Abstract
Background
Arsenic in drinking water is a global public health concern. This study aims to investigate the association between chronic low-level exposure to arsenic in drinking water and health outcomes in the volcanic area of Mt. Amiata in Italy, using a residential cohort study design.
Methods
Chronic exposure to arsenic in drinking water was evaluated using monitoring data collected by the water supplier. A time-weighted average arsenic exposure was estimated for the period 2005–2010. The population-based cohort included people living in five municipalities in the Mt. Amiata area between 01/01/1998 and 31/12/2019. Residence addresses were georeferenced and each subject was matched with arsenic exposure and socio-economic status. Mortality and hospital discharge data were selected from administrative health databases. Cox proportional hazard models were used to test the associations between arsenic exposure and outcomes, with age as the temporal axis and adjusting for gender, socio-economic status and calendar period.
Results
The residential cohort was composed of 30,910 subjects for a total of 407,213 person-years. Analyses reported risk increases associated with exposure to arsenic concentrations in drinking water > 10 µg/l for non-accidental mortality (HR = 1.07 95%CI:1.01–1.13) and malignant neoplasms in women (HR = 1.14 95%CI:0.97–1.35). Long-term exposure to arsenic concentrations > 10 µg/l resulted positively associated with several hospitalization outcomes: non-accidental causes (HR = 1.06 95%CI:1.03–1.09), malignant neoplasms (HR = 1.10 95%CI:1.02–1.19), lung cancer (HR = 1.85 95%CI:1.14–3.02) and breast cancer (HR = 1.23 95%CI:0.99–1.51), endocrine disorders (HR = 1.13 95%CI:1.02–1.26), cardiovascular (HR = 1.12 95%CI:1.06–1.18) and respiratory diseases (HR = 1.10 95%CI:1.03–1.18). Some risk excesses were also observed for an exposure to arsenic levels below the regulatory standard, with evidence of exposure-related trends.
Conclusions
Our population-based cohort study in the volcanic area of Mt. Amiata showed that chronic exposure to arsenic concentrations in drinking water above the current regulatory limit was associated with a plurality of outcomes, in terms of both mortality and hospitalization. Moreover, some signs of associations emerge even at very low levels of exposure, below the current regulatory limit, highlighting the need to monitor arsenic concentrations continuously and implement policies to reduce concentrations in the environment as far as possible.
Similar content being viewed by others
Background
Arsenic (As) is a ubiquitous element in the environment, and it occurs in both organic and inorganic forms. Human exposure to arsenic occurs mainly through the ingestion of contaminated food and water and, to a lesser extent, by inhalation and dermal contact. Organic compounds, which are less harmful than inorganic compounds, are most abundant in food, while inorganic forms are mainly found in water, including that intended for human consumption [1].
The health effects of exposure to high levels of arsenic in drinking water are well documented in studies of populations exposed in endemic areas, such as some Asian countries (Bangladesh, Taiwan, Vietnam, India), Argentina, Chile, and several areas of the United States (Arizona, California and Nevada) [2,3,4,5,6,7,8,9,10]. Based on sufficient evidence of carcinogenicity in humans and limited evidence of carcinogenicity in animals, arsenic and inorganic arsenic compounds have been classified by the International Agency for Research on Cancer (IARC) in Group 1 (carcinogenic to humans), due to an increased risk of lung, bladder and skin cancers [11]. There is also limited evidence of carcinogenesis reported for cancers of the liver, kidney and prostate, and there are reports of the effects of exposure to high levels of arsenic in drinking water on non-oncological outcomes, such as cardiovascular and respiratory diseases, diabetes, cognitive development and adverse pregnancy outcomes [3, 9, 10, 12].
At lower doses, the effects of prolonged exposure to arsenic have not yet been fully characterized [12,13,14,15]. Adverse effects have been reported on various systems, such as foetal development, glucose metabolism, skin pigmentation and peripheral vascular diseases have been reported [9, 16,17,18]. However, the evidence available is insufficient to identify a dose–response relationship or a threshold below which effects on health are excluded [13, 16, 18].
The widespread presence of arsenic in groundwater is a common feature of several areas of Italy. Mt. Amiata is the second tallest volcano in Italy located in southern Tuscany (Central Italy), and covers an area of about 80 km2. The volcanic rock is highly permeable due to fractures and houses an important potable phreatic aquifer. Due to the good hydraulic qualities and the relatively large volume of the volcanic rock, this aquifer represents the main water resource for a large part of southern Tuscany [19]. The Mt. Amiata area has been the subject of intensive geological and geophysical investigations, mainly for geothermal resources [20,21,22]. There are two geothermal reservoirs in the area and these have been exploited industrially for electricity production [23]. Epidemiological studies have also been conducted to evaluate the effects of exposure to hydrogen sulphide and other emissions from geothermal power plants on population health [24,25,26,27]. Moreover, in the past Mt. Amiata was also known as a world-class mercury (Hg) mining district, where the metal occurred as cinnabar and had been exploited since Etruscan times [28,29,30]. Production increased sharply in the second half of the 1800s [31, 32], as indicated by Ferrara et al. [33], who reported that from 1860 to 1980, when all Hg mines were permanently closed, more than 100,000 t of Hg were produced in the Mt. Amiata mining district, and it was ranked as the 4th largest producing district in the world [34].
