Abstract
Background
Low adoption of effective health technologies increases illness morbidity and mortality worldwide. In the case of malaria, effective tools such as malaria rapid diagnostic tests (RDTs) and artemisinin-combination therapies (ACTs) are both under-used and used inappropriately. Individuals’ confidence in RDTs and ACTs likely affects the uptake of these tools.
Methods
In a cohort of 36 households (280 individuals) in Western Kenya observed for 30 months starting in June 2017, we examined if experience with RDTs and ACTs changes people’s beliefs about these technologies and how those beliefs affect treatment behavior. Household members requested a free RDT from the study team any time they suspected a malaria illness, and positive RDT results were treated with a free ACT. We conducted annual, monthly, and sick visit surveys to elicit beliefs about the accuracy of malaria RDT results and the effectiveness of ACTs. Beliefs were elicited on a 5-point Likert scale from “very unlikely” to “very likely.”
Results
Over the study period, the percentage of survey respondents that said a hypothetical negative RDT result was “very likely” to be correct increased from approximately 55% to 75%. Controlling for initial beliefs, people who had been tested at least once with an RDT in the past year had 3.6 times higher odds (95% CI [1 1.718 7.679], P = 0.001) of saying a negative RDT was “very likely” to be correct. Confidence in testing was associated with treatment behavior: those who believed a negative RDT was “very likely” to be correct had 1.78 times higher odds (95% CI [1.079 2.934], P = 0.024) of adhering to a negative RDT result (by not taking ACTs) than those who were less certain about the accuracy of negative RDTs. Adherence to a negative test also affected subsequent beliefs: controlling for prior beliefs, those who had adhered to their previous test result had approximately twice the odds (OR = 2.19, 95% CI [1.661 2.904], P < 0.001) of saying that a hypothetical negative RDT was “very likely” to be correct compared to those who had not adhered.
Conclusions
Our results suggest that greater experience with RDTs can not only increase people’s confidence in their accuracy but also improve adherence to the test result.
Similar content being viewed by others
Background
In both high and low-income countries, underuse of effective health technologies, such as vaccinations and insecticide-treated bed nets, leads to high levels of avertable morbidity and mortality [1]. However, little is still known about how people’s beliefs evolve as they learn, and incorporate new information, about health technologies. There is some evidence that initial subsidies can encourage future adoption, while other research suggests that people can learn about the value of new technologies from their social networks [2,3,4,5,6].
In the case of malaria, there exist two very effective and accessible health tools that have contributed substantially to reductions in malaria burden: rapid diagnostic tests (RDTs) for malaria diagnosis and artemisinin-combination therapies (ACTs) for malaria treatment. Despite the availability of these tools, however, malaria remains a leading public health problem globally and especially in Sub-Saharan Africa. In 2020, approximately 241 million malaria cases were recorded globally and malaria killed an estimated 627,000 people [7].
Prompt diagnosis and appropriate treatment is key to preventing these unnecessary deaths. In 2010, the WHO recommended that all patients suspected of malaria be tested using either microscopy or RDT before receiving anti-malaria treatment [8]. In the last decade, there has been a massive scale up of RDT supply to Sub-Saharan Africa with the number of manufacturer-reported global RDTs sales increasing from less than 100 million in 2010 to approximately 419 million in 2020 [7]. This has made the WHO goal of “Test and Treat” much more feasible especially in resource-limited settings. RDTs are accurate, require minimal labor and training, and give results in approximately 15 min [9,10,11,12,13].
Despite these advantages of RDTs, presumptive treatment of fevers with anti-malarials has continued by both clinicians and patients, many of whom still believe all febrile illnesses are malaria [14,15,16]. Treating those without malaria with ACTs leads to delays in appropriate management of the illness, wastage of valuable drugs and potentially increases the spread of drug resistance [17,18,19,20]. Although Kenya adopted the WHO guidelines on “Test and Treat” a decade ago, studies in western Kenya indicate that presumptive malaria treatment, and lack of adherence to negative RDT results are common practices, especially in areas of high transmission [21,22,23].
At the same time, there is evidence that many children with malaria do not receive timely treatment with ACTs, drugs that are very effective in reducing morbidity and mortality from the disease [24,25,26,27]. Individuals’ under-estimation about the effectiveness of ACTs relative to older anti-malarial drugs could hamper uptake of the drug. A better understanding of people’s beliefs about health technologies such as RDTs and ACTs, including how these beliefs change over time, and how they relate to treatment behaviors, could improve design of interventions to encourage uptake and appropriate use [28].
