the 1-month follow-up in the QT group is conservatively estimated as 20% based on the data from California Smokers’ Helpline [19]; (4) an increase in abstinence rate of 15% in the WQ group compared with the QT group; (5) a dropout rate of 20%. Considering the random block size (4 or 6), 460 eligible patients (230 for each group) will be required in this study.
Data management
Data from all questionnaires and assessment scales will be collected electronically via the Tencent questionnaire platform. These data will be integrated with data on the use of smoking cessation services downloaded from the Quitline and WeChat platforms. Participants' cell phone numbers were used as unique identifiers.
All study files will be stored securely in password-protected computer files. Only the principal investigators of the study (SLC and LRL) will be given access to the cleaned full data sets. A data monitoring committee (DMC) has been established and composed of clinicians and statisticians independent of clinical trials, which will be responsible for monitoring study conduct, safety, effectiveness and adaptive design. It is independent from the sponsor and has no role in the design of this study and will not have any role during its execution, analyses, interpretation of the data, or decision to submit results.
Statistical analysis plan
All statistical analyses be analyzed and blinded to the intervention assignment by a statistician using SPSS version 22.0 software (SPSS, Inc., Chicago, IL). Data will be presented as mean (standard deviations) for continuous variables with normal distribution; median (inter-quartile range) for continuous variables without normal distribution; and proportions for categorical variables. Baseline characteristics of participants in the intervention and control groups will be compared using Student’s t-test or non-parametric test for continuous variables and Pearson's chi-square or Fisher's exact test for categorical variables.
Multiple logistic regression will be used for between-group comparisons of the primary outcome variable-biochemically validated 7-day PPA at the 1-month follow-up and the secondary outcomes- self-reported 7-day PPA at the 1-, 3-, 6-, and 12-month follow-up and self-reported continuous smoking abstinence from 1-month to 12-month, with odds ratio (OR) and 95% confidence intervals (CI) to assess between-group effects. By intent-to-treat analysis (ITT), participants who are lost to follow-up or refuse to participate in the validation tests will be considered as continuing smokers with no reduction in cigarette consumption compared with baseline. Participants who do not use smoking cessation services after randomization will be excluded from the ITT analysis data set. In addition, we will conduct a per-protocol (PP) analysis of the primary outcome among the participants in the QT group who receive at least one telephone session and those in the WQ group who use the QUIT WMP for at least 20 days and send more than 10 short messages in the QUIT WG. For comparisons of secondary continuous outcomes with non-normal distribution assumptions, Mann–Whitney tests will be conducted. Subgroup analyses will assess the efficacy in participants with different demographics, baseline smoking characteristics, combined chronic diseases, and use of intervention variables (including compliance of using NRT gum, frequency and duration of using WeChat- or quitline-based cessation services) using multiple logistic regression. No interim analysis will be conducted. A two-sided P value of < 0.05 will be considered as statistically significant difference.
An economic evaluation will be conducted alongside this RCT following the approach of Drummond et al. [45] and in concordance with the Consolidated Health Economic Evaluation Reporting Standards statement [46]. The ICER will be calculated as follows: ICER = (C1 − C0) / (E1 − E0), where C refers to costs, E refers to effect, and the subscripts 1 and 0 refer to the WQ and QT groups, respectively. We will generate 2500 replicate samples by bootstrap and estimate the corresponding incremental costs and effects for each replicate sample, which are then plotted on a cost-effectiveness plane.
Discussion
Currently, many mHealth-based interventions for smoking cessation are available, but they often have limited features [10]. Interventions based on SMS texting [14] or the web [47] frequently fail to deliver the recommended elements of behavioral treatments due to non-tailored content and lack of interactivity. The smartphone apps combine elements of texting and the web to create tailored and more interactive interventions [25]. The majority of them, however, failed to follow smoking cessation treatment guidelines and behavior change techniques, which led to poor quality interventions and lowered their effectiveness [48,49,50,51]. Moreover, their effectiveness was limited in real-world settings due to the high attrition and low utilization rates [52]. Social media could provide online guidance and increase the interactivity between smokers, but its effectiveness is still uncertain due to the low intensity of intervention [30,31,32]. In light of these above mentioned, the Surgeon General's 2020 report suggested integrating multiple treatment resources, as a potential strategy for increasing the reach and engagement of mHealth-based interventions for smoking cessation [10], while at the same time maintaining or improving their effectiveness.
