Abstract
Background
Over the past 20 years, excessive antibiotic use has led to serious antimicrobial resistance (AMR) worldwide, and the phenomenon is particularly serious in China. To this end, the Chinese health sector took a series of measures to promote rational antibiotic use. In this study, to reveal the impact of policies on antibiotic use, we explored the long-term trend and patterns of antibiotic use at public health care institutions from 2012 to 2020 in northwest China, taking Gansu Province as an example.
Methods
Antibiotic procurement data were obtained from the provincial centralized bidding procurement (CBP) platform between 2012 and 2020. Antibiotic use was quantified using the Anatomical Therapeutic Chemical (ATC)/defined daily doses (DDD) methodology and standardized using the DDD per 1000 inhabitants per day (DID). Twelve relevant quality indicators were calculated for comparison with the European Surveillance of Antimicrobial Consumption (ESAC) project monitoring results.
Results
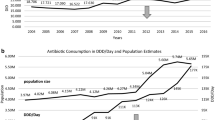
Total antibiotic use increased from 18.75 DID to 57.07 DID and then decreased to 19.11 DID, a turning point in 2014. The top three antibiotics used were J01C (beta-lactam antibacterials, penicillins), J01F (macrolides, lincosamides and streptogramins), and J01D (other beta-lactam antibacterials, cephalosporins), accounting for 45.15%, 31.40%, and 11.99% respectively. The oral antibiotics used were approximately 2.5 times the parenteral antibiotics, accounting for 71.81% and 28.19%, respectively. Different use preferences were shown in public hospitals and primary health care centres (PHCs), and the latter accounted for more than half of total use. The absolute use of all classes of antibiotics in Gansu is almost higher than any of the 31 European countries included in the ESAC, but the relative use of some focused antibiotics is lower than theirs.
Conclusions
The intervention policies of the health department reduced antibiotic use in Gansu Province, but the proportion of broad-spectrum and parenteral antibiotics was still high. It is necessary to further improve the quality of antibiotic prescriptions and pay more attention to the rationality of antibiotic use in PHCs.
Similar content being viewed by others
Introduction
Over the past 20 years, global antibiotic consumption has increased by 65%, with the majority being dispensed from low- and middle-income countries (LMICs) [1]. With the widespread use of antibiotics worldwide, antimicrobial resistance (AMR) has become a global public health problem following bacterial infections, endangering human health and life, and causing an enormous health economic burden [2]. An important assessment published in 2015 showed that at least 700,000 people worldwide die of AMR infections every year. This number was expected to rise to 10 million by 2050 if the foregoing circumstance was not controlled, and the total loss of the global economy may exceed $US100 trillion [3]. However, this analysis included only six pathogens and the acknowledged real number may be much higher [4].
As a representative of developing countries, China ranks second in the world in human antibiotic use, only lower than India [5]. Research has shown that prescriptions approved by Chinese doctors have always been characterized by excessive and unreasonable antibiotic use, and the antibiotic use rate in outpatients was close to or more than 50% [6], which was higher than in other developing countries (40%-50%). However, as recommended by the World Health Organization (WHO), the reasonable range of this index was 20.0%-26.8% [7], and China was higher than this recommended value. In addition, due to the lack of continuous high-quality education and training for health providers, this phenomenon was more common at primary health care centres (PHCs). Some research results showed that the antibiotic use rate in rural areas of China was generally 50%-70% [8,9,10].
The Chinese government never gave up using policy intervention to regulate antibiotic use. As early as 2004, the former Ministry of Health issued 'Guiding principles for clinical application of antibiotics' [11], which systematically standardized antibiotic use and management in health care institutions for the first time. However, due to the lack of mandatory requirements, the implementation of different health care institutions were uneven, and antibiotic use did not achieve the desired effect. To promote rational antibiotic use, China established the National Centre for Antibacterial Surveillance in the next year [12], but it was not until 2010 that all provinces and cities carried out the work in a comprehensive manner. After that, a series of relevant strategies and guidelines had little effect. By August 2012, the 'Administrative Measures for Clinical Use of Antimicrobial Agents' were implemented nationwide in the form of an order from the Ministry of Health. The policy required health care institutions to establish hierarchical management measures, monitor and analyse the trend of clinical antibiotic use and bacterial resistance, and strengthen interventions in irrational medicine use [13]. These steps gradually promoted the normalization and refinement of antibiotic use and management [14].