In 1998 according to new evidence from the literature [35], the legal limit for arsenic concentrations in drinking water was lowered from 50 µg/l to 10 µg/l (European Drinking Water Directive 98/83/EC, implemented in Italy through Legislative Decree 31/2001). For several years after the entry into force of the new regulatory standard, various municipalities in Italy, including in the Mt. Amiata area, were granted derogation. In 2010, the European Union resolved not to grant any further derogations and since then actions have been taken and structural works implemented to restore water quality. However, for decades populations in the Amiata area have been exposed to concentrations of arsenic in drinking water higher than the current regulatory limit.
The main objective of this study is to evaluate the long-term effects of chronic exposure to arsenic in drinking water in the area of Mt. Amiata, using a residential cohort study design.
Materials and methods
Definition of the cohort
The population-based cohort comprised all residents in the municipalities of Piancastagnaio, Abbadia San Salvatore, Arcidosso, Santa Fiora and Castel del Piano from 1 January 1998, and all those registered up to 31 December 2019. This is an open and dynamic cohort and all demographic movements were considered, such as births, immigration entries, emigration exits, deaths and changes of home address. The demographic archives transmitted by the registry offices of each municipality were subjected to quality control procedures, with the removal of duplicated entries, the correction of errors in the sequence of dates, and exclusion of subjects registered on the Registry of Italians Residing Abroad (AIRE). Geographical coordinates were assigned to each address and all subjects were included in a geographic information system (GIS). The georeferencing results were subjected to data quality analysis by overlapping with orthophotos and regional technical maps, to verify the degree of completeness and the level of precision of the geocoding process. Each subject of the study cohort was also assigned a value on the socio-economic deprivation index for the census tract where they live. This index is based on information on education, unemployment, number of rented dwellings, number of single-parent families with dependent children and housing density [36].
Arsenic concentrations in drinking water
Since 2005, the integrated water distribution system of the Mt. Amiata area has been operated by Acquedotto del Fiora Spa. According to the requirements established by Italian Legislative Decree 31/2001, arsenic is monitored at specific sampling points and a number of water samples are collected annually, proportional to population size and equally distributed in time and location. The analytical determination of arsenic is carried out by means of atomic absorption spectrometry and inductively coupled plasma mass spectrometry. Monitoring campaigns are carried out by the water supplier and the Local Health Authority. Acquedotto del Fiora provided data on arsenic concentration in tap water samples from 2005 to 2010. Data collected by the Local Health Authority have been available in paper format since 1988, but before 2001, when the new limit of 10 μg/l came into force, the arsenic measurements were fairly inaccurate and are not comparable with those taken in more recent years. Consequently, in our study we assumed that before 2005 the arsenic concentrations were stable in the study period based on the widely known levels of arsenic in groundwater, due to natural underlying geological processes and the absence of any mitigation action prior to 2010.
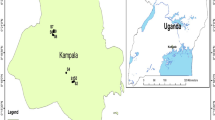
In addition to tap water data, the geographic coordinates of sampling points were also integrated into the geographic information system. Acquedotto del Fiora also divided the municipal territory into several supply units which deliver water directly to households. These supply units are portions of the territory considered to be homogeneous in terms of the type and quality of tap water distributed to households. They were identified by taking into account the quality of springs and groundwater, the characteristics of the water distribution system and the dilution procedures carried out by the water supplier. Figure 1 shows the map of the supply units for the five municipalities under study, with indication of the water sampling points (green stars) and the homes of the cohort members (blue dots).
Long-term exposure to arsenic concentrations in tap water was analysed for each indivudual. Each set of home coordinates was overlapped with the water supply unit using the GIS spatial join function, applying the average arsenic concentration in the period 2005–2010. For homes that did not fall within the supply units (rural locations) the subjects were assigned the arsenic concentrations of the nearest water utility, using the “point-to-point” spatial join.
To estimate the long-term exposure to arsenic in drinking water, we calculated a time-weighted average exposure (TWA) for each cohort member, adding for each home the arsenic levels multiplied by the time they lived at this address, divided by the total observation time, according to the following formula:
where Asi (in µg/l) is the average 2005–2010 concentration of arsenic at the i-th address and Di is the time lived at the i-th address.