Using the WQ modality, three mHealth-based interventions can be integrated into one platform, enabling information to be transferred between different services as well as mutual referrals, thereby increasing the reach of interventions and improving their effectiveness as a result of the cooperative effect [10]. Moreover, it was developed using the I-CET model, which incorporates several behavioral change theories (COM-B [20], EST [21], and TTM [22]), and provides evidence-based, comprehensive, stag-matched, and tailored smoking cessation interventions to fit the needs of the individual.
Through the QUIT WMP, smokers can develop skills to quit smoking, receive tailored tips, and obtain motivation to quit. It was developed based on guidelines for smoking cessation interventions [10, 29] and behavior change techniques [28], ensuring its efficacy and quality. Moreover, it could be accessed directly through the WeChat app without the need to download or install an application [41], which eliminates some limitations associated with smartphone apps. A pilot RCT has preliminarily found that WeChat mini-program might be effective in helping smokers quit smoking (biochemically verified 7-day PPA at 6 weeks in intervention group was 25%), but it was only self-help interventions, not combined with other interventions, such as online guidance by social media [53]. As part of the WQ modality, WeChat mini-program was integrated with other interventions, which is expected to be more effective than using WeChat alone. Our cohort study conducted in 12 provinces and cities in western China has preliminarily indicated that the WQ modality was effective in assisting Chinese smokers in quitting (self-reported 7-day PPA at one-month was 41.8%) [18].
The QUIT WG could offer group interventions led by professionals on a one-to-many base [54]. Intensive comprehensive cessation treatment at the group level likely brings to bear many key intervention functions of BCW [20], such as education, persuasion, training, modelling and environmental restructuring, all of which have consistently led to high quit rates [54]. And the online manner could broaden the reach and availability of group interventions [10]. Moreover, the QUIT WG can provide smokers with real-time online cessation counseling, which increases their knowledge about cessation, boosts their motivation and self-efficacy, and imparts their skills to quit [20, 28]. More importantly, “Quitting Pioneer” in the group could provide an example for other smokers to aspire to or imitate, share their experiences in quitting, motivate other smokers to quit and encourage them to participate in interactive activities [33, 34, 36]. In addition to enhancing the credibility and adherence of interventions among smokers, these interventions will facilitate the creation of reciprocal, strong, and long-lasting social bonds that promote smoking cessation. As well, the group administrator could supervise and guide smokers in using the QUIT WMP, which would increase its usage and improve its effectiveness.
The primary outcome of this study is slightly different from that of other related studies. The Society for Research on Nicotine and Tobacco recommends that the effectiveness outcome at the end of the intervention should be the primary outcome for determining whether the treatment is effective [42]. In light of this, we choose the short-term (1-month) outcome as the primary outcome based the intervention period (four weeks) rather than the long-term (such as 6-months or 12-months) outcomes selected by other studies [55]. Nevertheless, most of other studies have reported 1-month outcome [55], and we will also collect the long-term (3-month, 6-month, and 12-month) outcomes as secondary outcomes. Thus, the results of this study will be comparable to those of other studies.
Limitations should be mentioned. Firstly, the intervention period in this study has a shorter intervention period (four weeks) than other related studies (more than three months) [55]. This is primarily due to the relatively low engagement of mHealth-based approaches over a prolonged period, and the highest activity often occurs in the first month [56]. In the future, we will extend the intervention period once the effectiveness of this short-term intervention modality has been confirmed, with the intention of improving the long-term effectiveness of the WQ modality. Secondly, this study is an open label study, which may lead to information bias. In order to compensate for this type of bias, a variable random block length can be used instead of a fixed length, such as four or six. Furthermore, the staff performing the telephone follow-ups and statistical analysis will be blinded, and the participants’ self-reported abstinence will be biochemically validated by the exhaled CO test. Thirdly, this is a single-center study which could limit the generalizability of the results. Finally, all participants will be young to middle-aged, as elderly individuals have difficulty utilizing mobile devices skillfully [57]. Accordingly, the results should be extrapolated with caution to the elderly population.
