Abstract
Tuberculosis (TB) disproportionally affects persons and families who are economically and socially disadvantaged. Therefore, a patient cost survey was conducted in Tanzania to evaluate the costs incurred by patients and their households before and after the diagnosis of TB. It was the first survey in Tanzania to ascertain baseline information and experience for subsequent surveys. This paper aims to share the experience encountered during the survey to ensure a standardized approach and elimination of potential barriers for the implementation of future surveys. A total of 777 TB patients from 30 clusters selected based on probability proportional to the size were interviewed during the study period. As the sample size was calculated based on notification data from the previous year, some health facilities experienced an inadequate number of TB patients during the study to meet the allocated cluster size for the survey. Most facilities had poor recording and recordkeeping in TB registers where deaths were not registered, and some patients had not been assigned district identification numbers. Fixed days for TB drug refills in health facilities affected the routine implementation of the survey as the interviews were conducted when patients visited the facility to pick up the drugs. Tablets used to collect data failed to capture the geographic location in some areas. The households of TB patients lost to follow-up and those who had died during TB treatment were not included in the survey. When planning and preparing for patient costs surveys, it is important to consider unforeseen factors which may affect planned activities and findings. During the survey in Tanzania, the identified challenges included survey logistics, communications, patient enrollment, and data management issues. To improve the quality of the findings of future surveys, it may be reasonable to revise survey procedures to include households of TB patients who were lost to follow-up and those who died during TB treatment; the households of such patients may have incurred higher direct and indirect costs than households whose patient was cured as a result of receiving TB treatment.
Similar content being viewed by others
Background
Tuberculosis (TB) is a public health problem posing challenges to many families and countries worldwide [1]. The disease disproportionally affects disadvantaged persons both socially and economically. In most developing countries, TB care and treatment are provided free of charge to ensure universal access to care and reduce the economic burden on the individual and health system [2,3,4]. However, many TB patients and families still face high direct (medical and non-medical) and indirect costs due to TB illness and healthcare-seeking, which hampers access and puts the economically vulnerable population at higher risk of impoverishment [5,6,7,8,9]. These costs may be associated with longer treatment periods varying from six months for drug-sensitive TB (DS-TB) to two years for drug-resistant TB (DR-TB). Non-medical costs, such as costs for travel and food during health seeking, have been reported to be a major part of direct non-medical costs for patients, limiting access and adherence to treatment and care, which eventually affects the clinical health outcomes of patients [1, 5, 9]. By addressing barriers and reasons for the delay in initiation of TB treatment, costs incurred by TB patients can be effectively reduced [9].
Until recently, the magnitude of the economic burden incurred by TB patients and their households in Tanzania was unknown since programmatic data on costs incurred by patients while on TB care have not been collected. This information is critical for developing policies aligning with Universal Health Coverage (UHC) and aligning country efforts with global strategies to end TB by 2030 as outlined in the Sustainable Development Goals (SDGs) [10, 11]. As such, the Ministry of Health, Community Development, Gender, Elderly and Children (MOHCDGEC), in collaboration with the National Institute for Medical Research (NIMR), World Health Organization (WHO), and the U.S. Centers for Disease Control and Prevention (CDC) conducted a TB Patient Cost survey in Tanzania to evaluate the costs incurred by patients and their households before and after diagnosis of TB. The study methods and results are available elsewhere [12]. This was the first survey in Tanzania to derive baseline information and experience for subsequent surveys to be conducted regularly. The current publication aims to share the lessons learned during the implementation of the survey to inform researchers on appropriate planning and implementation of future surveys.
Survey implementation and lessons learned
-
1.
Survey organization
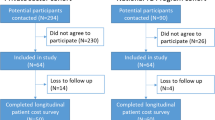
The first nationally representative cross-sectional facility-based TB Patient Cost survey was conducted in August–September 2019 at Tanzania's 30 facilities (clusters). The clusters were represented by facilities and selected based on probability proportional to the size of the respective facility according to the number of TB patients notified in the facility in 2017. Seven hundred seventy-seven TB patients, including children, were enrolled in the study and interviewed (no age limit was applied). All patients signed an informed consent form before the interview. In addition, parents/guardians of children under 18 years old provided informed consent for the child and responded to the interview questions. All responses were recorded using tablets in real-time in the field and uploaded to a secured web-based server for data storage. The generic electronic survey tool developed by WHO and adapted to the Tanzania context was utilized for data collection. Patients were compensated in cash for the travel and time spent during the interview. Parents or guardians of the children under 18 years who enrolled in the survey received the same compensation.