Follow-up procedures and health data
For each subject of the cohort, we performed the vital status follow-up from 01/01/1998 to 31/12/2019, using the municipal registry database. For subjects who died in the period 1998–2016, the cause of death was attributed using data from the Tuscany Regional Mortality Register (RMR), active since 1987, which records the deaths of residents of the Tuscany region, occurring both inside and outside the region. The procedure for identifying the cause of death was anonymous using a code applied by the Tuscany region for the purpose of protection of privacy.
Hospital admissions were analysed using data from the Hospital Discharge Records (HDR) of the Tuscany region, related to ordinary and day-hospital admissions, occurring inside and outside the region in the period 1998–2019. Considering incident cases only, a subject hospitalized several times for the same disease was counted only once, using the date of the first hospital admission. Furthermore, analyses only considered the primary diagnosis at discharge.
Statistical analysis
Each member of the cohort contributed to the calculation of person-years at risk starting from 1 January 1998 if living in the municipality on this date or starting from the date of their registration on the municipal registry if entering the municipality after 01/01/1998, up to the date of death, emigration or end of follow-up (31 December 2016 for mortality analyses and 31 December 2019 for hospitalization analyses).
The associations between long-term exposure to arsenic in drinking water and mortality/morbidity outcomes were evaluated using Cox proportional hazard models, to estimate hazard ratios (HR) and 95% confidence intervals [37]. The Cox models used age as the time scale and the confounding effects of gender, socio-economic deprivation indicator and calendar periods were taken into account. The assumption of the proportionality of the risks was tested using the Mantel-Cox test and, if violated, stratified Cox models were applied.
Two distinct approaches were used to assess exposure. In the first approach, the TWA variable was introduced to the models as a two-level categorical variable, comparing concentrations greater than 10 µg/l with concentrations equal to or lower than 10 µg/l. In the second approach, to assess the risks associated with arsenic concentrations below the regulatory limit of 10 µg/l, the TWA variable was introduced to the models as a three-level categorical variable, using concentrations ≤ 5 µg/ l as the reference group and two increasing exposure groups, 5–10 µg/l and ≥ 10 µg/l. P-values were calculated in the three-level models to identify trends.
Results
The cohort included 30,910 residents, 14,970 men and 15,940 women, for a total of 407,213 person-years. The main socio-demographic characteristics of the cohort are shown in Table 1.
Abbadia San Salvatore was the most populous municipality and made up 29.8% of the study cohort. About 60% of the population had lived in the study area for more than 15 years, and 54.8% had a high socio-economic status. A very high proportion of georeferenced addresses (98%) and good accuracy were achieved, also thanks to the manual recovery of non-geocoded addresses using automated procedures.
Table 2 shows the main characteristics of the drinking water supply units, reporting the number of water sampling points, the number of people served and the average arsenic concentrations in the period 2005–2010.
Table 2 shows the spatial variability of arsenic levels, with the highest value recorded in Unit 6 of Abbadia San Salvatore ASSDQ6 (mean arsenic concentration = 14.37 µg/l). Conversely, the lowest value is observed in Unit 2 of Arcidosso ARCDQ2 (mean arsenic concentration = 0.66 µg/l).
Cohort members were exposed to a time-weighted average (TWA) concentration of arsenic equal to 9.2 µg/l (range: 0.7–14.4 µg/l). Table 3 shows the distribution of the cohort members by gender, age and socio-economic status for the two classes of exposure to arsenic in drinking water.
Forty-four percent of the cohort members were exposed to arsenic concentrations higher than the current regulatory limit. There were no significant differences by gender and age. At the same time there was a significant association between arsenic exposure and socio-economic status, with a higher percentage of socio-economically disadvantaged people in the group of those exposed to arsenic values > 10 µg/l (p < 0.0001).