We followed a cohort of households in a high transmission malaria region in Western Kenya for 30 months. During this period, household members could request a free RDT from the study team for any suspected malaria illness. Individuals with RDT-positive infections were offered free treatment with an ACT. We investigated if individuals’ beliefs about RDTs affected their testing and treatment decisions, and how those decisions subsequently influenced their beliefs about these key health technologies. The main objective of this study was to determine if experience with RDTs increases confidence in RDTs, and whether confidence in RDTs in turn improves adherence to the test result. To the best of our knowledge, this is the first longitudinal study that has investigated malaria beliefs over an extended period.
Methods
Study setting and design
This study was part of a larger study that was designed to better understand the spatial scales of malaria transmission events in the Webuye Health and Demographic Surveillance System (HDSS). The Webuye HDSS was established in 2007 to provide reliable demographic, health and economic information for planning as well as provide a platform for health research [29]. The main study (our study was a sub-analysis) enrolled a subset of households into a longitudinal cohort using an open cohort design. “We enrolled an index household at random and then neighboring households radiating outwards until 12 households were enrolled per village in a natural grouping”[30]. The three villages enrolled into the study had higher than the average transmission in the area (malaria hotspots). All members of the household who were older than 12 months and regularly slept in the household were enrolled into the study. This resulted in a total sample size of 36 households and 280 participants who were followed between June 2017 and December 2019. Two households were replaced with neighboring households when the entire household migrated (the two migrating households are not included in this analysis). The three villages were located within two sub counties (Webuye East and Webuye West Sub-Counties) in western Kenya. This region has had high transmission of malaria throughout the year with small seasonal variations [31]. The study setting is described in detail elsewhere [32,33,34].
Study procedures
We conducted three types of surveys with households. The first type of survey was an “annual survey” conducted at baseline and repeated a year later. All members of these households including children above one year were included in the enrollment. The household respondent was either the male or female who heads the household and were generally a parent or primary caregiver of children under 18. We collected demographic information on household members, as well as details about housing characteristics, household assets and bed net use from a designated household respondent (usually the male or female household head). All adults aged 18 and above were also asked about their beliefs about malaria testing and treatment. This survey was repeated a year later to collect information on any new household members as well as changes in the past year.
We refer to the second type of survey as a “monthly survey.” Every month, participating households were visited by field workers who conducted interviews with all adults in the household. At this survey, we asked each person if they had had a malaria-like illness in the past month. If they did, we collected further information about the treatment steps they took for that illness (including whether they were tested and whether they took an ACT) as well as their beliefs about malaria testing and treatment. The household respondent provided this information for individuals under the age of 18.
The third type of survey is referred to as the “sick visit survey.” During the study period, household members were asked to contact the study team whenever they, or a child, felt unwell with suspected malaria. A trained field worker would visit the household to test the sick individual using an RDT. If the test result was positive, the participant was referred to the nearest pharmacy with a voucher to purchase a free Artemether Lumefantrine (AL) that was paid for by the study (this is the recommended first-line ACT for malaria in Kenya) [35]. If the individual tested negative, they were referred for further care at the nearest health facility. Severe cases were also referred for further management. During the visit, the individual (or the parent/guardian if the sick person was under age 18) was surveyed to ask their beliefs about the likelihood their illness was malaria (both immediately before the test and after receiving their result) as well as their confidence in malaria RDTs and ACTs.
Statistical analysis
Confidence in malaria RDTs and in ACTs was assessed in all three surveys but on different subsets of the sample. In the annual surveys everyone aged 18 and above was asked questions about their confidence in malaria testing and treatment. In the monthly surveys, beliefs were elicited only for those who reported having a malaria illness in the past month. Lastly, in the sick visit surveys, people requesting and receiving an RDT from the study team were asked about confidence in testing and treatment.
We examined the relationship between 1) confidence in RDTs and the decision to be tested/adherence to the test result 2) the decision to test/test adherence and subsequent reported confidence in RDTs and ACTs 3) how individual beliefs changed over time in relation to cumulative experience with testing (long-term trend) and 4) how beliefs about an illness are updated in response to information from a test in real-time.