In 2009, the Chinese government issued an essential medicines programmer and implemented a zero mark-up policy while establishing the provincial centralized bidding procurement (CBP) platform to ensure the supply of essential medicines [15], which provided a good channel for public health care institutions to purchase essential medicines. However, a systematic review revealed that despite the decline in the price of essential medicines, the impact of policy on antibiotic prescriptions in PHCs was very limited. Some results reported that number of antibiotic prescriptions had no statistically significant difference in PHCs before and after the policy [16].
The European Surveillance of Antimicrobial Consumption (ESAC) project recorded the information related to antibiotics in the sales records and reimbursement systems of 31 countries in Europe [17], which provided accurate data support to compare antibiotic use in China on an international level. The twelve quality indicators summarized by its scientific advisory board can be used to evaluate the potential unreasonable use of antibiotics in PHCs. This method has been proven to be effective in other studies [18]. This study collated antibiotic use data of Gansu Province from 2012 to 2020, analysed antibiotic use in different health care institutions and compared it to countries monitored by ESAC. This study aimed to (1) explore the long-term trend and mode of antibiotic consumption in northwest China, (2) reveal differences between this region and other countries and regions, and (3) provide clues and help decision-makers to formulate relevant policies and measures.
Methods
Study setting
Gansu is an underdeveloped province in northwest China. Its GDP was $US141.76 billion in 2020 —— the fifth lowest in the country, and the second-lowest in per capita GDP [19]. Since the beginning of the three-year new medical reform (2009–11), Gansu has included government-run medical and health institutions in the implementation of the national basic drug management approach and has achieved zero markups on drugs. By the end of 2012, the coverage rate of the essential medicines scheme reached 100% [20].
PHCs, in a broad sense, are medical institutions at the township (community) level and small medical and health service stations (village health offices, community health service stations, etc.). They are mainly responsible for providing comprehensive services such as public health services and the treatment of common and multimorbid diseases. China's primary health care institutions have been suffering from a shortage of doctors and low service capacity [21]. Due to the large rural population, small population density and large service radius, primary health care in Gansu is more difficult to carry out and of lower quality. Several relevant studies showed the phenomenon of excessive and unreasonable antibiotic use and serious AMR problems in Gansu, which were particularly prominent in PHCs [22,23,24].
Data source
The data on antibiotic use in this study were extracted from the CPB platform of the Gansu Public Resources Trading Centre. As the only medicine procurement system dominated by the government, operated online and faced by almost all public health care institutions, the platform was first established in 2009 [25]. By 2020, the number of public health care institutions purchasing antibiotics through this platform reached 1900 in the province, including 272 public hospitals and 1510 PHCs (Table 1), accounting for 93.39% of the total number of public medical institutions in the province. We collected data related to antibiotics on the platform from 2012 to 2020, and its main record information included the general name, dosage form, specification, procurement unit, procurement quantity, etc.
Data management
After deleting a few data with incomplete information, the Anatomical Therapeutic Chemical (ATC) system developed by WHO was used to code and classify medicine, and then was used to conveniently screen the specific medicine classes related to the study. In this paper, all "J01" (antimicrobials for system use) in "J" (anti-infectives for system use) were included in the study. The dataset was managed using Microsoft Office Excel 2016 and computed using Stata (version 15.0), and charts were plotted using GraphPad Prism 9.
Data analysis
The use of various antibiotics was calculated with the defined daily doses (DDD) as the minimum dose unit, and the total amount of purchases for all packaged units was expressed as DDDs and standardized with the DDD per 1000 inhabitants per day (DID). DDD, as the unit value of medication frequency analysis, represented the average maintenance dose per day in adults for its major therapeutic purposes; DDD equivalence per package (DPP) was an intermediate indicator for calculating total DDDs, and the calculation formula was DPP = (unit strength × pack size)/DDD, \(DDDs= {\sum }_{i=1}^{n}{DPP}_{i}\times {N}_{i}\), where \({N}_{i}\) represents the number of packages of a certain product (\(i\)). The standardized medication intensity was calculated by DID, which indicated the DDD per 1000 inhabitants per day.