Associations between health outcomes and two-level As exposure
Table 4 shows the results of the associations between arsenic concentrations in drinking water > 10 µg/l and mortality/hospitalization outcomes, compared to the reference class (TWA ≤ 10 µg/l). We found higher risks of non-accidental mortality (HR = 1.07 95%CI: 1.01–1.13), more pronounced in women (HR = 1.10 95%CI: 1.02–1.19) than in men (HR = 1.04 95%CI: 0.95–1.12). Mortality for malignant cancer showed a positive association for women only (HR = 1.14 95%CI: 0.97–1.35). Long-term exposure to arsenic concentrations > 10 µg/l resulted positively associated with multiple hospitalization outcomes. Higher risks were observed for hospital admissions for non-accidental causes (HR = 1.06 95%CI: 1.03–1.09), both in women (HR = 1.07 95%CI: 1.03–1.12) and in men (HR = 1.05 95%CI: 1.01–1.10), for malignant neoplasms (HR = 1.10 95%CI: 1.02–1.19; women HR = 1.19 95%CI: 1.07–1.32), for lung cancer (HR = 1.85 95%CI: 1.14–3.02) and breast cancer (HR = 1.23 95%CI: 0.99–1.51) in women, for endocrine disorders (HR = 1.13 95%CI: 1.02–1.26; women HR = 1.17 95%CI: 1.02–1.35), for cardiovascular diseases (HR = 1.12 95%CI: 1.06–1.18; women HR = 1.13 95%CI: 1.05–1.22; men HR = 1.10 95%CI: 1.03–1.19), particularly for ischaemic heart disease (HR = 1.11 95%CI: 0.99–1.24), hypertensive disease (HR = 1.21 95%CI: 0.99–1.48), heart failure (HR = 1.14 95%CI: 1.01–1.29; women HR = 1.17 95%CI: 0.99–1.37), cerebrovascular diseases (HR = 1.07 95%CI: 0.98–1.17) and respiratory diseases (HR = 1.10 95%CI: 1.03–1.18; women HR = 1.11 95%CI: 1.01–1.23; men HR = 1.10 95%CI: 1.00–1.20).
Associations between health outcomes and three-level As exposure
Table 5 shows the HR and 95% confidence intervals referring to associations between mortality/hospitalization outcomes and exposure to arsenic in drinking water, using the TWA metric as a three-level categorical variable. Arsenic concentrations < 5 µg/l were selected as the reference class and two increasing exposure groups were considered: exposure below the regulatory standard (5–10 µg/l) and above (> 10 µg/l). The excess for non-accidental mortality was confirmed in the highest exposure group (HR 1.13 95%CI: 1.02–1.26) and a significant trend was observed (p = 0.009). Several associations were also highlighted in hospital admissions, in relation to the exposure group 5–10 µg/l, though the division into three groups generates a greater instability of the estimates. Compared to models with two-level TWA metric, the excess risks associated with the highest exposure class are mostly confirmed, such as hospitalizations for non-accidental causes, for endocrine disorders and cardiovascular diseases. However, results also showed increased risks for the intermediate exposure group (5–10 µg/l) and significant exposure related trend. This is the case for hospitalizations for ischaemic heart disease (5–10 µg/l class HR = 1.26 95%CI: 1.00–1.59), prostate cancer (p = 0.041), endocrine disorders (p = 0.015), cardiovascular diseases (p < 0.001), heart failure (p = 0.019) and respiratory diseases (p = 0.014).
Discussion
This residential cohort study in the Mt. Amiata area found that chronic exposure to arsenic concentrations in drinking water above the current regulatory limit is associated with a plurality of diseases. Whilst some of these associations were observed in both men and women, women showed increased risks for several outcomes, in the analyses of both mortality and hospitalization. Positive associations were reported for non-accidental causes, malignant neoplasms, endocrine disorders, cardiovascular, and respiratory diseases. Moreover, our study also revealed some signs of associations even at very low exposure levels, below the current regulatory limit, in subjects exposed to long-term arsenic concentrations in the range 5–10 µg/l. Significant exposure-related trends were observed for ischaemic heart disease, prostate cancer, endocrine disorders, cardiovascular diseases, heart failure and respiratory diseases. However, there was high uncertainty of these estimates due to the low number of cases, mainly in the reference class of subjects exposed to arsenic concentrations < 5 µg/l.
We found a positive association between arsenic exposure and lung cancer in females. Conflicting results have been obtained in studies of arsenic and cancer conducted in non-endemic areas [38,39,40,41,42]. Many studies focus on the effects on lung and bladder cancer and the findings reported are inconsistent [14, 15, 43,44,45]. Considering the available evidence on biological plausibility, our findings are coherent with the IARC evaluation regarding the carcinogenic role of arsenic on lung cancer [11]. We also find a positive association with breast cancer, for which there is limited epidemiological evidence in both high and low dose studies [11].
Our results also showed a consistent association with cardiovascular diseases in both genders, specifically for ischaemic heart diseases, hypertension, heart failure and cerebrovascular diseases. These findings are supported by a series of recent surveys on populations exposed to low or moderate arsenic concentrations, which have shown preclinical heart damage, such as increased mean intimal thickness, carotid plaques, endothelial dysfunction and vascular inflammation [9, 46].
In addition, we found consistent associations between arsenic exposure and respiratory diseases in both genders. For chronic obstructive pulmonary disease (COPD), women showed a higher risk than men. Our findings are supported by many international studies that have analysed the effects of arsenic on symptoms and respiratory function [17]. Some possible mechanisms of action have been formulated, such as the accumulation of arsenic in the pulmonary epithelium [17] or early epithelial changes detected by serum Clara cell protein CC16 concentrations [47]. All these findings, including those from our population-based cohort study in the Mt. Amiata area, significantly contribute to the debate on lowering the limit for arsenic concentrations in drinking water [48, 49].