Survey teams
The survey implementation team consisted of officers from the National TB and Leprosy Program (NTLP), national consultants and researchers from the NIMR with different professional backgrounds, and external consultants from CDC and WHO. The implementation team coordinated all survey operations. Consultants from CDC and WHO monitored the survey implementation and provided technical support to ensure quality and scientifically sound results. Five field teams with three interviewers each were formed for data collection. The composition of the field teams took into consideration individual professional qualifications to ensure there was at least one clinician and one sociologist on each team. Each field team had a leader who was a senior and experienced researcher responsible for planning the team's daily activities, solving field challenges, and maintaining cohesion among team members.
-
2.
Survey implementation
Planning and execution of the TB patient cost survey is a comprehensive process and involves several steps which must be accomplished.
Planning and organization of the survey
The availability of an implementation plan with clearly defined strategies and actions made it possible to effectively collect quality and credible data within the intended period of the survey in Tanzania. In addition, the existence of a well-functioning NTLP infrastructure (TB clinics and TB health workers) and the availability of TB treatment documentation were key to the success of the survey data collection.
Recruitment and training of data collectors/interviewers
Appropriate selection of the interviewers was crucial to ensure effective data collection. The interviewers were recruited from the Tanzania National Institute of Medical Research (NIMR) staff. They had prior experience working in settings where the survey was conducted. After recruitment, the interviewers were trained for five days. The training focused on the study protocol, objectives, ethical issues, and data collection tools.
As a result, the experienced field team members, with the acquired skills and knowledge of their responsibilities, completed the enrollment and interviews within two months, in contrast with other similar studies [7, 13, 14] where the data collection took much longer.
Survey pilot
Field testing of the data collection tool and patient interviews was done in three health facilities that provided TB services; refinement of the questionnaire followed the pilot exercise. The health facilities where pilot testing was conducted were not among those selected for the main survey. We learned that allocating sufficient time for training the interviewers directly influenced the quality of collected information. The electronic data collection tool required sufficient time for training and rehearsal. In addition, training and piloting provided before the survey strengthened the ability of the interviewers to use the tablets and collect information in real time.
Availability of reliable transport
Each field team was facilitated with a well-maintained vehicle equipped with field-experienced drivers throughout the data collection period. Having a suitable vehicle and an experienced driver was an important logistical component of the successful implementation of the survey as it enabled timely visiting of the health facilities according to the field team schedule.
Communication and coordination
Rapid and effective communication was attained through the social networking application, e.g., WhatsApp on mobile devices, facilitating data collection for the survey. Mobile phones were used to communicate with the authorities of the district and facility, especially when an immediate response was required. Any challenge experienced during data collection in the field was communicated and shared immediately to minimize carrying forward mistakes in data collection. Challenges were shared, but no patient personal information was released through the social network. Prior communication with the selected sites made the survey implementation much easier and contributed to the successful survey operations. Scheduled interviews reduced the chances of jeopardizing the quality of interviews at the TB clinic by allowing sufficient time for each interview with 2–3 interviews per day.
The backup mechanism in case of electricity interruption
The Field teams were equipped with portable batteries, which had a sufficient storage capacity for charging the tablets and mobile phones in case the teams needed to travel long distances and work in rural areas where electricity was not available. Also, a few copies of the questionnaires in Kiswahili and English languages were printed and carried out by the interviewers for better familiarization with the questions. The paper questionnaires were used in case the tablets had technical faults during interviews. The team leaders were responsible for ensuring the team members charged their tablets and mobile phones before fieldwork.
Survey monitoring
Survey activities were monitored through coordination and supportive supervision to ensure the implementation of the survey according to the protocol and high quality of the collected data. Each field team provided a weekly report to the Survey Coordinator to allow documentation and evaluation of the team’s performance. Daily team meetings were convened in the field to discuss the challenges encountered before uploading the data to the central server. Challenges found to be too complex and requiring further technical inputs were elevated to the Survey Coordinator. The uploaded data were monitored and checked for consistency, accuracy, completeness, and quality daily by the survey data manager in the central survey office in Dar es Salaam who generated correspondent queries and shared them with the teams. This approach provided an opportunity for the survey implementation team to review collected data and provide their feedback and technical advice to the field teams as needed.