The number of inhabitants was represented by the average population every two years in the Gansu Statistics Yearbook [26]. According to these data, we found that approximately 60% of total outpatient visits were in hospitals and 40% were in PHCs. Outpatient visits by public health care institutions accounted for approximately 85% of all health care institutions [27]. Therefore, public hospitals shared approximately 51% of the total outpatient visits, and PHCs shared 34% in Gansu. We adjusted the share of antibiotic use in different health care institutions with the above data (assuming that patients chose health care institutions without crossing, that is, only choosing public or private health care institutions to visit).
Comparison with ESAC monitoring results
The purchasing units were divided into public hospitals and PHCs according to their levels. The consumption and use preferences of various antibiotics in different institutions were calculated, and the results were compared with monitoring results in the ESAC project [28]. Eighteen use indicators were calculated to indicate total antibiotic use, which were respectively J01, J01A (tetracyclines), J01B (amphenicols), J01C, J01D, J01E (sulfonamides and trimethoprim), J01F, J01G (aminoglycoside antimicrobials), J01M (quinolone antibacterials), J01CA (broad-spectrum penicillins), J01CE (beta-lactamase sensitive penicillins), J01CR (combinations of penicillins), J01DB (first-generation cephalosporins), J01DC (second-generation cephalosporins), J01DD (third-generation cephalosporins), J01FA (macrolides), J01FF (lincosamides) and J01MA (fluoroquinolones). Twelve relevant quality indicators were calculated to compare the difference in antibiotic use between different PHCs, which were the use of J01, J01C, J01D, J01F, J01M, and the percentages of J01CE, J01CR, J01DD + J01DE (fourth-generation cephalosporins), J01MA in total volume, as well as the ratio of broad/narrow-spectrum antibiotic use and seasonal variations of J01 and J01M use.
Results
Overview of antibiotic use in Gansu, 2012–20
The total antibiotic use in Gansu Province increased from 18.75 DID in 2012 to a peak of 57.07 DID in 2014, and then decreased to 19.11 DID in 2020. Consumption rose fastest in 2013 (95.97%) and fell fastest in 2015 (36.70%). Such a trend was mainly shaped by changes in J01F (Fig. 1). The changing trend of parenteral antibiotics was the same as that of total antibiotics and oral antibiotics peaked in 2017. The latter (23.55 DID, 71.81%) was approximately two times the former (10.33 DID, 28.19%) in average use.
Among of different antibiotic classes, J01C had the highest average use (14.96 DID, 45.15%). Its trend also increased first and then decreased, and the turning point took place in 2017. J01CA was dominant in penicillin use (13.77 DID, 91.45%). Although broad-spectrum penicillin use has gradually decreased in recent years, it is still the most used four-level subclass. In addition, the use of J01CR showed a slow-growth trend. The second most frequently used class of antibiotic was J01F (11.32 DID, 31.40%). Regarding its subclass, the proportion of J01FA (4.93 DID, 63.00%) was higher than that of J01FF (6.39 DID, 37.00%). J01FA has decreased significantly since 2014, and J01FF has changed slightly. J01D was the third most used antibiotic (3.97 DID, 11.99%), and its trend was the same as the total volume, but the decrease range was relatively small after the peak. The use of J01DB (1.75 DID, 43.34%), J01DC (1.13 DID, 28.38%) and J01DD (1.07 DID, 27.73%) decreased successively. J01DB use remained at a high level for several consecutive years. In addition to the above three antibiotics, other antibiotic use was relatively low (Fig. 2).
Antibiotic use at public hospitals in Gansu, 2012–20
The changing trend of antibiotic use in public hospitals in Gansu Province from 2012 to 2020 was the same as the total, rising from 14.48 DID in 2012 to 64.91 DID in 2014 and then falling to 13.09 DID in 2020. The use increased the most in 2013 (134.85%) and decreased the most in 2015 (63.72%). The average use of oral antibiotics (11.28 DID, 54.89%) was similar to parenteral antibiotics (13.59 DID, 45.11%), and they shared a similar varying trend with that in the whole province (Fig. 3).
In public hospitals, J01F use was the highest (13.87 DID, 47.93%). Among its subclass, J01FF use was higher in the first four years, while J01FA use was higher in the last five years. This was followed by J01C (5.76 DID, 26.37%), of which J01CA (4.53 DID, 77.75%) accounted for the highest proportion. The third and fourth most commonly used antibiotics were J01D (2.95 DID, 13.92%) and J01M (1.36 DID, 7.03%) (Fig. 4). It is worth mentioning that J01DC (1.15 DID, 39.68%) and J01DD (1.22 DID, 40.30%) use was higher than J01DB use (0.55 DID, 18.93%).