Our study has several important strengths. First, the study benefited from the considerable size of the investigated population, which enabled the evaluation of associations with various mortality and hospitalization outcomes. Second, the longitudinal study design allowed us to assess indidually the time at risk for each resident in the study region. The reconstruction of the open and dynamic historical cohort was very accurate, and all residential movements were taken into account, including intra-municipality changes of address. Links to regional health administrative data allowed us to follow up on the study participants continuously and very effectively, reducing the risk of selection, recall or non-response bias. The long follow-up period allowed us to evaluate the arsenic exposure effects for long-latency pathologies, such as cancer. The use of GIS technology is in line with other international studies [14, 50, 51] and it enabled us to estimate the long-term exposure to arsenic in drinking water on an individual level, considering the time lived at each address. Based on a complete and accurate regional geographical database, the geocoding process provided high-quality and complete geocoded addresses, limiting the risk of exposure misclassification. In the cohort study in Amiata a time-weighted average of arsenic levels in drinking water was derived from data collected by the water supplier over the period 2005–2010. Before 2005, the only data available were those collected by the Local Health Authority but, for reasons of poor accuracy and comparability, we chose not to use them in the exposure assessment. However, available data assume that arsenic concentrations were constant before 2005.
There are some limitations to our research. Our study is population-based and we used administrative health databases with routinely collected data. This approach means that data on other potential individual risk factors, such as consumption of tobacco and alcohol, physical activity, diet and obesity, were not available and results could involve confounding bias. However, many personal habits are associated with socio-economic status; we applied the social deprivation index to adjust in our models which could have been limited bias due to unmeasured confounders. Nevertheless, having used an aggregated socioeconomic indicator at the census tract level does not eliminate the risk of ecological fallacy that may have led to misclassification on an individual level. Our exposure assessment only considered arsenic levels in drinking water, and we cannot exclude the fact that the real population exposure may have been underestimated, due to all potential sources other than drinking water. The potential role of the use of water from non-controlled private wells or the ingestion of contaminated food, such as locally produced vegetables or fruits, were not considered. Real arsenic intake is not known and is very difficult to quantify; it has been estimated that 35–45% of inorganic arsenic exposure in populations residing in non-endemic areas is attributable to the consumption of drinking water, 30% to the use of water to prepare food and the remaining 25–30% to food intake [52]. Regarding other potential sources of environmental exposure, in the same study area we previously investigated the role of long-term exposure to emissions from geothermal power plants, especially hydrogen sulphide, using the same population-based cohort [27]. Results indicated no associations between increased hydrogen sulphide levels and mortality/hospitalization outcomes, except for respiratory diseases. We considered both environmental exposures, arsenic in drinking water and atmospheric hydrogen sulphide in sensitivity analyses. In bi-pollutant models, associations with long-term exposure to arsenic in drinking water persisted for all outcomes.
To conclude, our findings from the population-based cohort study in the volcanic area of Mt. Amiata reinforce the existing evidence of the adverse effects of long-term exposure to low-level arsenic in drinking water. These results contribute to the current debate on the dose–response model and the need for a more protective threshold for arsenic risk assessment. However, our study in an area of naturally occurring arsenic highlights the need for continuous monitoring of arsenic concentrations and for policies to reduce concentrations in the environment as far as possible.
Availability of data and materials
There are legal restrictions on sharing a de-identified data set because data contain potentially identifying and sensitive people information (age, gender, residence, health status). Aggregated data may be requested by writing to Daniela Nuvolone at: daniela.nuvolone@ars.toscana.it.
Abbreviations
- As:
-
Arsenic
- TWA:
-
Time-weighted average
- Hg:
-
Mercury
- HR:
-
Hazard ratio
- CI:
-
Confidence interval
- SD:
-
Standard deviation
- COPD:
-
Chronic obstructive pulmonary disease
- WHO:
-
World Health Organization
- IARC:
-
International Agency for Research on Cancer
- GIS:
-
Geographical information system
References
Agency for Toxic Substances and Disease Registry. Toxicological Profile for Arsenic. U.S. Department of health and human services. Public Health Service. 2007. https://www.atsdr.cdc.gov/toxprofiles/tp2.pdf.
Bates MN, Rey OA, Biggs ML, Hopenhayn C, Moore LE, Kalman D, Steinmaus C, Smith AH. Case-control study of bladder cancer and exposure to arsenic in Argentina. Am J Epidemiol. 2004;159(4):381–9.