We used data from the most recent monthly survey or annual survey to examine how prior confidence in testing affected an individual’s decision to be tested for a malaria-like illness and also their adherence to the test result. We also used the monthly surveys to assess how adherence to an RDT result was associated with subsequent confidence in malaria RDTs and ACTs. The monthly surveys were also used to observe trends in ACT and RDT confidence over the study period.
To examine how experience with RDT testing affects people’s confidence in testing and treatment, we combined data from the annual surveys with the sick visit data. The annual surveys assessed individuals’ confidence in malaria RDTs and ACTs regardless of whether they were tested for malaria, and therefore we could analyze changes in these beliefs between the two annual surveys based on testing experience. We used the sick visit surveys to determine whether the individual was tested with an RDT from the study team between the two annual surveys and the number of times tested.
Lastly, we used data from the sick visit surveys to determine whether people used information from their RDT results to update their beliefs about the likelihood that their illness is malaria.
Beliefs about RDT accuracy were assessed using the question “If you have a fever and your malaria RDT is negative, how likely is it that the test is correct?” (with similar framing for a positive test result). For beliefs about ACT effectiveness, we asked “If you have malaria, and you take AL, how likely is it that you will be completely better in 3 days?” Lastly, for questions about the likelihood that their illness is malaria, we asked “How likely is it that the illness is malaria?” For all beliefs questions, responses were given on a 5-point Likert scale from “Very Likely” to “Very Unlikely.” As in other studies [21, 36], in order to simplify the analysis and interpretation of the results, we dichotomized these beliefs into a binary variable that consisted of “Very Likely” compared to all other responses. In the case of children under the age of 18, we used the responses of the household respondent. The household respondent was either the male or female head of the household and was generally the child’s parent or primary caregiver. We defined adherence to the test result as taking an ACT if they tested positive for malaria, and not taking an ACT if they tested negative for malaria.
We first conducted a descriptive analysis and present summary statistics on the sample in terms of proportions and means/medians. We then examined associations between beliefs and behavior using logistic regressions. We adjusted our standard errors for clustering of the outcome by household. We conducted both bi-variate analyses as well as adjusted regressions that included village fixed effects as well as controls for age, gender, education (less than primary versus primary or more), whether they slept under a bed net the previous night, whether they own more than an acre of land, and whether they got water from a protected source. The last two variables were included as measures of socio-economic status that varied within the sample. In most cases, we present and discuss the adjusted results in the text. All analyses were conducted using STATA 15.1 [37].
Results
Sample characteristics
The sample included 36 households, which consisted of 280 individuals (60 of whom were added over the course of the study period). The additional number of participants was a result of new members joining the cohort households (for example through birth). The median age of the household respondent, who provided information for children under the age of 18, was 42 years (IQR = 23) and 15 (42%) were female. 21 respondents (58%) had at least a primary education (Table 1, Panel A).
Of the 280 individuals in the sample (including the household respondent), 151(54%) were female, and 110 (39%) were aged 18 or above. Among the 110 individuals aged 18 or older, 67 (61%) had at least a primary level education. At the baseline survey, 92 (88%) had previously heard of RDTs, and among those who had, 85 (94%) also had previous experience with an RDT (Table 1, Panel B).
At baseline, confidence in RDTs and ACTs was low, in spite of high levels of awareness and experience with this technology. Among those individuals above the age of 18, 82(92%) believed that a positive RDT result was “very likely” to be correct, however only 55 (63%) believed that a negative RDT result was “very likely” to be correct. In addition, only 60 (60%) believed that AL was “very effective” in treating malaria (Table 1, Panel B).
We conducted 5,617 household surveys between June 2017 and December 2019 (including both monthly and annual surveys). In those surveys, 909 people (16%) reported having a malaria-like illness in the past month and 638 (70%) of those were tested with an RDT (from either the study team or elsewhere). 337 (53%) of those tests were reported as being positive for malaria. While 323 (96%) people adhered to a positive test result, only 182 out of the 300 (61%) who reported testing negative adhered to their test result (Table 1, Panel C).