Antibiotic use at PHCs in Gansu, 2012–20
The peak time of antibiotic use at PHCs in Gansu was in 2017, where antibiotic use increased from 24.69 DID in 2012 to 62.60 DID in 2017 and then decreased to 26.32 DID in 2020. The use increased the most in 2013 (61.14%) and decreased the most in 2020 (42.17%). Oral antibiotic use (40.50 DID, 87.13%) was almost equal to the total (Fig. 3).
J01C use (28.20 DID, 60.64%) accounted for more than half of the total use in PHCs, with J01CA (27.14 DID, 95.59%) being widely used. J01F use was the second highest (6.88 DID, 15.43%), and J01FA had the highest use (6.07 DID, 87.98%) among it (Fig. 5). This was followed by J01D (5.20 DID, 12.20%) and J01M (3.88 DID, 8.64%).
Comparison with ESAC monitoring results
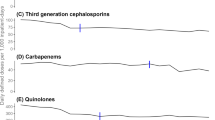
In comparison with the monitoring results of 31 European countries in the ESAC project in the same period, it was found that absolute antibiotic use in Gansu Province was at a high level, even higher than all countries included in the project for five consecutive years. This phenomenon was mainly reflected in J01C and J01F use, while the use of J01A and J01E was always low. In addition, although J01C use was high, J01CR use remained at a low level (Table 2).
Conversely, relative antibiotic use in Gansu Province was low. Especially regarding J01CR use and the ratio of broad/narrow-spectrum antibiotics, the results were lower than at least three-quarters of countries. The seasonal variation in total volume fluctuated greatly, but the seasonal variation in J01M was almost lower than that in all countries (Table 3).
Discussion
A global action plan on antimicrobial resistance pointed out that high total antibiotic consumption was the main factor leading to the occurrence of AMR and was also the key to controlling the problem [29]. The current study showed that total antibiotic consumption in Gansu was higher than in some high-income countries and even higher than in France (37% higher) — the country with the highest antibiotic consumption in ESAC before 2017 [30]. There is no doubt that compared with high-income countries, the disease burden faced by early LMICs (mainly referred to as the burden of infectious disease) was much larger than the adverse effects caused by AMR (burden of AMR bacterial infection). Accelerated urbanization and socioeconomic growth are two mutually reinforcing conditions, followed by the spread of various bacterial infectious diseases and the prevalence of nonbacterial infectious diseases [31]. The former made a great contribution to the extensive use of antibiotics, while the latter was an important driving factor for unreasonable antibiotic use. In addition, the high sales were also related to the production, sale, and management mode of antibiotics in China. Compared with other high-income countries, Chinese doctors had fewer restrictions in issuing antibiotic prescriptions, patients had more channels to buy antibiotics, and the general public had a higher rate of self-medication with antibiotics [32, 33]. This phenomenon was also common in other LMICs in Asia [34].
After 2014, although broad-spectrum antibiotic consumption was still high, total antibiotic sales showed significant negative growth in Gansu, which benefited from a series of top-down policies and measures issued by China's health department. After the reform, the rapid development of animal husbandry exacerbated antibiotic abuse. At the same time, it also gave birth to China's first medicine management regulation, 'The Drug Management Law of the People's Republic of China', but the regulation was only controlled from the overall medical level [35]. After entering the twenty-first century, relevant policies dedicated to antibiotic use and management were issued. In 2012, the former Ministry of Health issued the "strictest antibiotic management method" — 'The Administrative Measures for the Clinical Use of Antibacterial Drugs'. The project lasted for three years. During this period, antibiotic consumption in public hospitals in many provinces and cities fell precipitously [36].
The study found that J01C ranked first in the consumption of various antibiotics in Gansu Province all year round, followed by J01F, which was different from the use patterns of other provinces and cities [37]. In China, there were some differences in antibiotic use patterns in the eastern, middle and western regions divided by geographical location. In the final analysis, the reason was that their regional environment, economic, social, and cultural backgrounds were different, and the distribution of medical resources and the population literacy rate were also very different [38]. These socioeconomic factors were directly or indirectly related to antibiotic use. The natural climate and socioeconomic factors were similar among the provinces (cities and autonomous regions) in northwest China, and the disease spectrum had some common features. Therefore, the antibiotic use pattern in Gansu province can represent northwest China to some extent.