Celik I, Gallicchio L, Boyd K, Lam TK, Matanoski G, Tao X, Shiels M, Hammond E, Chen L, Robinson KA, Caulfield LE, Herman JG, Guallar E, Alberg AJ. Arsenic in drinking water and lung cancer: a systematic review. Environ Res. 2008;108(1):48–55.
Hopenhayn-Rich C, Biggs ML, Smith AH. Lung and kidney cancer mortality associated with arsenic in drinking water in Córdoba. Argentina Int J Epidemiol. 1998;27(4):561–9.
Yuan Y, Marshall G, Ferreccio C, Steinmaus C, Liaw J, Bates M, Smith AH. Kidney cancer mortality: fifty-year latency patterns related to arsenic exposure. Epidemiology. 2010;21(1):103–8.
Lewis DR, Southwick JW, Ouellet-Hellstrom R, Rench J, Calderon RL. Drinking water arsenic in Utah: A cohort mortality study. Environ Health Perspect. 1999;107(5):359–65.
Wadhwa SK, Kazi TG, Chandio AA, Afridi HI, Kolachi NF, Khan S, Kandhro GA, Nasreen S, Shah AQ, Baig JA. Comparative study of liver cancer patients in arsenic exposed and non-exposed areas of Pakistan. Biol Trace Elem Res. 2011;144(1–3):86–96.
Navas-Acien A, Silbergeld EK, Streeter RA, Clark JM, Burke TA, Guallar E. Arsenic exposure and type 2 diabetes: a systematic review of the experimental and epidemiological evidence. Environ Health Perspect. 2006;114(5):641–8.
Moon K, Guallar E, Navas-Acien A. Arsenic exposure and cardiovascular disease: an updated systematic review. Curr Atheroscler Rep. 2012;14(6):542–55.
Sanchez TR, Perzanowski M, Graziano JH. Inorganic arsenic and respiratory health, from early life exposure to sex-specific effects: A systematic review. Environ Res. 2016;147:537–55.
International Agency for Research on Cancer IARC. Arsenic and arsenic compounds. IARC Monogr Eval Carcinog Risks Hum. 2012;100C:41–93.
Maull EA, Ahsan H, Edwards J, Longnecker MP, Navas-Acien A, Pi J, Silbergeld EK, Styblo M, Tseng CH, Thayer KA, Loomis D. Evaluation of the association between arsenic and diabetes: a National Toxicology Program workshop review. Environ Health Perspect. 2012;120(12):1658–70.
Begum M, Horowitz J, Hossain MI. Low-dose risk assessment for arsenic: a meta-analysis approach. Asia Pac J Public Health. 2015;27(2):NP20-35.
Baastrup R, Sørensen M, Balstrøm T, Frederiksen K, Larsen CL, Tjønneland A, Overvad K, Raaschou-Nielsen O. Arsenic in drinking-water and risk for cancer in Denmark. Environ Health Perspect. 2008;116(2):231–7.
Mink PJ, Alexander DD, Barraj LM, Kelsh MA, Tsuji JS. Low-level arsenic exposure in drinking water and bladder cancer: a review and meta-analysis. Regul Toxicol Pharmacol. 2008;52(3):299–310.
Chen Y, Parvez F, Gamble M, Islam T, Ahmed A, Argos M, Graziano JH, Ahsan H. Arsenic exposure at low-to-moderate levels and skin lesions, arsenic metabolism, neurological functions, and biomarkers for respiratory and cardiovascular diseases: review of recent findings from the Health Effects of Arsenic Longitudinal Study (HEALS) in Bangladesh. Toxicol Appl Pharmacol. 2009;239(2):184–92.
Parvez F, Chen Y, Brandt-Rauf PW, Slavkovich V, Islam T, Ahmed A, Argos M, Hassan R, Yunus M, Haque SE, Balac O, Graziano JH, Ahsan H. A prospective study of respiratory symptoms associated with chronic arsenic exposure in Bangladesh: findings from the Health Effects of Arsenic Longitudinal Study (HEALS). Thorax. 2010;65(6):528–33.
Myers SL, Lobdell DT, Liu Z, Xia Y, Ren H, Li Y, Kwok RK, Mumford JL, Mendola P. Maternal drinking water arsenic exposure and perinatal outcomes in inner Mongolia. China J Epidemiol Community Health. 2010;64(4):325–9.
Doveri M, Nisi B, Cerrina Feroni A, Ellero A, Menichini M, Lelli M, Masetti G, Da Prato S, Principe C, Raco B. Geological, hydrodynamic and geochemical features of the volcanic aquifer of Mt Amiata (Tuscany, Central Italy): an overview. Acta Vulcanologica. 2012;23/24:51–72.