Confidence in malaria testing and treatment behavior
Table 2 shows the association between confidence in malaria RDTs and two key malaria treatment behaviors: whether an individual was tested with an RDT when they had a fever or malaria-like illness and whether an individual who was tested adhered to a negative RDT result. We focused on adherence to a negative test because adherence to a positive test was already very high. Compared to those with lower confidence in RDTs, those who believed a negative RDT was “very likely to be correct” were not more likely to get tested with an RDT (aOR = 1.31, 95% CI [0.866 1.976], P = 0.203), but, when they were tested with RDT, had 78% higher odds of adhering to a negative RDT result (aOR = 1.78, 95% CI [1.079 2.934], P = 0.024).
Experience with testing and confidence in testing and treatment
Over the 30-month study period, monthly beliefs data collected from all individuals who had a malaria-like illness, regardless of whether they were tested, show that confidence in both RDTs and ACTs increased steadily over time (Fig. 1). For RDTs, the proportion of people who said they believed a negative RDT was “very likely” to be correct increased from approximately 55% to 75%. The proportion of people who believed AL was “very likely” effective in treating malaria increased from approximately 75% to nearly 95%.
Confidence in RDTs (blue line, Panel A) and in AL (blue line, Panel B) over the survey period. Notes: Red lines indicate the proportion of illnesses tested with an RDT (Panel A) and the proportion of RDT-positives treated with AL (Panel B) over the same time period. Data is from monthly surveys and therefore only includes people who reported a malaria illness in the past month. Beliefs are those of the household respondent for children under 18
When we compared those who were tested for malaria with those who were not tested, we find further evidence that testing experience was associated with higher confidence in RDTs. Those who had any study RDT over the first study year had approximately three times higher odds of believing a negative RDT was “very likely” to be correct at the end of the year, controlling for their beliefs at the start of the study period (aOR = 3.63, 95% CI [1.718 7.679], P = 0.001). We find no evidence, however, that the number of tests people had over this time period was associated with their confidence in RDTs (Fig. 2).
Confidence in RDTs by the number of tests an individual had over the first year of the study. Notes: Number of tests are limited to those that were performed by the study team. Beliefs are those of the respondent for children under 18. The differences between no RDTs and 1, 2, or 3 + RDT categories are statistically significant at P < 0.05, none of the other pairwise comparisons are statistically significant
We also didn’t find strong evidence that the results of the test influenced changes in beliefs; those who received at least one positive RDT over the first year had slightly higher odds of strong confidence in RDTs at the end of the year but this association did not hold in the adjusted model and was not observed among those who had received at least one negative RDT during that time (Table 3).
Lastly, we did not find any statistically significant association between having been tested with an RDT and the odds of saying that AL was “very likely” to be effective in treating malaria at the end of the first year (Appendix Table A1).
Treatment behavior and confidence in testing and treatment
In Table 4 we show how adherence to the test result was associated with individuals’ subsequent confidence in RDT testing, controlling for their confidence in RDTs before the illness. We find that those who adhered to their malaria test result had approximately twice the odds of saying that a hypothetical negative RDT was “very likely” to be correct after the illness compared to those who did not adhere to the test result (aOR = 2.20, 95% CI [1.661 2.904], P < 0.001). When we split this out by adherence to a positive versus a negative test result, we find little effect of adherence to a positive test result (aOR = 1.07, 95% CI [0.316 3.594], P = 0.918), but a significant difference in confidence from those who adhered to a negative test result relative to those who did not adhere (aOR = 2.09, 95% CI [1.403 3.116], P < 0.001).
We also find some evidence that those who adhered to a positive test result were more likely to subsequently say that AL was “very effective” in treating malaria compared to those who did not adhere, however our results are not statistically significant (Appendix Table A2).
Updating beliefs with test result
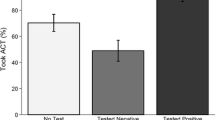
Figure 3 demonstrates that individuals used the information from the test to update their beliefs about the likelihood that their (or their child’s) illness was malaria. For example, we find that for individuals who tested positive on their RDT, 87% said it was “very likely” their illness was malaria before the test, compared to nearly 100% after the test result (P < 0.001). For those who tested negative, 61% said it was “very likely” their illness was malaria before testing, compared to only 14% after the test result (P < 0.001).