It is anticipated that this trial, along with its findings will provide evidence for the WQ modality as an effective, acceptable, and affordable mHealth-based approach to smoking cessation. It will be of paramount significance to reduce health disparities as well as address the imbalance in smoking cessation services between regions and socioeconomic groups.
Availability of data and materials
Data about individual identified participants of this study will be available from the corresponding author on reasonable request after the main results of the study have been published.
Abbreviations
- Apps:
-
Applications
- BCW:
-
Behavior change wheel
- CI:
-
Confidence intervals
- CO:
-
Carbon monoxide
- COM-B:
-
Capability, Opportunity, Motivation, and Behavior
- DMC:
-
Data monitoring committee
- EST:
-
Ecological Systems Theory
- FTND:
-
Fagerstrӧm test for nicotine dependence
- GATS:
-
Global Adult Tobacco Survey
- ICER:
-
Incremental cost-effectiveness ratio
- I-CET model:
-
Integrated COM-B/ Ecological systems/ Transtheoretical model
- ITT:
-
Intent-to-treat
- mHealth:
-
Mobile health
- MNWS:
-
Minnesota Nicotine Withdrawal Scale
- NRT:
-
Nicotine replacement therapy
- OR:
-
Odds ratio
- PP:
-
Per-protocol
- PPA:
-
Point prevalence of abstinence
- ppm:
-
Parts per million
- QR:
-
Quick Response
- QSU Brief:
-
Brief-Questionnaire of Smoking Urges
- QT:
-
Quitline-based treatment
- RCT:
-
Randomized controlled trial
- SMS:
-
Short message service
- SPIRIT:
-
Standardized protocol items recommendations for interventional trials
- TTM:
-
Transtheoretical Model
- WMP:
-
WeChat mini program
- WG:
-
WeChat group
- WOA:
-
WeChat official account
- WQ modality:
-
“Way to Quit” modality
References
World Health Organization. WHO Report on the Global Tobacco Epidemic, 2008: The MPOWER package. Geneva: World Health Organization; 2009.
World Health Organization. WHO Report on the Global Tobacco Epidemic, 2021: Addressing new and emerging products. Geneva: World Health Organization; 2021.
Chinese Center for Disease Control and Prevention. 2018 GATS China report. Beijing: Chinese Center for Disease Control and Prevention; 2018.
Lin H, Xiao D, Liu Z, Shi Q, Hajek P, Wang C. National survey of smoking cessation provision in China. Tob Induc Dis. 2019;17:25.
Matkin W, Ordóñez-Mena JM, Hartmann-Boyce J. Telephone counselling for smoking cessation. Cochrane Database Syst Rev. 2019;5(5):CD002850.
Wang J, Nan Y, Yang Y, Jiang Y. Quitline Activity in China. Asian Pac J Cancer Prev. 2016;17(S2):7–9.
Jing H, Zhang D, Liang LR, Chu SL, Yang Y, Yin RX, et al. Analysis on the change of call volume and the characteristics of callers of China professional quitline. Chin J Pre Contr Chron Dis. 2020;28(12):911–6 [Chinese].
Chen WL, Xiao D, Henderson S, Zhao L, Jing H, Wang C. Characteristics of callers accessing the tobacco cessation quitline in mainland China. Biomed Environ Sci. 2013;26(8):697–701.
Momin B, Neri A, McCausland K, Duke J, Hansen H, Kahende J, et al. Traditional and innovative promotional strategies of tobacco cessation services: a review of the literature. J Community Health. 2014;39(4):800–9.
U.S. Department of Health and Human Services. Smoking cessation: a report of the surgeon general. Washington, DC: Superintendent of Documents, U.S. Government Printing Office; 2020.
Patel SY, Mehrotra A, Huskamp HA, Uscher-Pines L, Ganguli I, Barnett ML. Variation in telemedicine use and outpatient care during the COVID-19 pandemic in the United States. Health Aff (Millwood). 2021;40(2):349–58.
Patel SY, Mehrotra A, Huskamp HA, Uscher-Pines L, Ganguli I, Barnett ML. Trends in outpatient care delivery and telemedicine during the COVID-19 pandemic in the US. JAMA Intern Med. 2021;181(3):388–91.