Calamai A, Cataldi R, Squarci P, Taffi L. Geology, Geophysics ah Hydrogeology of the Monte Amiata Geothermal Fields. Geothermics 1970 Special Issue 1.
Gianelli G, Puxeddu M, Batini F, Bertini G, Dini I, Pandeli E, Nicolich R. Geological model of a young volcanoplutonic system: the geothermal region of Monte Amiata (Tuscany Italy). Geothermics. 1988;17:719–34.
Brogi A. The structure of the Monte Amiata volcanogeothermal area (Northern Apennines, Italy): NeogeneQuaternary compression versus extension. Int J Earth Sci. 2008;97:677–703.
Razzano F, Cei M. Geothermal power generation in Italy 2010–2014 update report. Proceedings World Geothermal Congress 2015 Melbourne, Australia. 2015.
Bustaffa E, Minichilli F, Nuvolone D, Voller F, Cipriani F, Bianchi F. Mortality of populations residing in geothermal areas of Tuscany during the period 2003–2012. Ann Ist Super Sanita. 2017;53(2):108–17.
Minichilli F, Nuvolone D, Bustaffa E, Cipriani F, Vigotti MA, Bianchi F. Stato di salute delle popolazioni residenti nelle aree geotermiche della Toscana [State of health of populations residing in geothermal areas of Tuscany]. Epidemiol Prev. 2012;36(5 Suppl 1):1–104 (Italian).
Nuvolone D, Petri D, Biggeri A, Barbone F, Voller F. Health effects associated with short-term exposure to hydrogen sulfide from geothermal power plants: a case-crossover study in the geothermal areas in Tuscany. Int Arch Occup Environ Health. 2020;93(6):669–82.
Nuvolone D, Petri D, Pepe P, Voller F. Health effects associated with chronic exposure to low-level hydrogen sulfide from geothermoelectric power plants. A residential cohort study in the geothermal area of Mt. Amiata in Tuscany. Sci Total Environ. 2019;659:973–82.
Strappa D. Storia delle miniere di mecurio del M. Amiata Ind Min. 1977;28:252–9 (Italian).
Barghigiani C, Ristori T. The distribution of mercury in a Mediterranean area. In: Watras CJ, Huckabee JW, editors. Mercury Pollution: Integration and synthesis. Boca Raton: Lewis Publishers; 1994. p. 41–9.
Ferrara R, Maserti BE, Breder R. Mercury in abiotic and biotic compartments of an area affected by a geochemical anomaly (Mt. Amiata, Italy). Water Air Soil Pollut. 1991;56:219–33.
Cipriani C, Tanelli G. Risorse minerarie ed industria estrattiva in Toscana. Note storiche ed economiche. Atti e Memorie Accademia di Scienze e Lettere La Colombaria. 1983;28:241–83 (Italian).
Vaselli O, Higueras P, Nisi B, María Esbrí J, Cabassi J, Martínez-Coronado A, Tassi F, Rappuoli D. Distribution of gaseous Hg in the Mercury mining district of Mt. Amiata (Central Italy): a geochemical survey prior the reclamation project. Environ Res. 2013;125:179–87.
Ferrara R, Mazzolai UB, Edner H, Svanberg S, Wallinder E. Atmospheric mercury sources in the Mt. Amiata area. Italy Sci Total Environ. 1998;213:12–23.
Rimondi V, Gray JE, Costagliola P, Vaselli O, Lattanzi P. Concentration, distribution, and translocation of mercury and methylmercury in mine-waste, sediment, soil, water, and fish collected near the Abbadia San Salvatore mercury mine, Monte Amiata district. Italy Sci Total Environ. 2012;414:318–27.
World Health Organization. Guidelines for Drinking-water Quality, 2nd edition. Volume 1. Recommendations. WHO, Geneva; 1993. http://www.who.int/water_sanitation_health/dwq/2edvol1i.pdf?ua=1.
Caranci N, Biggeri A, Grisotto L, Pacelli B, Spadea T, Costa G. The Italian deprivation index at census block level: definition, description and association with general mortality. Epidemiol Prev. 2010;34:167–76.
Cox DR. Regression Models and Life-Tables. J R Stat Soc Series B. 1972;34:187–220.
Cantor KP. Lubin JH Arsenic, internal cancers, and issues in inference from studies of low-level exposures in human populations. Toxicol Appl Pharmacol. 2007;222(3):252–7.
Lewis DR, Southwick JW, Ouellet-Hellstrom R, Rench J. Calderon RL Drinking water arsenic in Utah: A cohort mortality study. Environ Health Perspect. 1999;107(5):359–65.
Ferreccio C, Gonzalez Psych C, Milosavjlevic Stat V, Marshall Gredis G, Sancha AM. Lung cancer and arsenic exposure in drinking water: a case-control study in northern Chile. Cad Saude Publica. 1998;14(suppl 3):193–8.