At some points, the survey field teams served as program review teams since programmatic issues and challenges needing immediate action were raised during some of the visits. These included high death rates of TB patients after starting treatment in some districts; shortage of anti-TB medications, especially pediatric formulations; delays in updating TB registers; delays in treatment initiation; the presence of some patients who had completed a full course of anti-TB treatment yet were still kept on treatment, and others. During the implementation of the survey, health care workers were mentored by the field teams to follow the National Guidelines for TB Care and Treatment. Observed and reported deviations from guidelines were immediately reported to the district and regional TB coordinators.
External technical assistance
Consultants from the CDC and WHO provided technical assistance during the survey implementation. Skype meetings between the in-country survey implementation team and external survey consultants were conducted regularly to evaluate the quality of the collected data. The guidance provided by subject matter experts enabled an efficient and high degree of success in implementing the survey.
-
3.
Challenges and lessons learned
Some challenges, including survey logistics, patient enrollment, communications, and data management, were encountered during the implementation of the survey.
Survey logistics and communications
Official letters to introduce the field teams to the regional and district administrative authorities were sent two weeks before the commencement of the survey. However, when field teams visited the regions and districts for data collection, it was reported that some of the letters had not been seen by the administrative offices. Arrangements for emergency officiation of the visits were done but caused substantial inconvenience for the survey teams. A similar situation caused a two-week delay in Zanzibar, where during the first site visit the field team learned approval from the Zanzibar ethical review committee was required in addition to a committee on the Tanzania mainland.
Some facilities selected for the survey did not have an adequate number of TB patients to meet the required cluster sample size requirement during the study period as the selection was based on the TB notifications from the previous year. In addition, some of the selected health facilities were not in operation at the time of the study visit, and TB patients had been transferred to other health facilities for various reasons. Thus tracking these patients was difficult. Based on our experience, it is important to contact the selected facilities before the visit to verify the status of their operations and performance and the number of patients who are registered and receiving treatment to confirm their eligibility for the survey. Also, it is urgent to plan and communicate in advance to ensure the involvement of appropriate stakeholders such as regional and district authorities and program staff; their involvement is critical for facilitating the movement of the field teams according to the survey plan and the smooth implementation of the survey.
Patient enrollment
Each field team consisting of 3 persons was able to enroll and interview a maximum of 9 patients per day (3 patients per team member) as the survey questionnaire was long, and some questions needed additional elaboration. The interview duration (1–1.5 h) and, in some cases, long waiting time for patients to be interviewed affected the enrollment/interview procedure as most patients had limited time for attending the clinic. This resulted in pressure on interviewers and consequent errors, including transcription, typos, and even swapped information. In some circumstances, the patients were asked to come back to clarify some information given during interviews.
In some facilities, TB patients refilled their drugs once per month, especially in the continuation phase. In this situation, even in facilities with a large number of patients, it was difficult to get enough patients for interviews during the facility visits. It was also found that some of the TB clinics were opened only on fixed days in a week. Furthermore, some patients lived more than 20 km away from the health facility. To come to the facility for an interview and travel back home, these patients hired motorcycles. Waiting to be interviewed meant more travel charges, contributing to the limited availability of some patients to participate in the survey.
Our experience demonstrated enrollment of the patients for the study could be challenging and required the development of comprehensive standardized operating procedures (SOP) to ensure patients were enrolled systematically, and consistently and enrollment does not interrupt the survey operations.
In this study, we followed the WHO guidelines on TB Patient Cost surveys and did not reach the households of TB patients who were lost to follow-up or died during TB treatment [15]. However, the households with such patients could incur higher costs than households whose patients were cured after receiving the treatment. Thus, the costs due to TB and the proportion of households that experienced catastrophic costs could be underestimated. As a long-term strategy, this is an area for future research to determine if it is feasible to include in the surveys households with patients who are lost to follow up or died, and the algorithm of the inclusion of such households in the survey.
Data collection and management
Poor recordkeeping and misplaced TB registers in health facilities were challenges during patient enrollment and potentially could affect the survey findings. During field visits, it was revealed that TB patient information in most facilities was not updated. As a result, there were records of unreported deaths and patients lost to follow-up. Some TB patients were taking their medication for more than the required period for the recommended regimen. In some areas, patients were reported to be sharing TB drugs with fellow TB patients, friends, or neighbors (those patients were not excluded from our analysis). Also, some TB patients were found to be on treatment but were not given a unique identification number as required by the program. All the above issues led to difficulties in finding their records in the unit registers.