We also found evidence that the degree to which people updated their beliefs based on the test results depended on their prior confidence in testing (Appendix Figure A1). Those who had said that a hypothetical negative RDT was “likely” or “very likely” to be correct, were more likely to revise downwards their belief that an illness was malaria after a negative test result, compared to those who said that they were less confident in a negative RDT result (P < 0.001).
Discussion
This study found three main results. First, we showed that people’s beliefs about malaria testing affected their treatment behavior. In particular, we found that those who had higher confidence in RDT testing were more likely to adhere to a negative test result. We did not find that confidence in testing affected whether an individual got tested, similar to a previous study [38]. However, as in that study, the bar to testing was very low for participants: the RDT was free, a member of the study team would come and test at the household, and participants could get a free ACT if they tested positive for malaria. Our results differ from Maffioli et al. [36] however, in that they did not find that beliefs predicted adherence to the test result, possibly because individuals had less experience with RDTs at that time.
Second, we found evidence that treatment experiences also affected people’s subsequent beliefs about testing. For example, we showed that those who had an RDT during the first year of the study period had higher confidence in RDTs at the end of the year than those who were not tested during that time (controlling for their initial confidence in testing). In addition, we also showed that those who adhered to a negative test result were more likely to express high confidence in a hypothetical negative test at the next survey than those who did not adhere (once again controlling for their initial confidence in a negative test). These results suggest that people learn from their experience with testing and treatment. Our results are consistent with findings from population-level studies that show that greater access to testing increases people’s confidence in malaria testing [38] and can reduce inappropriate use of ACTs [22, 39]. Unlike a previous study [40] however, we did not find any statistically significant effects of testing experience on confidence in AL, perhaps because confidence in AL was higher compared to confidence in RDTs, and adherence to a positive RDT was close to 100%, thus limiting the scope for learning about AL effectiveness.
Lastly, we demonstrated that people used the information from the test to update their beliefs in the way that we would expect. Those who tested positive revised upwards the likelihood that their illness was malaria, while those who tested negative revised downward their beliefs about the likelihood that their illness was malaria. This suggests that the information from malaria testing could play an important role in people’s treatment decisions.
There are several limitations in this study. Given that our study was conducted with 36 households in Western Kenya, it is possible that prevailing pre-conceptions among that group may not translate widely to other settings. Moreover, when considering individual-level beliefs, we do not account for the fact that household members could also learn from each other’s testing and treatment decisions (for example, a parent/guardian could learn from their own test results but also from those of their children). We did adjust our standard errors for clustering by household to account for the fact that observations within households were not independent from each other. Third, even though we controlled for initial beliefs as well as other demographic factors, it is likely that those who chose to be tested with an RDT were different from those who did not in unobservable ways. Lastly, the way people’s beliefs about malaria likelihood and beliefs about AL effectiveness were dichotomized (“very likely” compared to all others) means that the analysis focused on the degree to which respondents were fully confident in their response or had some uncertainty.
Nonetheless, our study design also has several strengths. Since we followed the same people over time, we could see how individual beliefs changed because of testing and treatment decisions, rather than focusing simply on population-level changes as testing becomes more available. Furthermore, we collected beliefs at multiple time points: before testing, after testing, and after treatment. This allowed us to observe how beliefs change at each treatment step.
Conclusions
Overall, our results suggest that people’s beliefs have an important role in treatment behavior but also that treatment behavior can in turn influence those beliefs. In terms of policy implications, our results suggest that lowering the barriers to testing would not only increase access to malaria RDTs thus potentially improving appropriate use of ACTs, but could also be beneficial in terms of community learning about the value of these new treatment technologies. Strategies for increasing uptake of RDTs (and other new health technologies) could include large subsidies [41,42,43], and making them more convenient and accessible such as at local drug shops, [44, 45] or through community health workers.[22, 46, 47]. With lower barriers, people can experiment with the technology thereby gaining confidence in its value, and promoting further uptake and appropriate use. These strategies could also potentially be used for increasing confidence and uptake of similar health tools for other diseases such as COVID-19 and HIV.
Availability of data and materials
The data is currently still in use by colleagues who are writing up on other aspects of the main study. However, these datasets used and/or analyzed during the current study can be availed by the corresponding author upon reasonable request.
Abbreviations
- ACT:
-
Artemisinin-Combination Therapies
- AL:
-
Artemether Lumefantrine
- HDSS:
-
Health and Demographic Surveillance Site
- RDT:
-
Rapid Diagnostic Test
References
Dupas P. Health Behavior in Developing Countries. Ann Rev Econ. 2011;3(1):425–49.