Karagas MR, Stukel TA, Morris JS, Tosteson TD, Weiss JE, Spencer SK, et al. Skin cancer risk in relation to toenail arsenic concentrations in a US population-based case-control study. Am J Epidemiol. 2001;153(6):559–65.
Karagas MR, Stukel TA, Tosteson TD. Assessment of cancer risk and environmental levels of arsenic in New Hampshire. Int J Hyg Environ Health. 2002;205(1–2):85–94.
Lamm SH, Engel A, Kruse MB, Feinleib M, Byrd DM, Lai S, et al. Arsenic in drinking water and bladder cancer mortality in the United States: an analysis based on 133 U.S. counties and 30 years of observation. J Occup Environ Med. 2004;46(3):298–306.
Heck JE, Andrew AS, Onega T, Rigas JR, Jackson BP, Karagas MR, et al. Lung cancer in a U.S. population with low to moderate arsenic exposure. Environ Health Perspect. 2009;117(11):1718–23.
García-Esquinas E, Pollán M, Umans JG, Francesconi KA, Goessler W, Guallar E, Howard B, Farley J, Best LG, Navas-Acien A. Arsenic exposure and cancer mortality in a US-based prospective cohort: the strong heart study. Cancer Epidemiol Biomarkers Prev. 2013;22(11):1944–53.
Wu F, Molinaro P, Chen Y. Arsenic Exposure and Subclinical Endpoints of Cardiovascular Diseases. Curr Environ Health Rep. 2014;1:148–62.
Parvez F, Chen Y, Brandt-Rauf PW, Bernard A, Dumont X, Slavkovich V, Argos M, D’Armiento J, Foronjy R, Hasan MR, Eunus HE, Graziano JH, Ahsan H. Nonmalignant respiratory effects of chronic arsenic exposure from drinking water among never-smokers in Bangladesh. Environ Health Perspect. 2008;116(2):190–5.
Schmidt CW. Low-dose arsenic: in search of a risk threshold. Environ Health Perspect. 2014;122(5):A130–4.
Sauvé S. Time to revisit arsenic regulations: comparing drinking water and rice. BMC Public Health. 2014;14:465.
Meliker JR, Slotnick MJ, Avruskin GA, Kaufmann A, Fedewa SA, Goovaerts P, Jacquez GJ, Nriagu JO. Individual lifetime exposure to inorganic arsenic using a space-time information system. Int Arch Occup Environ Health. 2007;80(3):184–97.
Hough RL, Fletcher T, Leonardi GS, Goessler W, Gnagnarella P, Clemens F, Gurzau E, Koppova K, Rudnai P, Kumar R, Vahter M. Lifetime exposure to arsenic in residential drinking water in Central Europe. Int Arch Occup Environ Health. 2010;83(5):471–81.
Kurzius-Spencer M, Burgess JL, Harris RB, Hartz V, Roberge J, Huang S, Hsu CH, O’Rourke MK. Contribution of diet to aggregate arsenic exposures-an analysis across populations. J Expo Sci Environ Epidemiol. 2014;24(2):156–62.
Acknowledgements
We would like to thank Acquedotto del Fiora Spa for making available to the researchers their arsenic sampling data and water distribution system information, and Giulia Bartoccini for her support in assembling the dataset.
Funding
This work was supported by a regional funding provided by Regione Toscana – Direzione Generale Politiche Ambientali, Energia e Cambiamenti Climatici (Resolution N. 973, 10 November 2014). The funding body took no part in the study design, data collection, analysis and interpretation of data, the writing of the report, the decision to submit the article for publication.
Author information
Authors and Affiliations
Contributions
DN: conceptualization, methodology, interpretation and manuscript writing. GS: methodology, formal analysis, interpretation and major contributor in writing the manuscript. DP: data management, formal analysis, interpretation and major contributor in editing the manuscript. FV: conceptualization, methodology, supervision and major contributor in editing the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable. In Italy, ethics committee approval and informed consent from patients were not required for observational studies based on administrative health database (Regulation (EU) 2016/679 of the European Parliament and of the Council of 27 April 2016, Legislative Decree 30 June 2003, n. 196). This study does not include experiments on humans and/or the use of human tissue samples. Consequently, for this study no institutional and/or licensing committee approval or informed consent were necessary. The study was carried out according to the principles and guidelines of the Helsinki Declaration.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Nuvolone, D., Stoppa, G., Petri, D. et al. Long-term exposure to low-level arsenic in drinking water is associated with cause-specific mortality and hospitalization in the Mt. Amiata area (Tuscany, Italy). BMC Public Health 23, 71 (2023). https://doi.org/10.1186/s12889-022-14818-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12889-022-14818-x