Friedman AB, Gervasi S, Song H, Bond AM, Chen AT, Bergman A, et al. Telemedicine catches on: changes in the utilization of telemedicine services during the COVID-19 pandemic. Am J Manag Care. 2022;28(1):e1–6.
Whittaker R, McRobbie H, Bullen C, Rodgers A, Gu Y, Dobson R. Mobile phone text messaging and app-based interventions for smoking cessation. Cochrane Database Syst Rev. 2019;10(10):CD006611.
Petkovic J, Duench S, Trawin J, Dewidar O, Pardo Pardo J, Simeon R, et al. Behavioural interventions delivered through interactive social media for health behaviour change, health outcomes, and health equity in the adult population. Cochrane Database Syst Rev. 2021;5(5):012932.
Top 50 Countries by Smartphone Users and Penetration. Newzoo. 2021. https://newzoo.com/insights/rankings/top-countries-by-smartphone-penetration-and-users. Accessed 20 Feb 2023.
WeChat Chinese and International versions combined monthly active users over 1.2 billion, mini-programme daily users over 400 million. https://tech.sina.cn/2020-05-13/detail-iircuyvi2911382.d.html. Accessed 20 Feb 2023.
Chu S, Tong Z, Zhang Y, Ye X, Liu Z, Chen H, et al. Usage, acceptability, and preliminary effectiveness of an mHealth-based integrated modality for smoking cessation interventions in Western China. Tob Induc Dis. 2023;21:07.
Zhu SH, Anderson CM, Tedeschi GJ, Rosbrook B, Johnson CE, Byrd M, et al. Evidence of real-world effectiveness of a telephone quitline for smokers. N Engl J Med. 2002;347(14):1087–93.
Michie S, van Stralen MM, West R. The behaviour change wheel: a new method for characterising and designing behaviour change interventions. Implement Sci. 2011;6:42.
Bronfenbrenner U. Toward an experimental ecology of human development. Am Psychol. 1977;32(7):513–31.
Prochaska JO, DiClemente CC. Stages of change in the modification of problem behaviors. Prog Behav Modif. 1992;28:183–218.
Prochaska JO, DiClemente CC, Norcross JC. In search of how people change. Applications to addictive behaviors. Am Psychol. 1992;47(9):1102–14.
Smith C. 110 amazing WeChat statistics and facts (2020). https://scholar.google.com/scholar_lookup?title=110+amazing+WeChat+statistics+and+facts+(2020)&author=C+Smith&publication_year=2020&. Accessed 20 Feb 2023.
Haskins BL, Lesperance D, Gibbons P, Boudreaux ED. A systematic review of smartphone applications for smoking cessation. Transl Behav Med. 2017;7(2):292–9.
Peiris D, Wright L, News M, Rogers K, Redfern J, Chow C, et al. A smartphone app to assist smoking cessation among aboriginal Australians: findings from a pilot randomized controlled trial. JMIR Mhealth Uhealth. 2019;7(4):e12745.
Bricker JB, Mull KE, Kientz JA, Vilardaga R, Mercer LD, Akioka KJ, et al. Randomized, controlled pilot trial of a smartphone app for smoking cessation using acceptance and commitment therapy. Drug Alcohol Depend. 2014;143:87–94.
Michie S, Hyder N, Walia A, West R. Development of a taxonomy of behaviour change techniques used in individual behavioural support for smoking cessation. Addict Behav. 2011;36(4):315–9.
National Health and Family Planning Commission of China. Clinical guideline for smoking cessation in China. http://www.cfchina.org.cn/uploadfile/2016/0622/%E4%B8%AD%E5%9B%BD%E4%B8%B4%E5%BA%8A%E6%88%92%E7%83%9F%E6%8C%87%E5%8D%9720150515-final.pdf. Accessed 20 Feb 2023. [Chinese].
May S, West R. Do social support interventions (“buddy systems”) aid smoking cessation? A review Tob Control. 2000;9(4):415–22.
Pechmann C, Delucchi K, Lakon CM, Prochaska JJ. Randomised controlled trial evaluation of Tweet2Quit: a social network quit-smoking intervention. Tob Control. 2017;26(2):188–94.