Dupas P. Short-run subsidies and long-run adoption of new health products: evidence from a field experiment. Econometrica. 2014;82(1):197–228.
Miller G, Mobarak AM. Learning About New Technologies Through Social Networks: Experimental Evidence on Nontraditional Stoves in Bangladesh. Mark Sci. 2015;34(4):480–99.
Saran I, Fink G, McConnell M. How does anonymous online peer communication affect prevention behavior? Evidence from a laboratory experiment. PLoS One. 2018;13(11):e0207679.
Rao N, Mobius MM, Rosenblat T. Social Networks and Vaccination Decisions. 2007.
Gunther Bensch JP. One-Off Subsidies and Long-Run Adoption—Experimental Evidence on Improved Cooking Stoves in Senegal. Am J Agr Econ. 2019;102(1):72–90.
WHO. World malaria report 2021. Geneva: World Health Organization; 2021.
WHO Guidelines for malaria,16 February 2021.Geneva: World Health Organization; 2021(WHO/UCN/GMP/2021.01). Licence: CC BY-NC-SA 3.0 IGO.
Shillcutt S, Morel C, Goodman C, Coleman P, Bell D, Whitty CJ, et al. Cost-effectiveness of malaria diagnostic methods in sub-Saharan Africa in an era of combination therapy. Bull World Health Organ. 2008;86(2):101–10.
Uzochukwu BS, Obikeze EN, Onwujekwe OE, Onoka CA, Griffiths UK. Cost-effectiveness analysis of rapid diagnostic test, microscopy and syndromic approach in the diagnosis of malaria in Nigeria: implications for scaling-up deployment of ACT. Malar J. 2009;8:265.
Ansah EK, Narh-Bana S, Epokor M, Akanpigbiam S, Quartey AA, Gyapong J, et al. Rapid testing for malaria in settings where microscopy is available and peripheral clinics where only presumptive treatment is available: a randomised controlled trial in Ghana. BMJ (Clinical research ed). 2010;340:c930.
Diggle E, Asgary R, Gore-Langton G, Nahashon E, Mungai J, Harrison R, et al. Perceptions of malaria and acceptance of rapid diagnostic tests and related treatment practises among community members and health care providers in Greater Garissa, North Eastern Province. Kenya Malar J. 2014;13:502.
Ajumobi O, Sabitu K, Nguku P, Kwaga J, Ntadom G, Gitta S, et al. Performance of an HRP-2 rapid diagnostic test in Nigerian children less than 5 years of age. Am J Trop Med Hyg. 2015;92(4):828–33.
Reyburn H, Mbatia R, Drakeley C, Carneiro I, Mwakasungula E, Mwerinde O, et al. Overdiagnosis of malaria in patients with severe febrile illness in Tanzania: a prospective study. BMJ (Clinical research ed). 2004;329(7476):1212.
Kabaghe AN, Visser BJ, Spijker R, Phiri KS, Grobusch MP, van Vugt M. Health workers’ compliance to rapid diagnostic tests (RDTs) to guide malaria treatment: a systematic review and meta-analysis. Malar J. 2016;15(1):163.
Boyce MR, O’Meara WP. Use of malaria RDTs in various health contexts across sub-Saharan Africa: a systematic review. BMC Public Health. 2017;17(1):470.
Lin JT, Juliano JJ, Wongsrichanalai C. Drug-Resistant Malaria: The Era of ACT. Curr Infect Dis Rep. 2010;12(3):165–73.
Ashley EA, Dhorda M, Fairhurst RM, Amaratunga C, Lim P, Suon S, et al. Spread of artemisinin resistance in Plasmodium falciparum malaria. N Engl J Med. 2014;371(5):411–23.
Sinclair D, Zani B, Donegan S, Olliaro P, Garner P. Artemisinin‐based combination therapy for treating uncomplicated malaria. Cochrane Database Syst Rev. 2009;3(CD007483). https://doi.org/10.1002/14651858.CD007483.
Thwing J, Eisele TP, Steketee RW. Protective efficacy of malaria case management and intermittent preventive treatment for preventing malaria mortality in children: a systematic review for the Lives Saved Tool. BMC Public Health. 2011;11 Suppl 3(Suppl 3):S14.