J01F consumption in Gansu was at an abnormally high level before 2016, and the main contribution (83.89%) came from public hospitals. It has been suggested that this was mainly due to the widespread use of clindamycin which is sensitive to methicillin-resistant Staphylococcus aureus (macrolide-lincosamide-streptomycin B-resistant) in hospitals. The incidence of infections caused by this class of resistant bacteria was higher in hospitals than in the community [39]. This trend in J01F was similar to that in other regions in China [40]. In addition, the extensive use of broad-spectrum antibiotics was another concern. Although the consumption of broad-spectrum penicillins has decreased in recent years, it was still the most used subclass in J01C. Before the reform of public hospitals, driven by seemingly ideal treatment effects and higher medicine profit returns, clinicians often chose high-level antibiotics for treatment — high-grade rather than low-grade, broad-spectrum rather than narrow-spectrum antibiotics [41], which led to the fact that most antibiotics used in hospitals at that time were three or more generations, seriously violating the principle of antibiotic use. The super indications medication was also another important culprit leading to the increase of AMR [42]. 'The guidelines for the clinical use of antibacterial drugs' issued in 2015 clarified the basic principles of antimicrobial use and the treatment principles of multiple bacterial infections and emphasized the indications and precautions of various antibiotics. At the same time, related regulations also revised and improved requirements for management indicators and clinical application evaluation of antibiotics, stipulating that the number of antibiotics used in secondary and tertiary hospitals' antibiotic catalogues shall not exceed 35 and 50, respectively [43]. All these factors can explain the obvious decline in consumption of many subclass antibiotics after 2016.
The overuse of parenteral antibiotics cannot be ignored. The proportion of parenteral antibiotic use in Gansu decreased from 39.47% in 2012 to 19.88% in 2020, which was a great breakthrough, but the figure was still higher than the estimated 7% in European countries [44]. The main share of this part also came from public hospitals. A series of medical consumable costs attached to parenteral antibiotic use was an integral part of the medical cost, and some doctors may issue such prescriptions driven by interest [45]. Compared with oral antibiotics, patients often thought that parenteral antibiotics had faster, better effects, and shorter course of treatment than oral antibiotics. Therefore, they might flow to higher-level hospitals due to the demand for parenteral antibiotics [46].
The number of outpatient visits undertaken by PHCs in Gansu accounted for only 40%, and this proportion was as high as 80% in most European countries [47]. Compared with their strong primary health care service capacities, China's PHCs have been weak in this regard. To make matters worse, PHCs had more antibiotic consumption (53.93%) while having fewer outpatient visits. We adjusted the intensity of antibiotic use in PHCs according to the proportion of outpatient visits. It was found that the total antibiotic and penicillin consumption used in PHCs in Gansu almost exceeded all monitoring countries in the ESAC project, and other provinces also reported similar results [18]. As the implementation objects of earlier antibiotic application management policies were mainly concentrated in public hospitals and the constraints on PHCs were not reflected until a long time later, the use of antibiotics increased steadily before 2017. Moreover, most medical personnel in PHCs had not received unified and high-quality preemployment learning and training, resulting in a lack of professional knowledge and insufficient diagnosis and treatment levels in terms of antibiotic indications and incompatibility. Therefore, the phenomenon of empirical medication, preventive medication, and nonstandard medication is common [48].
To achieve a reasonable pattern of medical treatment, under the background of 'the guiding opinions on promoting the construction of hierarchical diagnosis and treatment system', Gansu decided to implement the graded diagnosis and treatment system of a new rural cooperative medical system in three pilot cities in 2014, it was fully implemented in the whole province after 2016 [49]. Objectively speaking, the system indeed played a certain role in reducing the use of antibiotics in PHCs, but it was still far from its original purpose of "clear division of labour in health care institutions and promoting reasonable medical treatment". The key to the problem was that PHCs lack reliable and excellent medical resources [50]. The shortages in medical workers, medical equipment, and medical medicine have not been solved, so the effect of correct diagnosis and treatment and rational drug use has not been achieved. Regarding the ratio of broad/narrow-spectrum antibiotics use, the ratio of PHCs in Gansu was better than in most countries monitored by ESAC, but this result should be interpreted with caution. This cannot be completely attributed to the fact that antibiotic use at PHCs in Gansu was more reasonable than that in other countries. The consumption of broad-spectrum antibiotics was maintained at a high level. Rational analysis suggests that it may be due to high consumption of total antibiotics, and identical high consumption of narrow-spectrum antibiotics will hedge the ratio, so it masked some of the real results.