Baskerville NB, Azagba S, Norman C, McKeown K, Brown KS. Effect of a digital social media campaign on young adult smoking cessation. Nicotine Tob Res. 2016;18(3):351–60.
Zhao L, Yang G, Wang W, Chen Y, Huang JP, Ohashi H, et al. Herd behavior in a complex adaptive system. Proc Natl Acad Sci U S A. 2011;108(37):15058–63.
Saposnik G, Maurino J, Sempere AP, Ruff CC, Tobler PN. Herding: a new phenomenon affecting medical decision-making in multiple sclerosis care? Lessons learned from DIScUTIR MS. Patient Prefer Adherence. 2017;11:175–80.
Kelly JA, Amirkhanian YA, Kabakchieva E, Vassileva S, Vassilev B, McAuliffe TL, et al. Prevention of HIV and sexually transmitted diseases in high risk social networks of young Roma (Gypsy) men in Bulgaria: randomised controlled trial. BMJ. 2006;333(7578):1098.
Valente TW, Hoffman BR, Ritt-Olson A, Lichtman K, Johnson CA. Effects of a social-network method for group assignment strategies on peer-led tobacco prevention programs in schools. Am J Public Health. 2003;93(11):1837–43.
Soumerai SB, McLaughlin TJ, Gurwitz JH, Guadagnoli E, Hauptman PJ, Borbas C, et al. Effect of local medical opinion leaders on quality of care for acute myocardial infarction: a randomized controlled trial. JAMA. 1998;279(17):1358–63.
Shiffman S, Scholl SM, Mao J, Ferguson SG, Hedeker D, Primack B, et al. Using nicotine gum to assist nondaily smokers in quitting: a randomized clinical trial. Nicotine Tob Res. 2020;22(3):390–7.
Huang CL, Lin HH, Wang HH. The psychometric properties of the Chinese version of the Fagerstrom test for nicotine dependence. Addict Behav. 2006;31(12):2324–7.
Yu X, Xiao D, Li B, Liu Y, Wang G, Chen J, et al. Evaluation of the Chinese versions of the Minnesota nicotine withdrawal scale and the questionnaire on smoking urges-brief. Nicotine Tob Res. 2010;12(6):630–4.
How WeChat Became China's App For Everything. Fast Company. https://www.fastcompany.com/3065255/china-wechat-tencent-red-envelopes-and-social-money. Accessed 20 Feb 2023.
Hughes JR, Keely JP, Niaura RS, Ossip-Klein DJ, Richmond RL, Swan GE. Measures of abstinence in clinical trials: issues and recommendations. Nicotine Tob Res. 2003;5(1):13–25.
Cropsey KL, Trent LR, Clark CB, Stevens EN, Lahti AC, Hendricks PS. How low should you go? Determining the optimal cutoff for exhaled carbon monoxide to confirm smoking abstinence when using cotinine as reference. Nicotine Tob Res. 2014;16(10):1348–55.
Zhang L, Vickerman K, Malarcher A, Mowery P. Intermediate cessation outcomes among quitline callers during a national tobacco education campaign. Nicotine Tob Res. 2014;16(11):1478–86.
Drummond MF, Sculpher MJ, Claxton K, Stoddart GL, Torrance GW. Methods for the economic evaluation of health care programmes. Oxford, UK: Oxford University Press; 2015.
Husereau D, Drummond M, Petrou S, Carswell C, Moher D, Greenberg D, et al. Consolidated Health Economic Evaluation Reporting Standards (CHEERS) statement. Cost Eff Resour Alloc. 2013;11(1):6.
Taylor GMJ, Dalili MN, Semwal M, Civljak M, Sheikh A, Car J. Internet-based interventions for smoking cessation. Cochrane Database Syst Rev. 2017;9(9):CD007078.
Struik L, Rodberg D, Sharma RH. The behavior change techniques used in Canadian online smoking cessation programs: content analysis. JMIR Ment Health. 2022;9(3):e35234.
Bustamante LA, Gill Ménard C, Julien S, Romo L. Behavior change techniques in popular mobile apps for smoking cessation in France: content analysis. JMIR Mhealth Uhealth. 2021;9(5): e26082.