As the survey data were collected in real-time using an electronic data collection tool, it was important to consider all the possible issues which might arise during data collection while designing the electronic questionnaire. However, the Tanzania data collection tool inadvertently omitted some restricted inputs. As a result, it was difficult to detect some errors and discrepancies between questions during interviews using the tablets. For example, some tablets were accidentally set in auto-fill mode. Therefore, if the interviewer was not careful, a different word or number might be auto-typed without notice. Additionally, due to poor internet connection, the geographic location was not captured in some areas.
For successful survey implementation, it is critical to ensure the TB registers and patient cards in the selected facilities are maintained and updated properly. The facility staff should be informed about the study before the field team’s visits to allow cleaning of TB documentation in preparation for the visits. Also, if implementing real-time data entry, ensure the data collection tool includes all possible validation codes for data entry and is equipped with a data verification module to check and clean the data in the field.
Patient incentives
The amount of money set as patient compensation for transport appeared inadequate on some occasions. This was specifically related to patients residing far from the health facility requiring transport fares higher than the minimum amount budgeted by the survey. During our survey, some inconveniences were experienced when the transport compensation money was less than the actual transport costs for patients living very far from the selected cluster providing the patient’s care and treatment. A realistic and adequate budget is critical for the smooth execution of the survey. Therefore, appropriate budgetary considerations for patient transport costs and a modest allowance for the field support provided by health care workers from the selected facilities are important.
Conclusions
Preparation for patient cost surveys needs to take into consideration of unforeseen factors which can affect the planned survey activities and findings. The reported successes and identified challenges during the first Tanzania TB Patient Cost Survey were documented across categories, including survey logistics, communication, patient enrollment, and data collection. This information provides insights for appropriate planning and the implementation of similar future surveys. It would be reasonable for the WHO to review the recommended guidelines for conducting TB patient surveys to improve the quality of the findings. Future surveys could include households of patients lost to follow-up and those who died. Households such as these may have incurred higher direct and indirect costs than households whose patients were cured after receiving treatment.
Availability of data and materials
Not applicable.
Abbreviations
- TB:
-
Tuberculosis
- DS-TB:
-
Drug-sensitive tuberculosis
- DR-TB:
-
Drug-resistant tuberculosis
- UHC:
-
Universal Health Coverage
- SDGs:
-
Sustainable Development Goals
- MOHCDGEC:
-
Ministry of Health, Community Development, Gender, Elderly, and Children
- NIMR:
-
National Institute of Medical Research
- WHO:
-
World Health Organization
- CDC:
-
Centers for Disease Control and Prevention
- PPS:
-
Probability proportional to size
- NTLP:
-
National TB and Leprosy Program
References
World Health Organization. Global Tuberculosis Report 2020. Geneva: World Health Organization; 2020.
Lönnroth K, Jaramillo E, Williams BG, Dye C, Raviglione M. Drivers of tuberculosis epidemics: the role of risk factors and social determinants. Soc Sci Med. 2009;68(12):2240–6.
Fryatt RJ. Review of published cost-effectiveness studies on tuberculosis treatment programmes. Int J Tuberc Lung Dis. 1997;1(2):101–9.
Tanimura T, Jaramillo E, Weil D, Raviglione M, Lönnroth K. Financial burden for tuberculosis patients in low- and middle-income countries: a systematic review. Eur Respir J. 2014;43(6):1763–75. https://doi.org/10.1183/09031936.00193413.
KNCV Tuberculosis Foundation, WHO, the Japan Anti-Tuberculosis Association. The Tool to Estimate Patients' Costs. Tuberculosis Coalition for Technical Assistance, US Agency for International Development, 2009. Tool to Estimate Patients Costs. http://www.stoptb.org/wg/dots_expansion/tbandpoverty/spotlight.asp/Spotlight. Accessed 27 April 2020.
Mauch V, Bonsu F, Gyapong M, Awini E, Suarez P, et al. Free tuberculosis diagnosis and treatment are not enough: patient cost evidence from three continents. Int J Tuberc Lung Dis. 2013;17:381–7.
Ukwaja KN, Alobu I, Lgwenyi C, Hopewell PC. The high cost of free tuberculosis services: patient and household costs associated with tuberculosis care in Ebonyi State, Nigeria. PLoS ONE. 2013;8(8):e73134. https://doi.org/10.1371/journal.pone.0073134.