In addition, this study still has the following limitations. First, the data used in the study were from procurement records, so they may not directly reflect actual antibiotic use. Second, the purchasing units included in the system were all public health care institutions, so the results cannot reflect the contribution of private health care institutions to antibiotic consumption. Third, as a province with a large population outflow, the actual resident population in Gansu Province may differ from the statistical population, and since the number of mobile individuals cannot be obtained and estimated, we use the mid-year population as the denominator when calculating the intensity of antibiotic use, which also leads to small calculation results to some extent.
Conclusions
Based on the antibiotic purchase data of Gansu Province in the last nine years, taking different levels of health care institutions as the starting point, this study analysed antibiotic use patterns and long-term trends, and compared calculated quality indicators with ESAC monitoring results. It was found that antibiotic use in Gansu Province has decreased sharply since 2014, which was consistent with intervention policies issued by China's health department. The decline was most obvious in public hospitals and relatively slow in PHCs, so the follow-up control of antibiotic consumption should designate PHCs as the main target. The relatively large proportion of broad-spectrum antibiotics and the still high use of parenteral antibiotics are signs of irrational antibiotic use. Therefore, it is necessary to further standardize the quality of antibiotic-related prescriptions and pay particular attention to the rationality of antibiotic use in PHCs.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.
References
Klein EY, Van Boeckel TP, Martinez EM, et al. Global increase and geographic convergence in antibiotic consumption between 2000 and 2015. Proc Natl Acad Sci U S A. 2018;115(15):3463–70.
Downie L, Armiento R, Subhi R, et al. Community-acquired neonatal and infant sepsis in developing countries: efficacy of WHO’s currently recommended antibiotics–systematic review and meta-analysis. Arch Dis Child. 2013;98(2):146–54.
Lesho EP, Laguio-Vila M. The slow-motion catastrophe of antimicrobial resistance and practical interventions for all prescribers. Mayo Clin Proc. 2019;94(6):1040–7.
Laxminarayan R, Matsoso P, Pant S, et al. Access to effective antimicrobials: a worldwide challenge. The Lancet. 2016;387(10014):168–75.
Van Boeckel TP, Gandra S, Ashok A, et al. Global antibiotic consumption 2000 to 2010: an analysis of national pharmaceutical sales data. Lancet Infect Dis. 2014;14(8):742–50.
Yin X, Song F, Gong Y, et al. A systematic review of antibiotic utilization in China. J Antimicrob Chemother. 2013;68(11):2445–52.
Yimenu DK, Emam A, Elemineh E, et al. Assessment of antibiotic prescribing patterns at outpatient pharmacy using World Health Organization prescribing indicators. J Prim Care Community Health. 2019;10:2150132719886942. https://doi.org/10.1177/2150132719886942.
Zhang Z, Hu Y, Zou G, et al. Antibiotic prescribing for upper respiratory infections among children in rural China: a cross-sectional study of outpatient prescriptions. Glob Health Action. 2017;10(1):1287334.
Wei X, Zhang Z, Walley JD, et al. Effect of a training and educational intervention for physicians and caregivers on antibiotic prescribing for upper respiratory tract infections in children at primary care facilities in rural China: a cluster-randomised controlled trial. Lancet Glob Health. 2017;5(12):1258–67.
Duan L, Liu C, Wang D. The general population’s inappropriate behaviors and misunderstanding of antibiotic use in China: a systematic review and meta-analysis. Antibiotics (Basel). 2021;10(5):497.
Chang Y, Chusri S, Sangthong R, et al. Clinical pattern of antibiotic overuse and misuse in primary healthcare hospitals in the southwest of China. PLoS ONE. 2019;14(6):e0214779.
Qu X, Yin C, Sun X, et al. Consumption of antibiotics in Chinese public general tertiary hospitals (2011–2014): Trends, pattern changes and regional differences. PLoS ONE. 2018;13(5):e0196668.
Li H, Yan S, Li D, et al. Trends and patterns of outpatient and inpatient antibiotic use in China’s hospitals: data from the Center for Antibacterial Surveillance, 2012–16. J Antimicrob Chemother. 2019;74(6):1731–40.