Rajani NB, Weth D, Mastellos N, Filippidis FT. Adherence of popular smoking cessation mobile applications to evidence-based guidelines. BMC Public Health. 2019;19(1):743.
Cheng F, Xu J, Su C, Fu X, Bricker J. Content analysis of smartphone apps for smoking cessation in china: empirical study. JMIR Mhealth Uhealth. 2017;5(7):e93.
Zeng EY, Vilardaga R, Heffner JL, Mull KE, Bricker JB. Predictors of utilization of a novel smoking cessation smartphone app. Telemed J E Health. 2015;21(12):998–1004.
Chen J, Ho E, Jiang Y, Whittaker R, Yang T, Bullen C. Mobile social network-based smoking cessation intervention for Chinese male smokers: pilot randomized controlled trial. JMIR Mhealth Uhealth. 2020;8(10): e17522.
Stead LF, Carroll AJ, Lancaster T. Group behaviour therapy programmes for smoking cessation. Cochrane Database Syst Rev. 2017;3(3):CD001007.
Hartmann-Boyce J, Livingstone-Banks J, Ordóñez-Mena JM, Fanshawe TR, Lindson N, Freeman SC, et al. Behavioural interventions for smoking cessation: an overview and network meta-analysis. Cochrane Database Syst Rev. 2021;1: CD013229.
Lakon CM, Pechmann C, Wang C, Pan L, Delucchi K, Prochaska JJ. Mapping engagement in twitter-based support networks for adult smoking cessation. Am J Public Health. 2016;106(8):1374–80.
Zhu X, Cheng X. Staying connected: smartphone acceptance and use level differences of older adults in China. Univers Access Inf Soc. 2022;1–10. Epub ahead of print.
Acknowledgements
Not applicable.
Funding
This research is supported by Beijing Key Specialists in Major Epidemic Prevention and Control (Principal Investigators ZHT and LRL) from the Beijing Municipal Health Commission; Financial Budgeting Project of Beijing Institute of Respiratory Medicine (ysbz2022002, Principal Investigators: LRL) and Reform and Development Program of Beijing Institute of Respiratory Medicine (ysrh2022014, Principal Investigators: SLC) from Beijing Institute of Respiratory Medicine. The trial sponsors and contact information: Beijing Municipal Health Commission, Block B, Central Office Building 70, Zaolinqian Street, Xicheng District, Beijing, China, 100053, tel: + 8610- 83970601; Beijing Institute of Respiratory Medicine, No.8 Gong-Ti-Nan-Lu, Chaoyang District, Beijing, China, 100020, tel: + 8610–85231610. These funding agencies have no role in the design of this study and will not have any role during its execution, analyses, interpretation of the data, or decision to submit results. The authors are solely responsible for the design of the research and for the preparation of the manuscript.
Author information
Authors and Affiliations
Contributions
LRL and SLC conceived and designed the study, with contributions from LF, YTZ, HJ, ZHT, JS, HMM, and ZJZ in drafting the protocol manuscript. All authors contributed to the current manuscript through review and editing and have approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study protocol has been approved by the Institutional Review Board (IRB) of Beijing Chao-Yang Hospital, Capital Medical University in Beijing, China on December 30, 2020. IRB# 2020-Ke-545.The most recent continuing review was completed on December 15, 2022 (Protocol Version 1.5). The ethics committee granted a waiver of written documentation of informed consent. Electronic informed consent will be obtained from all participants (no e-signature). If there are many significant protocol modifications in the future, we will communicate the modifications to investigators, REC/IRBs, trial participants, trial registries and journals. All methods will be performed in accordance with the Declaration of Helsinki.
Consent for publication
Not applicable because we will not collect or videos of participants in this study. Additionally, no details on individuals are reported within the manuscript.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1.
SPIRIT 2013 Checklist: Recommended items to address in a clinical trial protocol and related documents*.
Additional file 2.
TheQuick Response code of the QUIT WeChat mini-program.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Chu, S., Feng, L., Zuo, Y. et al. Evaluation of an innovative mHealth-based integrated modality for smoking cessation in Chinese smokers: protocol for a randomized controlled trial. BMC Public Health 23, 561 (2023). https://doi.org/10.1186/s12889-023-15448-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12889-023-15448-7