Saran I, Maffioli EM, Menya D, O’Meara WP. Household beliefs about malaria testing and treatment in Western Kenya: the role of health worker adherence to malaria test results. Malar J. 2017;16(1):349.
Prudhomme O’Meara W, Menya D, Laktabai J, Platt A, Saran I, Maffioli E, et al. Improving rational use of ACTs through diagnosis-dependent subsidies: Evidence from a cluster-randomized controlled trial in western Kenya. PLoS Med. 2018;15(7):e1002607.
Laktabai J, Saran I, Zhou Y, Simmons RA, Turner EL, Visser T, et al. Subsidise the test, the treatment or both? Results of an individually randomised controlled trial of the management of suspected malaria fevers in the retail sector in western Kenya. BMJ Glob Health. 2020;5(11):e003378.
Bennett A, Bisanzio D, Yukich JO, Mappin B, Fergus CA, Lynch M, et al. Population coverage of artemisinin-based combination treatment in children younger than 5 years with fever and Plasmodium falciparum infection in Africa, 2003–2015: a modelling study using data from national surveys. Lancet Global Health. 2017;5(4):e418–27.
Cohen JL, Leslie HH, Saran I, Fink G. Quality of clinical management of children diagnosed with malaria: A cross-sectional assessment in 9 sub-Saharan African countries between 2007–2018. PLoS Med. 2020;17(9):e1003254.
Ogutu BR, Onyango KO, Koskei N, Omondi EK, Ongecha JM, Otieno GA, et al. Efficacy and safety of artemether-lumefantrine and dihydroartemisinin-piperaquine in the treatment of uncomplicated Plasmodium falciparum malaria in Kenyan children aged less than five years: results of an open-label, randomized, single-centre study. Malar J. 2014;13(1):1–10.
Kishoyian G, Njagi ENM, Orinda GO, Kimani FT, Thiongo K, Matoke-Muhia D. Efficacy of artemisinin-lumefantrine for treatment of uncomplicated malaria after more than a decade of its use in Kenya. Epidemiol Infect. 2021;149:e27.
Hansen KS, Lesner TH, Østerdal LP. Optimal price subsidies for appropriate malaria testing and treatment behaviour. Malar J. 2016;15(1):534.
Chrispinus JS, Violet N, Andrew AO, David OO, Paul A, Dinah C, Diana M, Patrick MC, Davy VB, Jan DM, Barasa O. Establishing Webuye Health and Demographic Surveillance Site in Rural Western Kenya: Challenges and Lessons Learned. Population Association of America, 2013 Annual meeting.
O’Meara WP, Simmons R, Bullins P, Freedman B, Abel L, Mangeni J, et al. Mosquito exposure and malaria morbidity: a microlevel analysis of household mosquito populations and malaria in a Population-Based longitudinal cohort in western Kenya. J Infect Dis. 2020;221(7):1176–84.
Bungoma District Health Management Team B health annual report, 1995. Bungoma Town, Kenya: 1996.
Mangeni J, Ongore D, Mwangi A, Vulule J, Ocala A, O’Meara W. Malaria, “hotspots” within a larger hotspot; what’s the role of behavioural factors in fine scale heterogeneity in western Kenya? East Afr Med J. 2017;94(5):369–84.
Mangeni J, Ongore D, Mwangi A, Vulule J, O’Meara WP, Obala A. Prevalence, heterogeneity of asymptomatic malaria infections and associated factors in a high transmission region. East Afr Med J. 2017;94(12):1067–79.
O’Meara WP, Simmons R, Bullins P, Freedman B, Abel L, Mangeni J, et al. Mosquito Exposure and Malaria Morbidity: A Microlevel Analysis of Household Mosquito Populations and Malaria in a Population-Based Longitudinal Cohort in Western Kenya. J Infect Dis. 2020;221(7):1176–84.
Ministry of Health Kenya M. NATIONAL GUIDELINES FOR THE DIAGNOSIS, TREATMENT AND PREVENTION OF MALARIA IN KENYA. Nairobi: Ministry of Public Health and Sanitation, Malaria Division; 2010.
Maffioli EM, Mohanan M, Saran I, O’Meara WP. Does improving appropriate use of malaria medicines change population beliefs in testing and treatment? Evidence from a randomized controlled trial. Health Policy Plan. 2020;35(5):556–66.