Xiao Y, Li L. Legislation of clinical antibiotic use in China. Lancet Infect Dis. 2013;13(3):189–91.
National Health Family Planning Commission (NHFPC). Further Standardize the Drug Centralized Bidding Procurement in Hospitals. 2009. http://www.nhc.gov.cn/wjw/zcjd/201304/a8ee38833d624d19a8eb7313dc5f8c2e.shtml. Accessed 18 Feb 2022.
Roque F, Herdeiro MT, Soares S, et al. Educational interventions to improve prescription and dispensing of antibiotics: a systematic review. BMC Public Health. 2014;14(1):1–20.
Coenen S, Ferech M, Haaijer-Ruskamp FM, et al. European Surveillance of Antimicrobial Consumption (ESAC): quality indicators for outpatient antibiotic use in Europe. BMJ Qual Saf. 2007;16(6):440–5.
Zhang X, Cui Y, Liu C, et al. Antibiotic Sales in primary care in Hubei Province, China: an analysis of 2012–2017 procurement records. Int J Environ Res Public Health. 2019;16(18):3376.
National Bureau of Statistics of China. China statistics yearbook. 2020. http://www.stats.gov.cn/tjsj/ndsj/. Accessed 22 Feb 2022.
Zhang T, Liu C, Ren J, et al. Perceived impacts of the national essential medicines system: a cross-sectional survey of health workers in urban community health services in China. BMJ Open. 2017;7(7):e014621.
Zhang W, Ung COL, Lin G, et al. Factors contributing to patients’ preferences for primary health care institutions in China: A qualitative study. Front Public Health. 2020;8:414.
Ning G, Wang X, Wu D, et al. The etiology of community-acquired pneumonia among children under 5 years of age in mainland China, 2001–2015: a systematic review. Hum Vaccin Immunother. 2017;13(11):2742–50.
Sang X-L, Liang X-C, Chen Y, et al. Estimating the burden of acute gastrointestinal illness in the community in Gansu Province, northwest China, 2012–2013. BMC Public Health. 2014;14(1):1–9.
Abotaleb BM, Al-Moraissi E, Zhiqiang W, et al. A detailed analysis of mandibular fractures epidemiology, treatment and outcomes: a 5-year retrospective study, Gansu Province-China. J Oral Maxillofacial Surg Med Pathol. 2018;30(3):197–205.
Song Y, Bian Y, Zhen T. Making medicines more accessible in China: An empirical study investigating the early progress of essential medicine system. PLoS ONE. 2018;13(8):e0201582.
Gansu Provincial Bureau of Statistics. Gansu Province statistics yearbook. 2020. http://tjj.gansu.gov.cn/tjj/c109464/info_disp.shtml. Accessed 22 Feb 2022.
Yip W, Hsiao W. Harnessing the privatization of China’s fragmented health-care delivery. Health Care Policy in East Asia: A World Scientific Reference: Volume 1: Health Care System Reform and Policy Research in China. 2020. p. 335–57.
ECDC. The European Centre for Disease Prevention and Control (ECDC) Quality Indicators for Antibiotic Consumption in the Community. 2020. https://ecdc.europa.eu/en/antimicrobial-consumption/database/quality-indicators. Accessed 3 March 2022.
WHO. Global action plan on antimicrobial resistance. 2015. https://www.who.int/publications/i/item/9789241509763. Accessed 3 March 2022.
Simmons B, Ariyoshi K, Ohmagari N, et al. Progress towards antibiotic use targets in eight high-income countries. Bull World Health Organ. 2021;99(8):550–61.
Steele AD, Hay Burgess DC, Diaz Z, et al. Challenges and opportunities for typhoid fever control: a call for coordinated action. Clin Infect Dis. 2016;62(Suppl 1):4–8.
Aslam A, Gajdacs M, Zin CS, et al. Evidence of the practice of self-medication with antibiotics among the lay public in low- and middle-income countries: a scoping review. Antibiotics (Basel). 2020;9(9):597.
Morgan DJ, Okeke IN, Laxminarayan R, et al. Non-prescription antimicrobial use worldwide: a systematic review. Lancet Infect Dis. 2011;11(9):692–701.
Widowati IGAR, Budayanti NNS, Januraga PP, et al. Self-medication and self-treatment with short-term antibiotics in Asian countries: a literature review. Pharm Educ. 2021;21:152–62.