Wynne A, Richter S, Banura L, Kipp W. Challenges in tuberculosis care in Western Uganda: health care worker and patient perspectives. Int J Africa Nurs Sci. 2014;1:6–10. https://doi.org/10.1016/j.ijans.2014.05.001.
Mauch V, Woods N, Kirubi B, Kipruto H, Sitienei J, Klinkenberg E. Assessing access barriers to tuberculosis care with the Tool to Estimate Patients' Costs: pilot results from two districts in Kenya. BMC Public Health. 2011;11(43). https://doi.org/10.1186/1471-2458-11-43.
World Health Organization. The End TB Strategy. Geneva, Switzerland: WHO; 2015. p. 1–16 WHO/HTM/TB/2015.19.
United Republic of Tanzania and United Nations Stop TB Partnership; Legal Environmental assessment for Tuberculosis (TB) in Tanzania. 2017. https://www.stoptb.org/united-republic-of-tanzania-dashboard.
Kilale AM, Pantoja A, Jani B, Range N, Ngowi BJ, et al. Economic burden of tuberculosis in Tanzania: a national survey of costs faced by tuberculosis-affected households. BMC Public Health. 2022;22(1):600. https://doi.org/10.1186/s12889-022-12987-3.
Hoa NB, Nhung NV. National tuberculosis patients cost survey: research findings lead to change in policy and practice. Viet Nam PHA. 2019;9(2):50–2.
Fuady A, Houweling TAJ, Mansyur M, Richardus JH. Catastrophic total costs in tuberculosis-affected households and their determinants since Indonesia’s implementation of universal health coverage. Infect Dis Poverty. 2018; 7(3). https://doi.org/10.1186/s40249-017-0382-3.
World Health Organization. Tuberculosis patient cost surveys: a handbook. Geneva: World Health Organization; 2017. Licence: CC BY-NC-SA 3.0 IGO.
Acknowledgements
We would like to acknowledge the Ministry of Health, Community Development, Gender, Elderly and Children, The Prime Minister’s Office, Regional and Local Governments, participated Health Facilities and the TB patients who made significant contributions in the acquisition of data. This survey would not have been possible without the financial and technical support from the U.S. Centers for Disease Control and Prevention and the World Health Organization.
Disclaimer
This project has been supported by the U.S. Centers for Disease Control and Prevention (CDC) and the President's Emergency Plan for AIDS Relief (PEPFAR) under the terms of Cooperative Agreement GH001180. The findings and conclusions in this Report are those of the authors and do not necessarily represent the official position of the U.S. Centers for Disease Control and Prevention.
Funding
Funding for the main study was provided by the US Agency for International Development (USAID) and the President’s Emergency Plan for AIDS Relief (PEPFAR) through the US Centers for Disease Control and Prevention (CDC) provided technical support in the design of the survey which is the basis for this publication; collection, analysis, and interpretation of data; and in writing the manuscript.
Author information
Authors and Affiliations
Contributions
AMK EM BJ and BM conceived the idea to conduct the study. AMK, NR, BN, CM, MM, CDM, BJ, EN, PH, SH and JE conceptualized the project. AK managed the survey implementation. CM, CDM, OK, SK, MM, and WM Led the field work during data collection. BJ and JE provided technical guidance during the survey implementation. AMK, BM, BN, MM, JE, VM, BJ, EN, EM and NR analyzed and interpreted the information for the study. All co-authors contributed in writing the manuscript. All authors read and approved the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The main study was approved by the National Health Research Ethics Committee of Tanzania (FWA00002632, expires: 9/22/2021) and Zanzibar Health Research Ethics Committee (ZAHREC) with Ref: No. ZAHREC /02/JULY/2019/12 of 31st July 2019. The study was reviewed in accordance with the U.S. Centers for Disease Control and Prevention (CDC) human research protection procedures and determined to research, but CDC investigators did not interact with human subjects or have access to identifiable data for the survey purposes (Project number: 2019–169). Individual consent for the source of data for this paper was not required.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1.
Patient cost report.
Additional file 2.
Report Nothern Zone
Additional file 3.
TB patient cost survey report
Additional file 4.
TBPCS field report.
Additional file 5.
Team number one.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Kilale, A.M., Makasi, C., Majaha, M. et al. Implementing tuberculosis patient cost surveys in resource-constrained settings: lessons from Tanzania. BMC Public Health 22, 2187 (2022). https://doi.org/10.1186/s12889-022-14607-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12889-022-14607-6