StataCorp. Stata Statistical Software: Release 15. College Station: StataCorp LLC; 2017.
Maffioli EM, Prudhomme O’Meara W, Turner EL, Mohanan M. Can individuals’ beliefs help us understand nonadherence to malaria test results? Evidence from rural Kenya. Rev Dev Econ. 2021;25(1):163–82.
Thiam S, Thior M, Faye B, Ndiop M, Diouf ML, Diouf MB, et al. Major reduction in anti-malarial drug consumption in Senegal after nation-wide introduction of malaria rapid diagnostic tests. PLoS ONE. 2011;6(4):e18419.
Adhvaryu A. Learning, Misallocation, and Technology Adoption: Evidence from New Malaria Therapy in Tanzania. Rev Econ Stud. 2014;81(4):1331–65.
Prudhomme O’Meara W, Mohanan M, Laktabai J, Lesser A, Platt A, Maffioli E, et al. Assessing the independent and combined effects of subsidies for antimalarials and rapid diagnostic testing on fever management decisions in the retail sector: results from a factorial randomised trial in western Kenya. BMJ Glob Health. 2016;1(2):e000101.
Laktabai J, Saran I, Zhou Y, Simmons RA, Turner EL, Visser T, et al. Subsidise the test, the treatment or both? Results of an individually randomised controlled trial of the management of suspected malaria fevers in the retail sector in western Kenya. BMJ Glob Health. 2020;5(11):e003378.
Cohen J, Dupas P, Schaner S. Price subsidies, diagnostic tests, and targeting of malaria treatment: evidence from a randomized controlled trial. Am Econ Rev. 2015;105(2):609–45.
Visser T, Bruxvoort K, Maloney K, Leslie T, Barat LM, Allan R, et al. Introducing malaria rapid diagnostic tests in private medicine retail outlets: a systematic literature review. PLoS ONE. 2017;12(3):e0173093.
Poyer S, Musuva A, Njoki N, Okara R, Cutherell A, Sievers D, et al. Fever case management at private health facilities and private pharmacies on the Kenyan coast: analysis of data from two rounds of client exit interviews and mystery client visits. Malar J. 2018;17(1):1–17.
Boyce MR, Menya D, Turner EL, Laktabai J, Prudhomme-O’Meara W. Evaluation of malaria rapid diagnostic test (RDT) use by community health workers: a longitudinal study in western Kenya. Malaria J. 2018;17(1):1–11.
Boyce MR, O’Meara WP. Use of malaria RDTs in various health contexts across sub-Saharan Africa: a systematic review. BMC Public Health. 2017;17(1):1–15.
Acknowledgements
We thank the study households for their participation in this study. We also are appreciative of the study implementation skills of project manager and field technicians in Webuye and Eldoret: J. Kipkoech Kirui, I. Khaoya, L. Marango, E. Mukeli, E. Nalianya, J. Namae, L. Nukewa, E. Wamalwa, and A. Wekesa.
Funding
This work was supported by the National Institute of Allergy and Infectious Diseases (R21AI126024 to WPO and R01 AI146849 to WPO and SMT).
Author information
Authors and Affiliations
Contributions
J.N.M, I.S, S.T and W.P.O designed the study, conducted the experiments, analysis and wrote the manuscript. L A and A. O designed the experiment, conducted the experiments and contributed to the writing of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study was approved by the ethical review boards of Moi University (2017/36) and Duke University (Pro00082000). All study participants provided written informed consent or assent (for children). All methods were carried out in accordance with relevant guidelines and regulations.
Consent for publication
Not applicable. All data used has been anonymized and de-identified.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Appendix Table A1.
Association between Testing Experience and Confidence in AL. Appendix Table A2. Association between Adherence to Test Result and Confidence in AL. Appendix Figure A1. Change in beliefs illness likely malaria before and after negative test result by confidence in the test. Data source is sick visit surveys. Beliefs are those of the respondent for children under 18.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Mangeni, J.N., Abel, L., Taylor, S.M. et al. Experience and confidence in health technologies: evidence from malaria testing and treatment in Western Kenya. BMC Public Health 22, 1689 (2022). https://doi.org/10.1186/s12889-022-14102-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12889-022-14102-y