Dong H, Bogg L, Rehnberg C, et al. Drug policy in China: pharmaceutical distribution in rural areas. Soc Sci Med. 1999;48(6):777–86.
Bao L, Peng R, Wang Y, et al. Significant reduction of antibiotic consumption and patients’ costs after an action plan in China, 2010–2014. PLoS ONE. 2015;10(3):e0118868.
Yin J, Li Q, Sun Q. Antibiotic consumption in Shandong Province, China: an analysis of provincial pharmaceutical centralized bidding procurement data at public healthcare institutions, 2012–16. J Antimicrob Chemother. 2018;73(3):814–20.
Zhen X, Zhang H, Hu X, et al. A comparative study of catastrophic health expenditure in Zhejiang and Qinghai province, China. BMC Health Serv Res. 2018;18(1):844.
Lall M, Sahni AK. Prevalence of inducible clindamycin resistance in Staphylococcus aureus isolated from clinical samples. Medical Journal Armed Forces India. 2014;70(1):43–7.
Liu W, Hassan Gillani A, Xu S, et al. Antibiotics (Macrolides and Lincosamides) consumption trends and patterns in China’s healthcare institutes. Based on a 3 Year Procurement Records, 2015–2017. Int J Environ Res Public Health. 2021;18(1):113.
Liu Y, Zhong L, Yuan S, et al. Why patients prefer high-level healthcare facilities: a qualitative study using focus groups in rural and urban China. BMJ Glob Health. 2018;3:e000854.
Du B, Chen D, Liu D, et al. Restriction of third-generation cephalosporin use decreases infection-related mortality. Crit Care Med. 2003;31(4):1088–93.
Yan K, Xue M, Ye D, et al. Antibiotic prescribing practices in secondary and tertiary hospitals in Shaanxi province, western China, 2013–2015. PLoS ONE. 2018;13(12):e0207229.
Versporten A, Bolokhovets G, Ghazaryan L, et al. Antibiotic use in eastern Europe: a cross-national database study in coordination with the WHO Regional Office for Europe. Lancet Infect Dis. 2014;14(5):381–7.
Fleming-Dutra KE, Hersh AL, Shapiro DJ, et al. Prevalence of Inappropriate Antibiotic Prescriptions Among US Ambulatory Care Visits, 2010–2011. JAMA. 2016;315(17):1864–73.
Wang HH, Wang JJ, Wong SY, et al. Epidemiology of multimorbidity in China and implications for the healthcare system: cross-sectional survey among 162,464 community household residents in southern China. BMC Med. 2014;12(1):1–12.
Ashiru-Oredope D, Budd EL, Bhattacharya A, et al. Implementation of antimicrobial stewardship interventions recommended by national toolkits in primary and secondary healthcare sectors in England: TARGET and Start Smart Then Focus. J Antimicrob Chemother. 2016;71(5):1408–14.
Zhan Q, Wang YL, Chen X. Evaluation of antibacterial use in outpatients of township and community primary medical institutions in a district of Sichuan Province. China J Glob Antimicrob Resist. 2019;19:201–6.
Li HM, Chen YC, Gao HX, et al. Changes in inpatients’ distribution and benefits under institution level-based quota payment for specific diseases in rural China: An interrupted time-series analysis. Int J Health Plann Manage. 2019;34(1):436–46.
Wu Q, Xie X. Implementation performance evaluation of hierarchical diagnosis and treatment system in China based on a sustainable perspective. Int J Health Plann Manage. 2022;37:214–27.
Acknowledgements
We truly appreciate the support and assistance provided by Prof. Hai Lin in the data processing.
Funding
This study was supported by the Gansu Provincial Department of Finance Collaborating Program 'Special Research on Lanzhou University Serving Gansu Economic and Social Development' (contract number 2019-FWZX-11).
Author information
Authors and Affiliations
Contributions
Yongheng Ma and Defang Zhao provided data and technical support, Hu Feng carried on partial data management, Xiaobin Hu provided critical comments and polished the language, and Wenxuan Cao analyzed the data and wrote the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The data for the study was obtained from the institutional drug procurement database, and not involved human participants or human material, so ethics approval and consent to participate were not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Cao, W., Feng, H., Ma, Y. et al. Long-term trend of antibiotic use at public health care institutions in northwest China, 2012–20 —— a case study of Gansu Province. BMC Public Health 23, 27 (2023). https://doi.org/10.1186/s12889-022-14944-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12889-022-14944-6